Abstract
Ecological communities that occupy similar habitats may exhibit functional convergence despite significant geographical distances and taxonomic dissimilarity. On coral reefs, steep gradients in key environmental variables (e.g. light and wave energy) restrict some species to shallow depths. We show that depth-generalist reef fishes are correlated with two species-level traits: caudal fin aspect ratio and diet. Fishes with high aspect ratio (lunate) caudal fins produce weaker vortices in the water column while swimming, and we propose that ‘silent swimming’ reduces the likelihood of detection and provides an advantage on deeper reefs with lower light irradiance and water motion. Significant differences in depth preference among trophic guilds reflect variations in the availability of different food sources along a depth gradient. The significance of these two traits across three geographically and taxonomically distinct assemblages suggests that deep-water habitats exert a strong environmental filter on coral reef-fish assemblages.
Keywords: environmental filtering, caudal fin, aspect ratio, trophic guild, mesophotic, depth range
1. Background
Community assembly is generally considered to be deterministic at the functional level, because geographically isolated communities with different species pools often show similarity in community structure [1,2]. Consequently, functional trait-based approaches allow for generality in describing ecological communities, providing many advantages for understanding community assembly over traditional methods based on species identities [3]. Analysis of functional traits can explain community structure by disentangling the effects of two key factors that influence community assembly: competition between species (limiting similarity) and adaptation to local environmental conditions (environmental filtering) [4,5]. Environmental filtering selects species that share certain adaptive traits that enable them to persist in a particular environment [6], leading to greater similarity in the traits present within a community existing in a particular environment. Limiting similarity has the opposite effect, because species with very similar traits will compete for shared resources [7].
Environmental filtering causes convergence of traits among communities from similar habitats, because comparable habitats will impose similar environmental filters [2,8]. The importance of environmental filters in generating functionally similar communities has been demonstrated across a range of marine and terrestrial ecosystems [2,9]. Consequently, identifying functional traits shared among geographically and taxonomically disparate assemblages provides valuable insights into environmental filters influencing community structure. For example, taxonomically dissimilar fish communities in freshwater streams in Europe and North America showed considerable convergence in biological traits along hydraulic and geomorphic gradients [10]. However, few studies have examined community convergence of functional traits in marine ecosystems.
Coral reefs are the most diverse marine ecosystems on earth, occurring across all tropical oceans. Globally, coral reefs are becoming increasingly degraded by numerous disturbances operating at multiple spatial scales [11]. Fishes play a critical role in maintaining functioning coral reef ecosystems and avoiding shifts to alternative, undesirable ecosystem states (e.g. macroalgal-dominated reefs) [12,13]. However, disturbances such as habitat degradation and overfishing are altering the abundance and structure of fish communities [13–15]. Fish species capable of using deep-water or ‘mesophotic’ coral reef habitats (depths more than 30 m; [16]) are considered to be at lower risk of local extinction than fishes confined to shallow habitats [17], because habitat disturbances and fishing pressure often decline with increasing depth [18]. Data on the composition and structure of fish communities on reefs at depths greater than 20 m are rare due to logistical restrictions associated with SCUBA surveys, whereas remote data collection techniques such as baited videos effectively target higher trophic levels only. Consequently, patterns and causes of depth distributions in coral reef fishes are poorly described, and alternative methods are needed for estimating vulnerability of reef fishes to local extinction. Living in deeper waters presents many challenges to coral reef fishes, including higher pressure, decreased light irradiance and lower and/or more variable temperatures [19]. Since environmental filtering is generally more prominent in harsher environments [20], mesophotic fish assemblages could be expected to show convergence in functional traits across biogeographic regions.
Here, we identify ecological and morphological traits of depth-generalist fishes to predict which functional groups are likely to be capable of using deep-water refuges. We compared mesophotic and shallow reef communities in three coral reef regions across two ocean basins, separated for over three million years and differing in taxonomic composition: Puerto Rico in the Western Atlantic, the Northwestern Hawaiian Islands (NWHI) in the Central Pacific and Pohnpei in the Caroline Islands (Western Pacific). We identify common characteristics among ‘depth-generalist’ fishes that are widespread among both shallow (less than 30 m depth) and mesophotic (deeper than 30 m) reef habitats, and test how ecological and morphological traits relate to the ability of species to span a broad depth range. We also identify environmental filters leading to convergence in functional diversity of deep-water fish assemblages across disparate geographical locations. We focused on Puerto Rico, NWHI and Pohnpei (electronic supplementary material, figure S1), because (i) their mesophotic reef-fish fauna have been recently surveyed with the use of SCUBA, yielding a detailed taxonomic composition along a depth gradient; (ii) the species composition of shallow reef fish communities, and their traits, are well-known; and (iii) they are geographically distant and support taxonomically distinct fish assemblages, thus providing an opportunity to test assumptions regarding community convergence theory and to verify the generality of our results [3]. Our hypotheses (electronic supplementary material, table S1) were based on expectations that traits related to resource acquisition, species movement and morphological adaptations to low-light environments will be positive predictors of depth generality. Our results provide a mechanistic understanding of the environmental filters influencing the depth ranges of coral reef fishes and provide new insights into their extinction vulnerability.
2. Methods
(a). Data collection
Depth ranges of coral reef fishes derived from empirical observations collected using open- and closed-circuit SCUBA by the authors (R.R.C. and C.N.K. for Pohnpei and NWHI, respectively), from published literature [21,22] and from US National Oceanic and Atmospheric Administration (NOAA) Coral Reef Ecosystem monitoring data accessed via the NOAA Data Catalogue http://www8.nos.noaa.gov/bpdmWeb/queryMain.aspx. Diver-based surveys used 25 × 2 m belt transects at 10 m depth intervals (see [21]). We use empirical observations on species depth ranges rather than global-scale databases, because the latter are generally derived from fisheries records, are biased towards certain species and geographical locations and are too imprecise for our analyses, which require accurate data on species composition among depths [23]. Locations were chosen because their fish faunas have been surveyed using comparable methods across a wide bathymetric range spanning both shallow and mesophotic reef habitats. The fish assemblage at each location was divided into ‘shallow specialists’ and ‘depth generalists’, based on their occurrence either side of 30, 40 and 50 m depth contours (see §2b). Shallow specialists occurred exclusively above and depth-generalists both above and below the specified depth contour. Relatively few species occurred exclusively at less than 50 m; therefore, deep specialists were not included as a separate group.
We selected ecological and morphological traits that could potentially influence the ability of shallow-water species to associate with mesophotic reefs (electronic supplementary material, table S1). For each species, data on level occupied in the water column, aspect ratio of the caudal fin, proportion of eye diameter (a proxy for visual acuity in low light), nocturnal activity, diet, body length, body height and schooling behaviour were obtained for the 298 reef fish species recorded across all sites (electronic supplementary material, table S2). We recorded 114 species from the Puerto Rico, 137 species from the NWHI and 70 species from Pohnpei, with 23 species recorded in both the NWHI and Pohnpei. Species location (Puerto Rico, NWHI or Pohnpei) was included as an interactive predictive trait to test the generality of our results among locations.
Highly mobile species are more capable of moving between shallow and deep habitats on diel or seasonal timeframes [24]. The level in the water column in which species usually forage correlates with swimming ability [12]; therefore, we assigned species to one of three categories based on the position in the water column where they usually forage as adults and/or juveniles: (i) bottom-dwellers are species that remain on or within benthic habitats and are usually sedentary or territorial; (ii) low-level swimmers are demersal fishes that roam just above the reef (usually less than 2 m from the substrate); and (iii) high-level swimmers are semi-pelagic species that normally swim well above (more than 2 m) the substrate, usually roaming from one reef to another within the same day or over a short period of time. Similarly, the shape of the caudal fin is associated with swimming efficiency [25]. Species with a higher aspect ratio of the caudal fin (i.e. lunate or forked fins) are more mobile and are predicted to occur across a broad depth range.
Light irradiance in the ocean declines exponentially with depth, therefore, species with large eyes or exhibiting nocturnal behaviour may be better suited to deeper, low-light habitats. We therefore included eye size and nocturnal activity as predictor variables in our analyses. Nocturnal behaviour was determined by the time of day when a species primarily forages. Diminishing light irradiance with increasing depth can also influence resource acquisition. For example, low light availability constrains the growth of photosynthetic algae, causing a reduction in the abundance of herbivorous fishes [22,26]. In contrast, planktivorous species tend to be relatively more abundant in deeper waters [22,27,28]. We assigned species to one of four trophic guilds: (i) carnivores; (ii) herbivores; (iii) piscivores; and (iv) zooplanktivores based on the published literature (see electronic supplementary material, table S1). Low ambient light reduces the capacity for prey species to visually detect predators in deeper mesophotic habitats, making them vulnerable to predation in twilight [29]. Therefore, we tested three traits related to predation avoidance: body length, body height and schooling behaviour. We designated as schooling species those that regularly form polarized, cohesive groups of 20 or more conspecific individuals [30].
To assess the generality of the relationships between depth-generalism and species traits we included additional terms in our model representing interactions between locality (Puerto Rico, NWHI or Pohnpei) and each of our predictor variables. Significant interactions between locality and traits indicate that relationships between depth-generalism and traits vary among locations. If no significant interactions are found, then the results can be generalized among locations.
Data on the location, level occupied in the water column, nocturnal activity, diet, maximum body length and schooling behaviour were obtained from the literature (see electronic supplementary material), the global database FishBase (www.fishbase.org) and from authors’ experience observing fishes in the field. Morphometric data (eye diameter, body height and aspect of the caudal fin ratio) were also obtained from FishBase. Aspect ratio of the caudal fin is defined by the equation
where h is the height of the caudal fin and s is its surface area [31].
As a conservative measure to avoid problems associated with species detection and identification by scuba divers in the mesophotic environment, we included only species in non-cryptic and conspicuous families: Acanthuridae, Apogonidae, Balistidae, Caesionidae, Carangidae, Chaetodontidae, Diodontidae, Epinephelidae, Haemulidae, Holocentridae, Labridae (including Scarinae), Lethrinidae, Lutjanidae, Monacanthidae, Mullidae, Ostraciidae, Pomacanthidae, Pomacentridae, Priacanthidae, Serranidae, Siganidae, Tetraodontidae and Zanclidae. For the purposes of our analysis, the speciose family Pomacentridae was divided into two groups: sedentary and actively swimming genera, based on their swimming mode. The genera Acanthochromis, Amblyglyphidodon, Amphiprion, Dascyllus, Microspathodon, Plectroglyphidodon and Stegastes generally employ mean or paired fin (MPF) swimming and were classified as sedentary, whereas Abudefduf, Chromis and Chrysiptera commonly use body or caudal fin (BCF) swimming and were considered active swimmers. Trait data for all species used in our analysis are presented in electronic supplementary material, table S2.
(b). Data analysis
The effects of different species-level traits on depth-generalism were analysed using generalized linear mixed-effects models (GLMMs), assuming a binomial distribution for the response variable (0, shallow specialists; 1, depth-generalists) and a logit link function for the predictor variables [32]. Models combined species from all three regions, with each species assigned to a locality (Puerto Rico, NWHI or Pohnpei) that was included in the model as a fixed variable to test for interactions with traits. Taxon (genus nested within family) was included as a random variable to account for the non-independence of species owing to shared ancestry. Nested random variation is represented as taxon-level differences of families and genera around the overall ‘fixed’ effects, attributable to other variables that can then be generalized to the entire fauna [33]. For the predictor variable ‘diet’, we also used an exact binomial test to determine whether the average number of depth-generalists within the four trophic guilds was significantly different from all other trophic guilds. Results are presented as the proportional difference between focal groupings relative to all other groupings (i.e. bars above zero indicate that a group has more depth-generalists than expected by chance). We used 95% confidence intervals to indicate any overlap with zero, indicating ‘no difference’. All variables were tested for collinearity prior to analyses (|r| > 0.7). For model selection, we followed the backward stepwise procedure outlined in [32], which entailed sequential removal of non-significant fixed-effect terms (p > 0.05) from the full model based on log-likelihood ratio tests. The GLMMs were fitted using the function ‘lmer’ in the package ‘lme4’ [34] in R.
To explicitly test whether our results could be attributed to shared ancestry, we conducted additional models that applied a phylogenetic correction. We generated a phylogenetic tree for 201 of the 299 species in our model for which suitable data are available [35,36] (electronic supplementary material, figure S2), then applied a phylogenetic correction to our models using phylogenetic generalized linear models (PGLMs) using the function ‘phyloglm’ in the package ‘phylolm’ [37].
3. Results
Our results were similar for models using 30, 40 and 50 m depth thresholds. Our figures refer to the 50 m model, but the results for 30, 40 and 50 m models are presented in table 1. GLMMs and PGLMs also showed similar results, indicating that our results are not attributable to shared ancestry. Phylogenies are not available for many reef fish families, resulting in a lower sample size in the PGLMs. The results below therefore refer to GLMMs, but the results of PGLMs are presented in the electronic supplementary material.
Table 1.
Final predictive generalized linear mixed-effects models with the lowest Akaike information criterion (AIC) values (showing estimates, standard error and p-values). Italicized values indicate statistical significance at p < 0.05. Reference level for diet is set as ‘carnivore’ and as ‘northwestern Hawaiian Islands’ for region.
| variables | est. | s.e. | z-value | p-value |
|---|---|---|---|---|
| 50 m threshold | ||||
| intercept | −0.385 | 0.362 | −1.064 | 0.287 |
| aspect ratio of the caudal fin | 1.694 | 0.496 | 3.410 | <0.001 |
| diet | ||||
| herbivores | −1.286 | 0.470 | −2.736 | 0.006 |
| piscivores | 0.122 | 0.510 | 0.241 | 0.809 |
| zooplanktivores | 0.166 | 0.494 | 0.336 | 0.736 |
| 40 m threshold | ||||
| intercept | −0.275 | 0.333 | −0.826 | 0.408 |
| aspect ratio of the caudal fin | 1.574 | 0.495 | 3.181 | 0.001 |
| 30 m threshold | ||||
| intercept | 0.169 | 0.375 | 0.451 | 0.651 |
| aspect ratio of the caudal fin | 1.538 | 0.486 | 3.167 | 0.001 |
| diet | ||||
| herbivores | −0.935 | 0.429 | −2.176 | 0.029 |
| piscivores | 0.312 | 0.517 | 0.603 | 0.546 |
| zooplanktivores | 0.138 | 0.480 | 0.289 | 0.772 |
| region | ||||
| Pohnpei | 1.000 | 0.408 | 2.450 | 0.014 |
| Puerto Rico | −0.119 | 0.339 | −0.352 | 0.725 |
In Pohnpei, 50% of fish species were shallow specialists occurring exclusively above 50 m depth, compared with 43% for the NWHI and 42% for Puerto Rico. Aspect ratio of the caudal fin was the best predictor of depth-generalist species in all three models (table 1). Diet was also significant in the 30 and 50 m models. There was a significant interaction among predictor variables and region for the 30 m model, indicating that the effects of aspect ratio and diet were stronger in Puerto Rico than in the NWHI or Pohnpei.
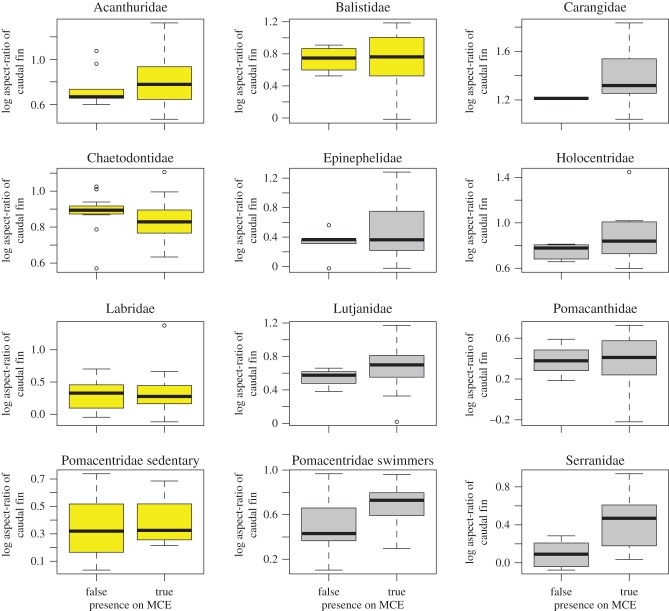
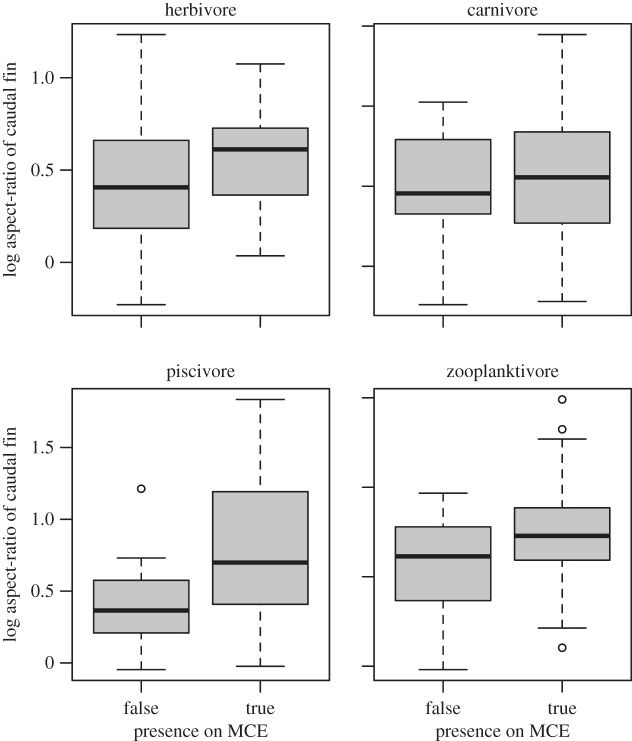
For all three depth thresholds, species with higher aspect ratios (lunate or forked caudal fins) were significantly more likely to occur across a broad depth range than species with low aspect ratios (rounded caudal fins). The relationship was consistent among the majority of the trophic groups and families, although the strength of the relationship varied among groups. Among families with at least 12 representatives in our analysis, a positive correlation between caudal fin aspect ratio and depth-generalism occurred in the Lutjanidae, Carangidae, Holocentridae, Serranidae and swimming Pomacentridae, but not for Acanthuridae, Chaetodontidae and Labridae, Balistidae, Epinephalidae or sedentary Pomacentridae (figure 1). The relationship between depth-generalist species and aspect ratio was present for all trophic groups (figure 2), independent of body size (electronic supplementary material, figure S3), and occurred in both the GLMMs and PGLMs (electronic supplementary material, table S3).
Figure 1.
Relationship between caudal fin aspect ratio and depth-generalism for the 12 most species-rich families (n > 12). Yellow boxes indicate families in which species are medium or paired fin (MPF) swimmers; grey boxes indicate families in which species are body or caudal (BCF) swimmers. Pomacentridae were split into sedentary (MPF-swimming) species and active (BCF-swimming) genera. MCE, mesophotic coral ecosystem.
Figure 2.
Relationship between caudal fin aspect ratio and depth-generalism among trophic guilds. MCE, mesophotic coral ecosystem.
Diet was also a significant predictor of depth-generalist species in the 30 and 50 m models (table 1). The effect of diet was driven by herbivores, which exhibited much shallower depth ranges than expected by chance (figure 3).
Figure 3.

Differences in trophic guild proportions of depth-generalists relative to the expected proportion for all trophic guilds: Planktivores (dotted line); piscivores (dotted and dashed); carnivores (solid line); herbivores (dashed line).
4. Discussion
We found that the same two traits—caudal fin aspect ratio and diet—predicted depth-generalist reef fish species across three geographically and taxonomically distinct assemblages. Detailed surveys on depth distributions in coral reef fish assemblages over a broad depth range are sparse; therefore, our analysis was necessarily limited to a subset of species from three geographical locations. However, the fish faunas of the Atlantic and central-western Pacific have been isolated at least since the closure of the Central American Seaway 3.4 mya [38]. The extensive geographical distance and taxonomic dissimilarity between the locations and assemblages provide strong evidence that environmental filtering plays a significant role in determining the composition of mesophotic coral reef assemblages.
High aspect ratios are commonly associated with pelagic fishes, but to the best of our knowledge, caudal fin aspect ratio has never previously been associated with depth distributions. We propose three potential explanations why depth-generalist fish species may possess more lunate caudal fins than shallow-water specialists, none of which are mutually exclusive. Lunate caudal fins may (i) assist movement between deep and shallow reef habitats; (ii) allow for a rapid evasion from predators, when feeding in the water column in a low-light environment (especially for zooplanktivores); and (iii) generate less wake, presenting an advantage in deeper habitats with lower light and less turbulent water flow than shallower habitats.
Caudal fin aspect ratio influences swimming speed, acceleration and manoeuvrability, with high aspect ratios facilitating either long-distance ‘cruising’ or rapid acceleration and low aspect ratios associated with greater manoeuvrability [39]. Many large reef-associated species (e.g. Carangids) have large home ranges that encompass both shallow and mesophotic reefs [22,24]. In addition, they often exhibit considerable diel and/or seasonal migrations [24]. High caudal fin aspect ratios would facilitate migration among habitats, including between deep and shallow reefs. Families containing large, mobile species (e.g. Carangidae) showed a positive relationship between depth-generalist and aspect ratio. However, the relationship between depth range and caudal fin aspect ratio was independent of body size, indicating that smaller, less-mobile depth-generalist taxa also exhibit higher aspect ratios than shallow specialist counterparts (figure 4). Consequently, the pattern reported here is not due solely to deeper assemblages being dominated by large, mobile predators.
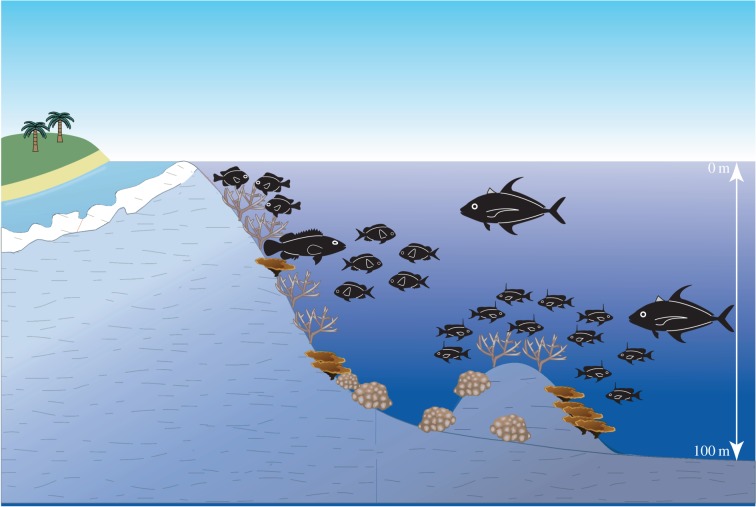
Figure 4.
Schematic diagram of the distribution of functional groups along a depth gradient. Species with high caudal fin aspect ratios (lunate caudal fins) and trailing fin filaments are significantly more likely to occur across a broad depth range than conspecifics with more rounded caudal fins. Adapted from Bridge et al. [18].
Planktivores often exhibit high aspect ratios [40], and their greater proportion of zooplanktivores at mesophotic depths [22,26,27] could be responsible for a greater number of species with lunate caudal fins in the deeper assemblages. Zooplanktivores must move into the water column to feed, and lunate caudal fins provide a more efficient method of locomotion under such circumstances. Given that zooplankton provides a major source of nutrients at mesophotic depths, the ability to capture zooplankton efficiently likely provides a significant advantage for species with lunate caudal fins. However, our results showed that the relationship between aspect ratio and depth-generalism occurred across all four trophic guilds (figures 2 and 3), demonstrating that the trend is not solely due to the greater proportion of zooplanktivores in mesophotic assemblages. Although most herbivores exhibit relatively shallow depth distributions, those species that do occur in deeper waters also show significantly higher aspect ratios. The propensity for higher aspect ratios across all trophic guilds and body sizes suggests that lunate caudal fins present an advantage in deeper habitats beyond simply facilitating more efficient movement through the water.
We propose that the hydrodynamic properties of lunate caudal fins may enable species with high aspect ratios to swim more ‘silently’. High aspect ratios produce weak vortices that do not travel far from their source [41], making this design efficient for travelling long distances and resulting in its convergent evolution across a diverse range of taxa, including pelagic teleosts, sharks and cetaceans [42]. The lack of turbulence generated by lunate fins may be advantageous in deeper habitats by reducing rheotactic detectability. Water flow in the marine environment decreases with depth—particularly turbulent flow generated by surface waves [43]. Consequently, turbulence generated by swimming would be detected more easily in mesophotic habitats. Fish use both vision and mechanosensory cells of the lateral line for predation and predator avoidance [44,45]. Species capable of occupying low-light habitats could be expected to rely less on vision but more on mechanosensory organs to detect prey and avoid predators than fish on shallow-water reefs. High caudal fin aspect ratios would enable deep-water species to swim ‘silently’, presenting a significant advantage in a habitat where vibrations are likely to play a key role for both predators and prey. In addition to high aspect ratios, many mesophotic coral reef fishes exhibit long, trailing filaments on their caudal, dorsal or anal fins (e.g. Pseudanthias thompsoni and Clepticus parrae) (T.B. and O.J.L., personal observation). Among zooplankton, similar trailing filaments are associated with ‘stealth swimming’ that reduces hydrodynamic disturbance in the surrounding water, and thus exposure to rheotactic predators [46]. Similarly, hindwing tails in moths were recently discovered to confer a significant advantage in avoiding predation by insectivorous bats by deflecting the bats' sonar [47], suggesting that acoustic and rheotactic diversion may be more common in predator–prey interactions than currently appreciated. Lunate caudal fins and potentially trailing filaments may therefore present a significant advantage to fishes in mesophotic habitats, and the ability to swim silently to avoid predators may act as an environmental filter on community membership on deeper reefs.
Examining the swimming modes employed by different families of reef fishes provides further support for the relationship between caudal fin aspect ratio and depth range. At a broad level, fish swimming modes can be divided into two groups: body and/or caudal fin (BCF) and median and/or pectoral fin (MPF) swimming [48]. BCF swimming achieves greater speed and acceleration, whereas MPF swimming provides greater manoeuvrability and stability at slow speeds. Considering the families in our analysis containing 12 or more species, four of the five families that primarily use MPF swimming (Chaetodontidae and Labridae, Balistidae and sedentary Pomacentridae) show little or no relationship between caudal fin aspect ratio and depth range. In contrast, five of the seven families that primarily use BCF swimming show a positive relationship between caudal fin aspect ratio and depth range. The lack of a relationship in the other two families (Epinephalidae and Pomacanthidae) may be due to the fact that these families are largely sedentary, reducing the advantages provided by high aspect ratios. All species of Epinephalidae are essentially ambush predators, and the thrust provided by low aspect ratios for occasional bursts of speed to capture prey may be more beneficial than the rheotactic camouflage or swimming efficiency provided by a high aspect ratio. Depth-generalist Pomacanthids are dominated by the genus Centropyge, comprising small, relatively cryptic species with small home ranges. Centropyge sp. rarely venture far from the reef matrix, and may therefore have little reason to develop high aspect ratios.
High aspect ratios are significantly over-represented in depth-generalists of all other BCF-swimming genera. Interestingly, the relationship is clearly evident in the Pomacentridae when the family is split into BCF- and MPF-swimming genera. This is likely due to the relatively large number of species that feed high up in the water column and rely more heavily on their caudal fins to swiftly return to shelter when threatened by a predator [29]. Pectoral fin aspect ratios are associated with swimming efficiency in MPF swimmers [40,49], and could potentially be correlated with depth-generalism in MPF swimming species.
Diet was also a significant predictor of depth-generalist species in our analysis. Differences in the proportion of depth-generalist species among trophic guilds reflect variations in the availability of different food sources along the depth gradient. Among the four trophic groups examined, herbivores were the only species to show a significant relationship, exhibiting significantly shallower depth distributions than expected by chance (figure 3). Zooplanktivores were more likely to occur in deeper water than expected by chance, although their representation in deeper assemblages was not significantly different from carnivores or piscivores. Similar patterns in the depth distributions of herbivores and zooplanktivores have been reported from the western Pacific [27], the Red Sea [26] and the Caribbean [22], attributed to the effects of decreasing light irradiance with increasing depth on food availability. The lack of a significant relationship between zooplanktivores and depth in our study was probably because the deepest surveys occurred at 60–70 m, and the inclusion of additional data from greater depths would probably strengthen this relationship.
Light is often considered an important factor limiting the depth range of coral reef fishes, particularly for diurnal zooplanktivores that rely on vision for prey capture [29]. A trend towards larger eyes in species with occupying deeper habitats has been documented for rockfishes in the North Pacific [6]. Light irradiance declines exponentially with increasing depth, and even in clear tropical waters light irradiance falls to 10% of surface values at a depth of approximately 40 m, and 1% by 80 m [16]. Consequently, we expected traits associated with adaptation to low-light habitats (eye size and nocturnal behaviour) to be over-represented in deeper coral reef fish assemblages; however, neither trait showed any relationship with depth range. One potential explanation for the lack of a relationship between eye size and depth range in our study is that fish populations in deeper water can adapt their vision to low-light relative to shallow-water conspecifics, enabling a single species to exist across a broad depth range [50]. Although ambient light irradiance at depths more than 40 m is at least an order of magnitude lower than at the surface, irradiance may not be diminished sufficiently to act as an environmental filter. Eye size was shown to predict species' depth range for rockfish (Sebastes sp.) along a much larger depth gradient of 0–500 m in the northeast Pacific [6], suggesting larger eyes do influence depth distributions over broader depth ranges. Visual acuity develops rapidly in coral reef fishes, and at least some taxa are capable of feeding at depths of more than 80 m very early in life [51]. Although large eyes are clearly important in very low light, our results suggest that the decrease in ambient light over the depth range examined here (less than 70 m) does not significantly effect the composition of fish assemblages.
5. Conclusion
Our results indicate that environmental filtering is important for structuring mesophotic coral reef fish assemblages. Traits characteristic of depth-generalist species are remarkably similar across three distinct fish assemblages in similar habitats but different biogeographic and evolutionary contexts. Given that depth range is an important predictor of extinction risk [17], our results can provide some insight into vulnerability to population declines following disturbance events. Since species with high caudal fin aspect ratios are significantly more likely to be depth-generalists, they are therefore more likely to be capable of using deep-water refuges to persist through disturbance events. In contrast, species with low caudal fin aspect ratios are significantly more likely to be restricted to shallow habitats, making them more vulnerable to depth-dependent habitat degradation. Although herbivores are disproportionately shallow-water specialists and provide key ecosystem services, they appear less vulnerable to population declines following disturbances than more specialized taxa such as obligate corallivores [17,52]. Surveying deep-water habitats, and thus determining population trends, remains challenging for many fish species. Our results suggest that lunate caudal fins enable fish to occur over a broad depth range, and these species may therefore hold an advantage in terms of extinction vulnerability. However, we caution that additional factors, such as vulnerability to fishing pressure, must also be considered when assessing extinction vulnerability in coral reef fishes.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank S. Binning, A. Grech and N. Graham for their valuable comments on the manuscript, and D.R. Barneche and M. Prazeres for assistance with compiling the morphological trait data.
Authors' contributions
T.C.L.B. designed the experiment and wrote the first draft of the manuscript. O.J.L. performed statistical analyses. All authors contributed to the collection and collation of data, interpretation of results and manuscript preparation.
Competing interests
We declare we have no competing interests.
Funding
T.C.L.B. was supported by the Australian Research Council. O.J.L. was supported by the Quantitative Ecology and Evolution Laboratory at Macquarie University. R.R.C. was supported by a fellowship from the National Science Foundation grant no. DGE-329626 and the Office of the National Marine Sanctuaries Dr. Nancy Foster Scholarship Award no. NA15NOS4290067 (RRC). C.N.K. was funded by Papahānaumokuākea Marine National Monument and the Nancy Foster Scholarship programme. R.K.K. was supported by Papahānaumokuākea Marine National Monument. Surveys conducted in Pohnpei were supported by the Seaver Institute (to J. M. Copus, R. L. Pyle and B. W. Bowen). Surveys conducted in the NWHI were supported by the Office of National Marine Sanctuaries through Papahānaumokuākea Marine National Monument.
References
- 1.Wilson JB. 1999. Assembly rules in plant communities. In Ecological assembly rules: perspectives, advances, retreats (eds Weiher E, Keddy P), pp. 130–164. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Fukami T, Martijn Bezemer T, Mortimer SR, Putten WH. 2005. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett. 8, 1283–1290. ( 10.1111/j.1461-0248.2005.00829.x) [DOI] [Google Scholar]
- 3.McGill BJ, Enquist BJ, Weiher E, Westoby M. 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185. ( 10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 4.Weiher E, Clarke GDP, Keddy PA. 1998. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 81, 309–322. ( 10.2307/3547051) [DOI] [Google Scholar]
- 5.Chase JM, Leibold MA. 2003. Ecological niches: linking classical and contemporary approaches. Chicago, IL: University of Chicago Press. [Google Scholar]
- 6.Ingram T, Shurin JB. 2009. Trait-based assembly and phylogenetic structure in northeast Pacific rockfish assemblages. Ecology 90, 2444–2453. ( 10.1890/08-1841.1) [DOI] [PubMed] [Google Scholar]
- 7.MacArthur R, Levins R. 1967. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 101, 377–385. ( 10.1086/282505) [DOI] [Google Scholar]
- 8.Fox BJ. 1987. Species assembly and the evolution of community structure. Evol. Ecol. 1, 201–213. ( 10.1007/BF02067551) [DOI] [Google Scholar]
- 9.Wiens JA. 1991. Ecological similarity of shrub-desert avifaunas of Australia and North America. Ecology 72, 479–495. ( 10.2307/2937189) [DOI] [Google Scholar]
- 10.Lamouroux N, Poff NL, Angermeier PL. 2002. Intercontinental convergence of stream fish community traits along geomorphic and hydraulic gradients. Ecology 83, 1792–1807. ( 10.1890/0012-9658(2002)083%5B1792:ICOSFC%5D2.0.CO;2) [DOI] [Google Scholar]
- 11.Bellwood DR, Hughes TP, Folke C, Nyström M. 2004. Confronting the coral reef crisis. Nature 429, 827–833. ( 10.1038/nature02691) [DOI] [PubMed] [Google Scholar]
- 12.Mouillot D, et al. 2014. Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc. Natl Acad. Sci. USA 111, 13 757–13 762. ( 10.1073/pnas.1317625111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham NAJ, Bellwood DR, Cinner JE, Hughes TP, Norstrom AV, Nystrom M. 2013. Managing resilience to reverse phase shifts in coral reefs. Front. Ecol. Environ. 11, 541–548. ( 10.1890/120305) [DOI] [Google Scholar]
- 14.Wilson SK, Fisher R, Pratchett MS, Graham NAJ, Dulvy NK, Turner RA, Cakcaka A, Polunin NVC, Rushton SP. 2008. Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob. Change Biol. 14, 2796–2809. ( 10.1111/j.1365-2486.2008.01696.x) [DOI] [Google Scholar]
- 15.Halford AR, Caley MJ. 2009. Towards an understanding of resilience in isolated coral reefs. Glob. Change Biol. 15, 3031–3045. ( 10.1111/j.1365-2486.2009.01972.x) [DOI] [Google Scholar]
- 16.Kahng SE, Garcia-Sais JR, Spalding HL, Brokovich E, Wagner D, Weil E, Hinderstein L, Toonen RJ. 2010. Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29, 255–275. ( 10.1007/s00338-010-0593-6) [DOI] [Google Scholar]
- 17.Graham NAJ, et al. 2011. Extinction vulnerability in coral reef fishes. Ecol. Lett. 14, 341–348. ( 10.1111/j.1461-0248.2011.01592.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridge TC, Hughes TP, Guinotte JM, Bongaerts P. 2013. Call to protect all coral reefs. Nat. Clim. Change 3, 528–530. ( 10.1038/nclimate1879) [DOI] [Google Scholar]
- 19.Srinivasan M. 2003. Depth distributions in coral reef fishes: the influence of microhabitat structure, settlement, and post-settlement processes. Oecologia 137, 76–84. ( 10.1007/s00442-003-1320-6) [DOI] [PubMed] [Google Scholar]
- 20.Fischer AG. 1960. Latitudinal variations in organic diversity. Evolution 14, 64–81. ( 10.2307/2405923) [DOI] [Google Scholar]
- 21.Kane C, Kosaki RK, Wagner D. 2014. High levels of mesophotic reef fish endemism in the Northwestern Hawaiian Islands. Bull. Mar. Sci. 90, 693–703. ( 10.5343/bms.2013.1053) [DOI] [Google Scholar]
- 22.Bejarano I, Appeldoorn RS, Nemeth M. 2014. Fishes associated with mesophotic coral ecosystems in La Parguera, Puerto Rico. Coral Reefs 33, 313–328. ( 10.1007/s00338-014-1125-6) [DOI] [Google Scholar]
- 23.Robertson DR. 2008. Global biogeographical data bases on marine fishes: caveat emptor. Divers. Distrib. 14, 891–892. ( 10.1111/j.1472-4642.2008.00519.x) [DOI] [Google Scholar]
- 24.Papastamatiou YP, Meyer CG, Kosaki RK, Wallsgrove NJ, Popp BN. 2015. Movements and foraging of predators associated with mesophotic coral reefs and their potential for linking ecological habitats. Mar. Ecol. Prog. Ser. 521, 155–170. ( 10.3354/meps11110) [DOI] [Google Scholar]
- 25.Alexander RM. 1967. Functional design in fishes. London, UK: Hutchinson and Co. Publishing Ltd. [Google Scholar]
- 26.Brokovich E, Einbinder S, Shashar N, Kiflawi M, Kark S. 2008. Descending to the twilight zone: changes in coral reef fish assemblages along a depth gradient down to 65 m. Mar. Ecol. Prog. Ser. 371, 253–262. ( 10.3354/meps07591) [DOI] [Google Scholar]
- 27.Thresher RE, Colin PL. 1986. Trophic structure, diversity and abundance of fishes of the deep reef (30–300 m) at Enewetak, Marshall Islands. Bull. Mar. Sci. 38, 253–272. [Google Scholar]
- 28.Kahng SE, Copus JM, Wagner D. 2014. Recent advances in the ecology of mesophotic coral ecosystems (MCEs). Curr. Opin. Environ. Sustainability 7, 72–81. ( 10.1016/j.cosust.2013.11.019) [DOI] [Google Scholar]
- 29.Hobson ES. 1991. Trophic relationships of fishes specialized to feed on zooplankters above coral reefs. In The ecology of fishes on coral reefs (ed. Sale PF.), pp. 69–95. San Diego, CA: Academic Press. [Google Scholar]
- 30.Luiz OJ, Allen AP, Robertson DR, Floeter SR, Kulbicki M, Vigliola L, Becheler R, Madin JS. 2013. Adult and larval traits as determinants of geographic range size among tropical reef fishes. Proc. Natl Acad. Sci. USA 110, 16 498–16 502. ( 10.1073/pnas.1304074110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westneat MW, Wainwright SA. 2001. Mechanical design for swimming: muscle, tendon, and bone. In Fish physiology, vol. 19 (eds Block BA, Stevens DE), pp. 271–311. San Diego, CA: Academic Press. [Google Scholar]
- 32.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 33.Pinheiro J, Bates D, DebRoy S, Sarkar D. 2012. nlme: linear and nonlinear mixed effects models. R package v3.1–104. See http://cran.r-project.org/web/packages/nlme/index.html (accessed November 2014).
- 34.Bates D, Maechler M, Bolker B, Walker S.2014. lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-7. See http://CRAN.R-project.org/package=lme4 .
- 35.Rabosky DL, Santini F, Eastman J, Smith SA, Sidlauskas B, Chang J, Alfaro ME. 2013. Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat. Commun. 4, 1958 ( 10.1038/ncomms2958) [DOI] [PubMed] [Google Scholar]
- 36.Rabosky DL, Santini F, Eastman J, Smtih SA, Sidlauskas B, Chang J, Alfaro ME. 2013. Data from: rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Dryad Digital Repository. ( 10.5061/dryad.j4802) [DOI] [Google Scholar]
- 37.Ho LST, Ana C. 2014 phylolm: a linear-time algorithm for Gaussian trait evolution models. R package version 2.3. See https://cran.r-project.org/web/packages/phylolm/ . [Google Scholar]
- 38.Bellwood DR, Wainwright SA. 2002. The history and biogeography of fishes on coral reefs. In Coral reef fishes, dynamics and diversity in a complex ecosystem (ed. Sale PF.), pp. 5–32. San Diego, CA: Academic Press. [Google Scholar]
- 39.Pauly D. 1990. Food consumption by tropical and temperate marine fishes: some generalizations. J. Fish Biol. 35, 11–20. ( 10.1111/j.1095-8649.1989.tb03041.x) [DOI] [Google Scholar]
- 40.Wainwright PC, Bellwood DR, Westneat MW. 2002. Ecomorphology of locomotion in labrid fishes. Environ. Biol. Fish. 65, 47–62. ( 10.1023/A:1019671131001) [DOI] [Google Scholar]
- 41.Chopra MG. 1976. Large amplitude lunate-tail theory of fish locomotion. J. Fluid Mech. 74, 161–182. ( 10.1017/S0022112076001742) [DOI] [Google Scholar]
- 42.Donley J, Sepulveda CA, Konstantinidis P, Gemballa S, Shadwick RE. 2004. Convergent evolution in mechanical design of lamnid sharks and tunas. Nature 429, 61–65. ( 10.1038/nature02435) [DOI] [PubMed] [Google Scholar]
- 43.Sebens KP. 1997. Adaptive responses to water flow: morphology, energetics, and distribution of reef corals. Proc. Int. Coral. Reef. Symp. 2, 1053–1058. [Google Scholar]
- 44.Helfman GS. 1989. Threat-sensitive predator avoidance in damselfish–trumpetfish interactions. Behav. Ecol. Sociobiol. 24, 47–58. ( 10.1007/BF00300117) [DOI] [Google Scholar]
- 45.Stewart WJ, Nair A, Jiang H, McHenry MJ. 2014. Prey fish escape by sensing the bow wave of a predator. J. Exp. Biol. 217, 4328–4336. ( 10.1242/jeb.111773) [DOI] [PubMed] [Google Scholar]
- 46.Kiorboe T, Jiang H, Goncalves RJ, Nielsen LT, Wadwha N. 2014. Flow disturbances generated by feeding and swimming zooplankton. Proc. Natl Acad. Sci. USA 111, 11 738–11 743. ( 10.1073/pnas.1405260111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barber JR, Leavell BC, Keener AL, Breinholt JW, Chadwell BA, McClure CJW, Hill GM, Kawahara AY. 2015. Moth tails divert bat attack: evolution of acoustic deflection. Proc. Natl Acad. Sci. USA 112, 2812–2816. ( 10.1073/pnas.1421926112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webb PW. 1984. Form and function in fish swimming. Sci. Am. 251, 58–68. ( 10.1038/scientificamerican0784-72) [DOI] [Google Scholar]
- 49.Binning SA, Roche DG. 2015. Water flow and fin shape polymorphism in coral reef fishes. Ecology 96, 828–839. ( 10.1890/14-0426.1) [DOI] [PubMed] [Google Scholar]
- 50.Brokovich E, Ben-Ari T, Kark S, Kiflawi M, Dishon G, Iluz D, Shashar N. 2010. Functional changes of the visual system of the damselfish Dascyllus marginatus along its bathymetric range. Physiol. Behav. 101, 413–421. ( 10.1016/j.physbeh.2010.07.006) [DOI] [PubMed] [Google Scholar]
- 51.Job SD, Bellwood DR. 2000. Light sensitivity in larval fishes: implications for vertical zonation in the pelagic zone. Limnol. Oceanogr. 45, 362–371. ( 10.4319/lo.2000.45.2.0362) [DOI] [Google Scholar]
- 52.Graham NAJ, Wilson SK, Jennings S, Polunin NVC, Robinson J, Bijoux JP, Daw TM. 2007. Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries and ecosystems. Conserv. Biol. 21, 1291–1300. ( 10.1111/j.1523-1739.2007.00754.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.