Abstract
Biological invasions as drivers of biodiversity loss have recently been challenged. Fundamentally, we must know where species that are threatened by invasive alien species (IAS) live, and the degree to which they are threatened. We report the first study linking 1372 vertebrates threatened by more than 200 IAS from the completely revised Global Invasive Species Database. New maps of the vulnerability of threatened vertebrates to IAS permit assessments of whether IAS have a major influence on biodiversity, and if so, which taxonomic groups are threatened and where they are threatened. We found that centres of IAS-threatened vertebrates are concentrated in the Americas, India, Indonesia, Australia and New Zealand. The areas in which IAS-threatened species are located do not fully match the current hotspots of invasions, or the current hotspots of threatened species. The relative importance of biological invasions as drivers of biodiversity loss clearly varies across regions and taxa, and changes over time, with mammals from India, Indonesia, Australia and Europe are increasingly being threatened by IAS. The chytrid fungus primarily threatens amphibians, whereas invasive mammals primarily threaten other vertebrates. The differences in IAS threats between regions and taxa can help efficiently target IAS, which is essential for achieving the Strategic Plan 2020 of the Convention on Biological Diversity.
Keywords: alien species, biological invasions, non-native species, threatened species, vertebrates
1. Introduction
More than 10 years ago, Gurevitch & Padilla [1] asked whether invasive alien species (IAS) are a major cause of extinction. They found that more than five times as many species are categorized as threatened or endangered by habitat loss than by IAS. More recently, Thomas & Palmer [2] and Pearce [3] have also questioned the importance of biological invasions as a threat to biodiversity. Clavero & García-Berthou [4] have challenged this view and have demonstrated that of the 170 animal extinctions for which the causes of extinction are known, 54% are partly due to IAS, and 20% are due to only IAS. Despite recent efforts [5,6], the influences of IAS on biodiversity loss are still poorly understood [7,8], and the role of IAS in the global distribution of threatened species is understudied. This issue is critical because neither biodiversity [9] nor the drivers of its decline are evenly distributed [10,11], therefore, there should be substantial spatial variation in the vulnerability of biodiversity to biological invasions.
Because resources for the control of IAS are limited, we must understand whether IAS are a major problem for biodiversity, and if so, for which species and locations. The need to focus action on priority species is also noted in the Strategic Plan 2020 of the Convention on Biological Diversity (CBD), which calls for parties to identify priority IAS for responses [12]. Currently, marine and terrestrial hotspots of invasions (Europe, North America, Australia and New Zealand) [13,14] are of primary concern regarding the prevention of new invasions. However, this approach may not be particularly useful if there is a mismatch between the hotspots of invasions and the IAS-threatened species. Additionally, the current management strategies are predominantly implemented on islands [15]. However, a global assessment of IAS threats to biodiversity in mainland areas has never been conducted, although such an assessment could enable increased efficiency in management of IAS, through the identification of areas in which the influence of IAS on native species is elevated.
Here, we examine the spatial and taxonomic relationships between IAS and threatened vertebrates (i.e. mammals, birds, reptiles and amphibians). To our knowledge, this is the first time that the role of IAS as a threat to vertebrates has been quantified and spatially analysed on a global scale. Specifically, this approach will help to (i) identify regions or countries on which control efforts should be focused, (ii) prioritize known IAS that should be controlled or eradicated and (iii) evaluate the efficiency of conservation measures implemented by countries.
2. Material and methods
To identify high-risk regions regarding IAS, we used the International Union for the Conservation of Nature (IUCN) and Global Invasive Species Database (GISD) databases to determine both vertebrates that are threatened by IAS and which IAS are responsible for these threats. Below are descriptions of these databases.
(a). Species data
The species assessments of the IUCN Red List are conducted by experts who place each species into one of the following categories of extinction risk: extinct (EX), extinct in the wild (EW), critically endangered (CR), endangered (EN), vulnerable (VU), near threatened (NT), least concern (LC) and data deficient (DD) [16]. These Red List categories are based on a number of criteria that indicate the level of extinction risk and include rate of population decline (criterion A), the size and decline of the geographical range (criterion B), the population size (criteria C and D) or quantitative analyses (criterion E) [17]. All the species assessments are reviewed and accepted by the IUCN and then published online (www.iucnredlist.org). The Red List process has been extensively described [17] and used in many other articles to provide guidelines regarding the conservation of species and habitats [9,18].
We also used the redesigned Global Invasive Species Database (GISD), which interlinks the IUCN Red List species with IAS information [19]. For each IUCN Red List species, this database compiles the scientific name, RL category, threat code, scientific and common names of the IAS involved, and the source. The information in the GISD is compiled from an array of sources including scientific papers and regional databases that have been reviewed by international expert contributors. The combination of the IUCN Red List and the GISD is used to verify and nominate each IAS as related to an IAS-threatened species. We therefore extracted information about the IAS that affect the IUCN Red List species.
We also collected the IAS spatial distributions from online databases (e.g. the Global Biodiversity Information Facility) by using the gbif () function of the dismo R package [20] for the species that have been identified by the IUCN-GISD database (the spatial distributions of N = 197 species were available).
(b). Threat category
During the species assessment process, data regarding the threat drivers are collated for each species [21]. The IUCN and Birdlife International supervise a process by which 11 major threats to biodiversity are identified and classified (i.e. the IUCN threat classification scheme v. 3.0). We compiled the external threats for each species from the Red List of Threatened Species published by the IUCN. These threats are the following: (1) residential and commercial development; (2) agriculture and aquaculture; (3) energy production and mining; (4) transportation and service corridors; (5) biological resource use; (6) human intrusion and disturbance; (7) natural system modifications; (8) invasive and other problematic species, genes and diseases; (9) pollution; (10) geological events; and (11) climate change and severe weather [21]. We used the same classification scheme as IUCN with the exception of category number 8, which we subdivided into IAS (i.e. invasive non-native, alien species and diseases) and other problematic species (i.e. native species and species of unknown origin) to conservatively include only alien species because IAS do not include native species. When available, we also collected information about the threat severity (i.e. significant decline, causing/could cause fluctuations, negligible declines and no decline) and the scope of the IAS threat (i.e. whether the majority (more than 50%) of the range is threatened).
We associated the threats to each species with the information available in the IUCN Red List. For example, if a given species was threatened by Agriculture and Aquaculture according to the IUCN, it was assigned a ‘1’ for this category in the data matrix. Otherwise, if the species was not threatened by Agriculture and Aquaculture, it received a value of ‘0’ (zero). We repeated this process for all external threats listed above. Therefore, more than one single factor could threaten a species, but each threat was treated independently. To describe the spatial distributions of the threats, we had to assume that the listed threats affecting a species throughout its distribution range accurately described the spatial patterns [22]. We found that for most birds and amphibians (for which data were available, N = 438), IAS threats occurred in the majority of the localities in the species area distributions and that for the large majority of birds and amphibians (more than 70%), IAS had caused significant declines in their population sizes (electronic supplementary material, figure S5).
(c). Threat occurrences and prevalence
For each taxonomic group, we calculated the number of threatened species and the number of IAS-threatened species (i.e. those for which IAS were cited as a threat). This procedure allowed us to calculate the proportion of species for which IAS have been reported as factors related to extinction risk.
(d). Species distribution and maps of the threat processes
For our analyses, we categorized the VU, EN and CR species as threatened species, and the other categories, including NT and LC, were categorized as non-threatened species. We obtained data regarding the spatial distributions of four vertebrate groups, i.e. 1251 birds classified as threatened by other factors and 415 threatened by IAS, 1058 mammals classified as threatened by other factors and 179 threatened by IAS, 1815 amphibians classified as threatened by other factors and 565 threatened by IAS and 714 reptiles classified as threatened by other factors and 132 threatened by IAS and mapped the species' spatial ranges as polygons [23]. We produced maps of the richness of threatened species and IAS-threatened species by overlaying a hexagonal grid with 10-min resolution onto the aggregated species' distributions.
(e). Spatial units
We considered various scales of analyses including islands versus mainlands, countries and continents. We defined islands using the IUCN island shapefile, which encompass nearly 180 000 islands worldwide (http://www.iucnredlist.org/technical-documents/red-list-training/iucnspatialresources). We discriminated by continents as follows: Africa, Asia, Europe, North America, Oceania and South America. A species was considered present in a given spatial unit (island versus mainland, country or continent) whenever its mapped range overlapped with that unit.
All analyses were conducted in R v. 3.2.0 (R core team v. 2015). The spatial analyses were performed at a spatial resolution of 10 min and an equal area projection. All raster layers and R code will be freely accessible on Dryad.
3. Results
(a). Are invasive alien species a significant threat to vertebrates worldwide?
A total of 1352 (27%) mammals, birds, reptiles and amphibians are IAS-threatened worldwide compared to the 4917 species threatened due to other factors (table 1). The absolute numbers of IAS-threatened species are particularly high for amphibians (N = 565) and birds (N = 443) compared with mammals (N = 183) and reptiles (N = 161). These overrepresentations of amphibians and birds were not found among species threatened by other factors (table 1). Consequently, one-quarter of all threatened amphibians and birds are currently considered to be threatened by IAS, whereas the proportions of IAS-threatened species among mammals and reptiles are approximately 15% and 18%, respectively. However, we also found that IAS concurred with other threats and were never the sole cause of the threat to a species (electronic supplementary material, table S1). Interestingly, the proportion of IAS-threatened species among all threatened species increased with the extinction risk in all vertebrate groups. For example, 28% of the critically endangered reptiles are threatened by IAS compared with only 13% of the vulnerable reptiles, and the number of threats was stable across the species that are CR, EN or VU (electronic supplementary material, table S1).
Table 1.
Numbers of mammal, bird, reptile and amphibian species threatened (CR, EN and VU) by IAS and other threats.
| IUCN category | no. other threatened sp. | no. IAS-threatened sp. | % IAS-threatened among all | |
|---|---|---|---|---|
| mammals | CR | 178 | 36 | 17% |
| EN | 437 | 81 | 16% | |
| VU | 456 | 66 | 13% | |
| total | 1071 | 183 | 15% | |
| birds | CR | 190 | 94 | 33% |
| EN | 394 | 137 | 26% | |
| VU | 693 | 212 | 23% | |
| total | 1277 | 443 | 26% | |
| reptiles | CR | 142 | 54 | 28% |
| EN | 290 | 65 | 18% | |
| VU | 282 | 42 | 13% | |
| total | 714 | 161 | 18% | |
| amphibians | CR | 503 | 234 | 32% |
| EN | 772 | 201 | 21% | |
| VU | 580 | 130 | 18% | |
| total | 1855 | 565 | 23% |
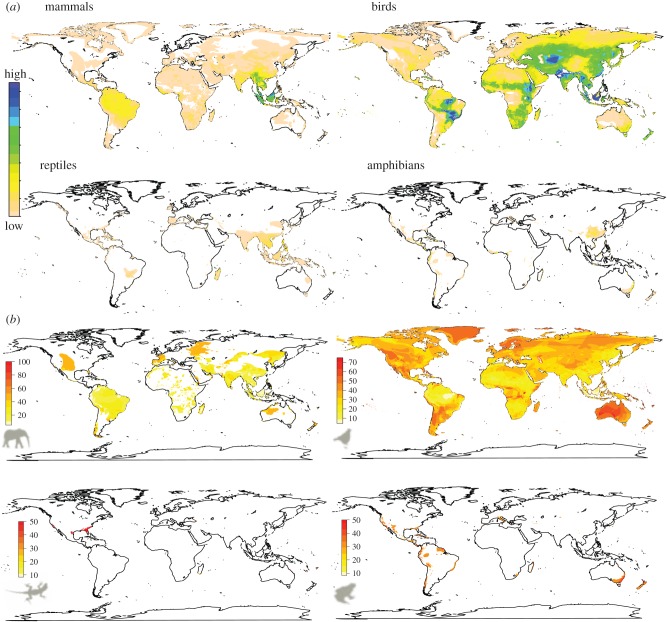
Examination of the spatial distributions of the IAS-threatened vertebrates revealed distinct differences between the four vertebrate groups (figure 1). The IAS-threatened mammals and birds are currently widespread over all continents. This trend represents the current state and might hide spatial discrepancies over time (electronic supplementary material, figure S3). However, the IAS-threatened species follow a similar pattern similar to those of species threatened by other factors, although the area covered by IAS-threatened species is smaller (figure 1; electronic supplementary material, figure S4). In contrast, the IAS-threatened reptile and amphibian distributions are so small and isolated that no location has more than seven and 12 species overlapping ranges of these vertebrates, respectively. These areas are mostly restricted to Central and South America, Australia and the Indonesian islands. Although the spatial distribution of IAS-threatened amphibians is congruent with that of the other threatened amphibian species, we observed a divergent pattern for the IAS-threatened reptiles, which are more restricted to Florida, New Caledonia and the Fiji islands (figure 1; electronic supplementary material, figure S4).
Figure 1.
Spatial distribution of IAS-threatened (CR, EN and VU IUCN Red List) mammals, birds, reptiles and amphibians, in absolute number (a) and given as a proportion of all threatened species (b). For instance, a proportion of 50% for a given location means that 50% of the threatened species there are threatened by IAS.
(b). Which countries have particularly high absolute numbers of invasive alien species-threatened vertebrates?
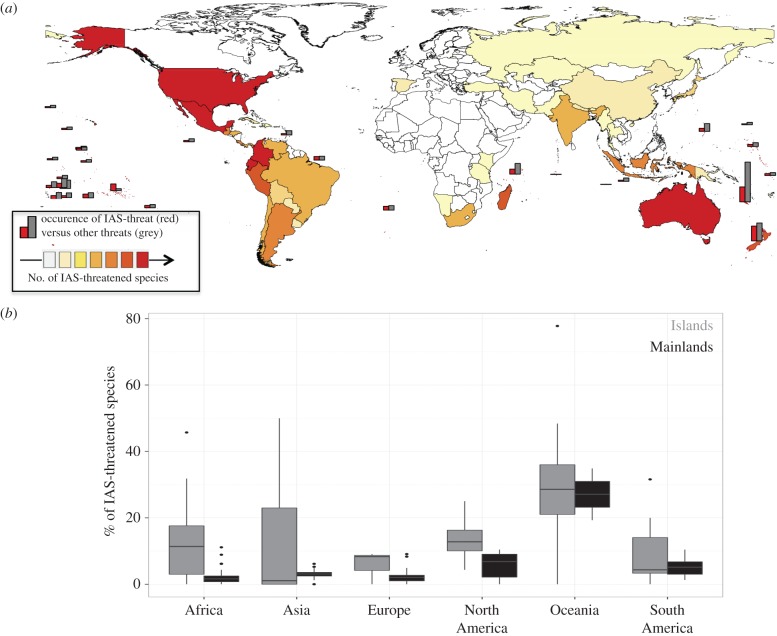
The spatial distributions of the absolute numbers of IAS-threatened vertebrates vary greatly between regions (figure 2a). The Americas (North [Nmax (maximum number of IAS-threatened species per country) = 89], Central [Nmax = 129] and South [Nmax = 107]), Australia (N = 108), New Caledonia (N = 63), New Zealand (N = 62) and Madagascar (N = 52) host the most IAS-threatened species (electronic supplementary material, table S2). We observed moderate numbers of IAS (17–51) in rapidly developing countries including India, Brazil, Argentina, Peru and Indonesian islands. We also observed that the occurrence of IAS threats compared with other threats is greater on islands than mainland areas. A more detailed classification of the countries confirmed that the proportion of IAS-threatened species tends to be higher on islands than mainland areas, although there are exceptions to this pattern (figure 2b).
Figure 2.
(a) Maps of the distribution of CR, EN and VU IAS-threatened vertebrates per country, and occurrences of IAS-threat (red) versus other threats (grey). Note that only countries and islands are illustrated here where more than 25% of the threatened species are threatened due to IAS. (b) Boxplots showing the percentages of IAS-threatened species compared to all threatened species on islands versus mainlands.
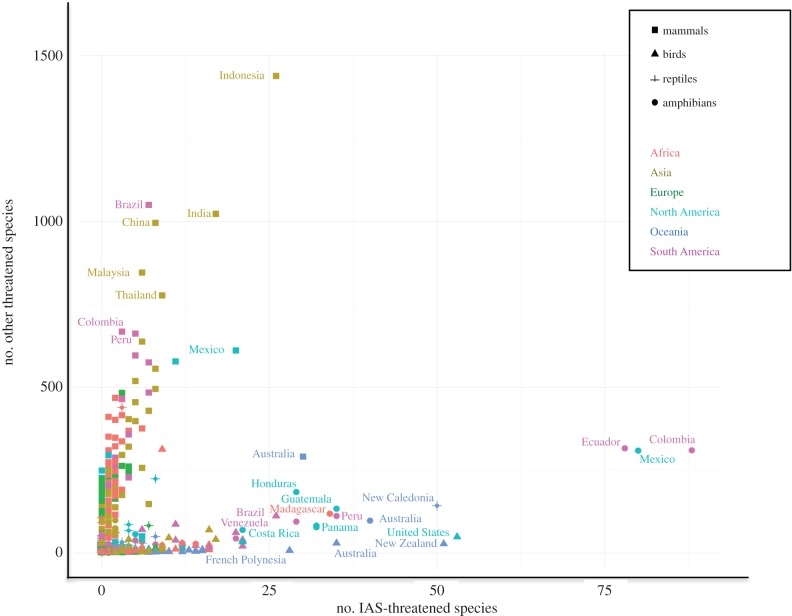
Comparison of the numbers of IAS-threatened vertebrates with the numbers of vertebrates threatened by other factors revealed that the IAS threat is concentrated in a subset of countries and that IAS are not the most important contributor to the number of species that are threatened globally (figure 3). We distinguished two clusters of countries. The first cluster includes many countries that harbour high numbers of Red List vertebrates (particularly mammals; e.g. Indonesia, India, China, Brazil, Malaysia and Thailand) that are threatened by factors other than IAS and harbour only a few IAS-threatened species. The second cluster includes countries that harbour relatively high numbers of IAS-threatened vertebrates and relatively low numbers of vertebrates threatened by other factors. Examples of these countries include the US, New Zealand and Australia for birds, New Caledonia for reptiles, and Colombia, Mexico, Ecuador, Australia, Peru, Madagascar, Guatemala and Panama for amphibians. Many of the other countries are of relatively low concern regarding IAS-threatened vertebrates.
Figure 3.
Number of IAS-threatened species compared to number of species threatened by other factors per country and taxonomic group. Please note the different scales of the two axes.
(c). Which species are of primary concern among invasive alien species-threatened vertebrates?
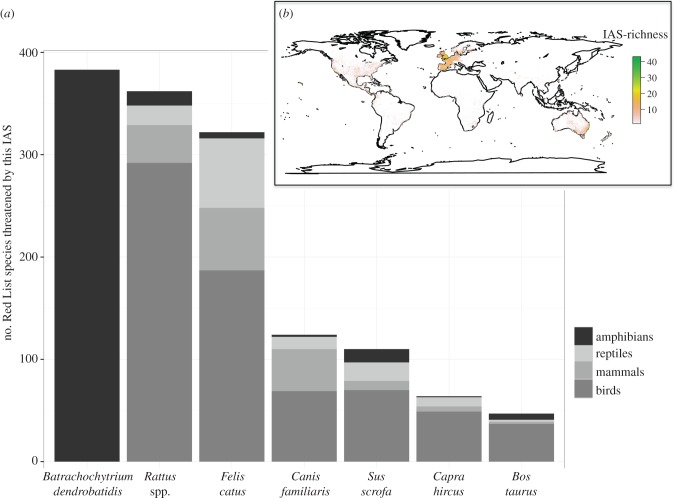
The IAS that threatens the greatest number of vertebrates is Batrachochytrium dendrobatidis (figure 4a). This chytrid fungus is the main driver of amphibian declines (see also [24,25]) and causes the disease chytridiomycosis. All rats (Rattus spp.) together are in second position. A finer subdivision into the different rat species is hampered by the available data, as for more than a hundred vertebrates threatened by rats, the exact rat species is not given in our database (electronic supplementary material, table S3). Still, the black rat (Rattus rattus) alone threatens at least 148 vertebrate species. All following ranks on the list of IAS threatening the highest numbers of vertebrates are also occupied by mammals: cats (Felis catus) on position 3, which primarily threaten birds; rank 4 is occupied by dogs (Canis familiaris), but they threaten much fewer vertebrates than cats, so there is a gap between position 3 and 4; wild boar (Sus scrofa), goats (Capra hircus) and cattle (Bos taurus) in positions 5, 6 and 7, respectively. Interestingly, 184 different IAS in total threaten bird species, whereas only 74, 53 and 48 IAS threaten mammals, amphibians and reptiles, respectively. We identified a total of 231 IAS (in 31 families) that threaten vertebrates including 197 for which we were able to collect spatial information. Regarding the IAS that threaten at least five vertebrates, we found that most of these species are omnivores (37%), followed by carnivores and herbivores (26% each), primary producers (9%) and scavenger predators (2%). The resulting map revealed that the known occurrences of IAS that threaten vertebrates are concentrated in Europe, North America, New Zealand and Australia (figure 4b), although IAS are also found in South and Central America, Africa and Asia.
Figure 4.
(a) Top 7 of IAS that globally threaten the highest numbers of mammals, birds, reptiles and amphibians. (b) Spatial distribution of IAS that have been identified as threatening vertebrates and for which spatial distribution data were available (N = 197). We calculated Pearson's correlation coefficient between IAS distribution (here) and IAS-threatened vertebrates (combined figure of figure 1b): Pearson's r = −0.05.
4. Discussion
Our results indicate that the effects of IAS on Red List vertebrates are not equally distributed across the globe but are spatially concentrated in a subset of countries. The current major centres of IAS-threatened vertebrates are in the Americas (North, Central and South), India, Indonesia, Australia and New Zealand. These areas are crucially important for biodiversity. This pattern is the result of both past (e.g. through colonization) and modern (e.g. through international trade) species introductions [26,27]. The distribution of IAS-threatened vertebrates is only in partial agreement with the current known hotspots of invasions based on the most recent Global Biodiversity Outlook report [28], especially regarding European, Asian and South American countries (figure 1; electronic supplementary material, figure S4). Our findings also contradict previous results regarding threatened fish species (which were not included in our study) for which the six major invasion hotspots have been characterized as containing the greatest proportions of threatened fish species [29]. Indeed, the hotspots of introductions ([30], figure 4b) are more spatially restricted than the hotspots of IAS-threatened species. This spatial mismatch is probably the consequence of many factors, for example, (i) one IAS can threaten multiple native species (figure 4); (ii) some native species have life-history or other traits that make them disproportionately vulnerable to IAS exposure; (iii) the number of introduced species is likely to be underestimated in most countries, and there is a geographical bias in the severity of this underestimation [8,31,32]; and (iv) there is a time lag between the arrival of IAS and their effects on native species [33,34]. Such spatial mismatches have implications regarding current management practices for IAS because management strategies are mainly based on known IAS distributions [35]. We also found that the proportion of IAS-threatened species tends to be higher on islands than in mainland areas, and this pattern is in line with the island susceptibility hypothesis [36]. However, this pattern was not observed in Asia and South America; hence, future research is needed to explain these differences.
We also found that IAS are mostly spatially associated with other threats and are unlikely to be the sole drivers of biodiversity loss in the large majority of countries, particularly those in mainland areas. In contrast, we found strong spatial disparities between countries that harbour threatened species in general and IAS-threatened species in particular. For example, although Africa is the second-most important continent in terms of the number of threatened vertebrates in general (especially due to habitat loss), it is the least important continent in terms of the number of IAS-threatened vertebrates (with the exceptions of Madagascar and South Africa). This pattern might be due to a geographical reporting bias [8,31,32] and/or the fact that Africa's long-standing ecological communities have not been heavily invaded by IAS yet. We also found that some American countries (i.e. Colombia, Ecuador and Mexico) and Oceanic islands face the highest risk of species extinctions due to IAS; these countries and islands harbour the highest absolute numbers of vertebrates that are threatened by IAS, and IAS are the predominant threat in these countries relative to other threats.
Meeting Aichi Target 9 of the CBD also requires the identification of the priority IAS. We found that IAS—including predators (e.g. cats and rats) and pathogens (i.e. the chytrid B. dendrobatidis)—play major roles in the threats to birds [6] and amphibians, but play lesser roles in the threats to mammals and reptiles [10,22] (see also table 1, and electronic supplementary material, figures S1 and S2). Previous studies have demonstrated that alien mammalian predators and the chytrid fungus frequently threaten native species, but these studies were mainly performed in specific regions or with particular species groups [37–40]. Although predators and pathogens represent the most threatening IAS, unknown invaders might also spread and affect biodiversity in the future. This study is the first to examine the current threats to vertebrates posed by IAS at the global scale.
It is striking that positions 2–7 on the list of IAS threatening the highest numbers of vertebrates are all occupied by mammals. This taxonomic group has previously been demonstrated to elicit particularly strong invader effects [41,42]. Many of these species including pigs, goats and cattle, are also major threats to threatened plants [1]. However, the management of the effects of these high-impact mammals is challenging because, with the exception of rats, they all also provide clear and direct benefits to humans. Fortunately, approximately 88% of eradication programmes have successfully removed R. rattus from islands [43]. Regarding other invasive mammals, management measures that seek to limit reproduction are generally preferable to measures that involve the killing of mammals. Additionally, B. dendrobatidis can explain most of the patterns of threatened amphibians. Although the disease caused by this fungus was entirely unknown until the late 1990s [44], the current literature describes a variety of disease mitigation strategies that can be applied to amphibians, and the application of these strategies needs to become a more active conservation policy [45]. Other highly problematic IAS, such as the little fire ant Wasmania auropunctata (ranked 3 for reptiles), have also been successfully eradicated in most cases. We found that birds are threatened by more than 100 different IAS, particularly in New Zealand, the US, Australia and French Polynesia. Therefore, we strongly recommend that these countries pursue more aggressive and innovative strategies for the management of IAS to protect their native species (see e.g. [46] the Predator-Free New Zealand campaign).
The current knowledge of the IUCN Red List species and their respective threats is imperfect and is likely to be more complete for richer rather than poorer countries [9]. For example, the spatial distributions of IAS-threatened species are based on polygons drawn by experts and are actually only rough approximations. Furthermore, information about the intensities and severities of threats is often lacking. For example, IAS have caused significant declines in the majority of threatened amphibian and bird populations (electronic supplementary material, figure S5), which are the two vertebrate groups most affected by IAS. However, we lack such data for other groups and thus assume that IAS that are cited as threats are also causing significant declines in these species. Moreover, IAS threats are often associated with other threats, and despite the current knowledge provided by the newly designed GISD, it remains difficult to disentangle the relative roles of IAS compared with other threats. Another important finding of this study is that the proportion of IAS-threatened species increases with extinction risk among mammals, birds, reptiles and amphibians (table 1), whereas the number of threats remains stable across the IUCN categories. One possible interpretation of this finding is that IAS cause a greater risk of extinction than do some other threats.
Meeting the Aichi Target 9 requires that appropriate management measures control IAS be implemented within each country. In contrast to other threats, such as hunting and fishing, for which the effects immediately end when specific activities are stopped, IAS will continue to represent severe threats even if very effective biosecurity policies that halt any movement of alien species worldwide are enforced, because established IAS will continue to affect native biodiversity. Although the IUCN Red List has some limitations (e.g. [47]), it is currently the most comprehensive list of threatened species worldwide.
Based on our results, we have three main recommendations to contribute to the achievement of Aichi Target 9. First, most current eradication programmes are implemented on individual islands, but the design of eradication programmes has significantly improved, and there is a growing number of multispecies programmes [28]. Therefore, funding and efforts should be coordinated to eradicate one or a subset of targeted IAS from multiple islands, to provide the opportunity for native populations to significantly recover (e.g. New Zealand [46]). Over the past decades, across the 1128 successful eradication programmes that have been implemented on individual islands [48], the overall risks of extinction due to IAS have been substantially reduced for only 11 bird, five mammal and one amphibian species [6]. Eradication programmes on islands should be prioritized on the basis of not only eradication feasibility, economic cost, reinvasion potential and the most problematic IAS [15,49,50], but also the potential outcome for global biodiversity. We were able to determine where the most important clusters of IAS-threatened species are, and which IAS are responsible for these effects. These findings should aid decision-making regarding the implementation of eradication programmes.
Second, there have been very few attempts and successful eradication programmes in mainland areas due to limited funding and feasibility [51]. However, IAS are also threatening species in mainland areas as highlighted by our results, and thus IAS control programmes must be strengthened in these areas. However, IAS are rarely the only cause of extinction in mainland areas and should not be considered in isolation but should instead be considered jointly with other factors that threaten biodiversity as demonstrated, for example, by the complex case of the amphibian chytrid fungus [52]. Thus, we advocate that control of IAS in mainland areas be based on multiple-threat analyses to protect biodiversity [53].
Third, some countries that are highly vulnerable to IAS (such as those reported here) still lack IAS policies, e.g. countries in Central and South America [40]. It is crucial that the governments of these countries improve their actions in this regard.
Supplementary Material
Acknowledgements
We are grateful to the IAS and IUCN experts for sharing data with the community and to G. Mace, G. Luque, P. Staniczenko, B. Gallardo and the referees for valuable comments that improved the manuscript.
Data accessibility
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.d4p98.
Authors' contributions
C.B. and J.M.J. designed the research. P.G. provided the data. C.B. analysed the data. C.B., J.M.J. and P.G. wrote the paper.
Competing interests
We have no competing interest.
Funding
This study was funded by an Axa Fellowship (C.B.) and the DFG projects JE 288/8-1 and JE 288/9-1 (J.M.J.), and the ERA-Net BiodivERsA (project FFII, JE 288/7-1) with the national funders Agence Nationale de la Recherche and DFG (JE 288/7-1), part of the 2012-13 BiodivERsA call for proposals.
References
- 1.Gurevitch J, Padilla DK. 2004. Are invasive species a major cause of extinctions? Trends Ecol. Evol. 19, 470–474. ( 10.1016/j.tree.2004.07.005) [DOI] [PubMed] [Google Scholar]
- 2.Thomas CD, Palmer G. 2015. Non-native plants add to the British flora without negative consequences for native diversity. Proc. Natl Acad. Sci. USA 112, 4387–4392. ( 10.1073/pnas.1423995112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce F. 2015. Trial by repetition. New Sci. 227, 26–27. ( 10.1016/S0262-4079(15)31125-8) [DOI] [Google Scholar]
- 4.Clavero M, García-Berthou E. 2005. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 20, 110 ( 10.1016/j.tree.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 5.Burbidge AA, Manly BFJ. 2002. Mammal extinctions on Australian islands: causes and conservation implications. J. Biogeogr. 29, 465–473. ( 10.1046/j.1365-2699.2002.00699.x) [DOI] [Google Scholar]
- 6.McGeoch MA, Butchart SHM, Spear D, Marais E, Kleynhans EJ, Symes A, Chanson J, Hoffmann M. 2010. Global indicators of biological invasion: species numbers, biodiversity impact and policy responses. Divers. Distrib. 16, 95–108. ( 10.1111/j.1472-4642.2009.00633.x) [DOI] [Google Scholar]
- 7.Scalera R, Genovesi P, Essl F, Rabitsch W. 2012. The impacts of invasive alien species in Europe. EEA Technical report no. 16/2012.
- 8.Lowry E, Rollinson EJ, Laybourn AJ, Scott TE, Aiello-Lammens ME, Gray SM, Mickley J, Gurevitch J. 2012. Biological invasions: a field synopsis, systematic review, and database of the literature. Ecol. Evol. 3, 182–196. ( 10.1002/ece3.431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues ASL, Brooks TM, Butchart SHM, Chanson J, Cox N, Hoffmann M, Stuart SN. 2014. Spatially explicit trends in the global conservation status of vertebrates. PLoS ONE 9, e113934 ( 10.1371/journal.pone.0113934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Böhm M, et al. 2013. The conservation status of the world's reptiles. Biol. Conserv. 157, 372–385. ( 10.1016/j.biocon.2012.07.015) [DOI] [Google Scholar]
- 11.Schipper J, et al. 2008. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230. ( 10.1126/science.1165115) [DOI] [PubMed] [Google Scholar]
- 12.Tittensor DP, et al. 2014. A mid-term analysis of progress toward international biodiversity targets. Science 346, 241–244. ( 10.1126/science.1257484) [DOI] [PubMed] [Google Scholar]
- 13.Bellard C, Thuiller W, Leroy B, Genovesi P, Bakkenes M, Courchamp F. 2013. Will climate change promote future invasions? Glob. Change Biol. 19, 3740–3748. ( 10.1111/gcb.12344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molnar JL, Gamboa RL, Revenga C, Spalding MD. 2008. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 6, 485–492. ( 10.1890/070064) [DOI] [Google Scholar]
- 15.Brooke MDL, Hilton GM, Martins TLF. 2007. Prioritizing the world's islands for vertebrate-eradication programmes. Anim. Conserv. 10, 380–390. ( 10.1111/j.1469-1795.2007.00123.x) [DOI] [Google Scholar]
- 16.IUCN. 2001. IUCN Red List Categories and Criteria, v. 3.1. IUCN Species Survival Commission. Gland, Switzerland and Cambridge, UK: IUCN.
- 17.Mace GM, Collar NJ, Gaston KJ, Hilton-Taylor C, Akçakaya HR, Leader-Williams N, Milner-Gulland EJ, Stuart SN. 2008. Quantification of extinction risk: IUCN's system for classifying threatened species. Conserv. Biol. 22, 1424–1442. ( 10.1111/j.1523-1739.2008.01044.x) [DOI] [PubMed] [Google Scholar]
- 18.Heard MJ, Smith KF, Ripp KJ, Berger M, Chen J, Dittmeier J, Goter M, McGarvey ST, Ryan E. 2013. The threat of disease increases as species move toward extinction. Conserv. Biol. 27, 1378–1388. ( 10.1111/cobi.12143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagad S, Genovesi P, Carnevali L, Scalera R, Clout M. 2015. IUCN SSC Invasive Species Specialist Group: invasive alien species information management supporting practitioners, policy makers and decision takers. Manage. Biol. Invasions 6, 127–135. ( 10.3391/mbi.2015.6.2.03) [DOI] [Google Scholar]
- 20.Hijmans ARJ, Phillips S, Leathwick J, Elith J. 2011. Packagedismo: species distribution modeling. R package version 1.0-12. See https://cran.r-project.org/web/packages/dismo/index.html.
- 21.Salafsky N, et al. 2008. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conserv. Biol. 22, 897–911. ( 10.1111/j.1523-1739.2008.00937.x) [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Suarez M, Revilla E. 2014. Generalized drivers in the mammalian endangerment process. PLoS ONE 9, e90292 ( 10.1371/journal.pone.0090292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IUCN. 2010. IUCN Red List of Threatened Species, v. 2010.3. See http://www.iucnredlist.org.
- 24.Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, Bielby J, Garner TWJ, Weaver G, Fisher MC. 2013. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS ONE 8, e56802 ( 10.1371/journal.pone.0056802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786. ( 10.1126/science.1103538) [DOI] [PubMed] [Google Scholar]
- 26.Hulme PE. 2009. Trade, transport and trouble: managing invasive species pathways in an era of globalization. J. Appl. Ecol. 46, 10–18. ( 10.1111/j.1365-2664.2008.01600.x) [DOI] [Google Scholar]
- 27.Seebens H, Gastner MT, Blasius B. 2013. The risk of marine bioinvasion caused by global shipping. Ecol. Lett. 16, 782–790. ( 10.1111/ele.12111) [DOI] [PubMed] [Google Scholar]
- 28.Leadley PW, et al. 2014. Progress towards the Aichi targets: an assessment of biodiversity trends, policy scenarios and key actions. Montreal, Canada: Secretariat of the Convention on Biological Diversity. [Google Scholar]
- 29.Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S. 2008. Fish invasions in the world's river systems: when natural processes are blurred by human activities. PLoS Biol. 6, e28 ( 10.1371/journal.pbio.0060028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seebens H, et al. 2015. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Change Biol. 21, 4128–4140. ( 10.1111/gcb.13021) [DOI] [PubMed] [Google Scholar]
- 31.Pyšek P, Richardson DM, Pergl J, Jarošík V, Sixtová Z, Weber E. 2008. Geographical and taxonomic biases in invasion ecology. Trends Ecol. Evol. 23, 237–244. ( 10.1016/j.tree.2008.02.002) [DOI] [PubMed] [Google Scholar]
- 32.Bellard C, Jeschke J. 2015. A spatial mismatch between invader impacts and research publications. Conserv. Biol . ( 10.1111/cobi.12611) [DOI] [PubMed] [Google Scholar]
- 33.Crooks JA. 2005. Lag times and exotic species: the ecology and management of biological invasions in slow-motion. Ecoscience 12, 316–329. ( 10.2980/i1195-6860-12-3-316.1) [DOI] [Google Scholar]
- 34.Essl F, Dullinger S, Rabitsch W, Hulme PE, Pyšek P, Wilson JRU, Richardson DM. 2015. Historical legacies accumulate to shape future biodiversity in an era of rapid global change. Divers. Distrib. 21, 534–547. ( 10.1111/ddi.12312) [DOI] [Google Scholar]
- 35.Convention on Biological Diversity. 2014. Pathways of introduction of invasive species, their prioritization, and management. Subsidiary Body on Scientific, Technical and Technological advice, Montreal, Canada. [Google Scholar]
- 36.Jeschke JM. 2008. Across island and continents, mammals are more successful invaders than birds. Divers. Distrib. 14, 913–916. ( 10.1111/j.1472-4642.2009.00583.x) [DOI] [Google Scholar]
- 37.Clavero M, Brotons L, Pons P, Sol D. 2009. Prominent role of invasive species in avian biodiversity loss. Biol. Conserv. 142, 2043–2049. ( 10.1016/j.biocon.2009.03.034) [DOI] [Google Scholar]
- 38.Blackburn TM. 2004. Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958. ( 10.1126/science.1101617) [DOI] [PubMed] [Google Scholar]
- 39.Pounds JA, et al. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167. ( 10.1038/nature04246) [DOI] [PubMed] [Google Scholar]
- 40.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ.2010. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl Acad. Sci. USA107, 9689–9694. (idoi:10.1073/pnas.0914111107) [DOI] [PMC free article] [PubMed]
- 41.Vilà M, et al. 2010. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ. 8, 135–144. ( 10.1890/080083) [DOI] [Google Scholar]
- 42.Kumschick S, Bacher S, Blackburn TM. 2012. What determines the impact of alien birds and mammals in Europe? Biol. Invasions 15, 785–797. ( 10.1007/s10530-012-0326-6) [DOI] [Google Scholar]
- 43.DIISE. 2015. The database of island invasive species eradications, developed by island conservation, costal conservation action. See http://diise.islandconservation.org.
- 44.Berger L, et al. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl Acad. Sci. USA 95, 9031–9036. ( 10.1073/pnas.95.15.9031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodhams DC, et al. 2011. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front. Zool. 8, 8 ( 10.1186/1742-9994-8-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell JC, Innes JG, Brown PH, Byrom AE. 2015. Predator-free New Zealand: conservation country. BioScience 65, 520–525. ( 10.1093/biosci/biv012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayward MW. 2009. The need to rationalize and prioritize threatening processes used to determine threat status in the IUCN Red List. Conserv. Biol. 23, 1568–1576. ( 10.1111/j.1523-1739.2009.01260.x) [DOI] [PubMed] [Google Scholar]
- 48.Pluess T, Cannon R, Jarošík V, Pergl J, Pyšek P, Bacher S. 2012. When are eradication campaigns successful? A test of common assumptions. Biol. Invasions 14, 1365–1378. ( 10.1007/s10530-011-0160-2) [DOI] [Google Scholar]
- 49.Capizzi D, Baccetti N, Sposimo P. 2010. Prioritizing rat eradication on islands by cost and effectiveness to protect nesting seabirds. Biol. Conserv. 143, 1716–1727. ( 10.1016/j.biocon.2010.04.020) [DOI] [Google Scholar]
- 50.Harris DB, Gregory SD, Bull LS, Courchamp F. 2011. Island prioritization for invasive rodent eradications with an emphasis on reinvasion risk. Biol. Invasions 14, 1251–1263. ( 10.1007/s10530-011-0153-1) [DOI] [Google Scholar]
- 51.Baker SJ. 2010. Control and eradication of invasive mammals in Great Britain: the Neolithic period to the 18th Century. Rev. Sci. Tech. 29, 311–327. [DOI] [PubMed] [Google Scholar]
- 52.Hof C, Araújo MB, Jetz W, Rahbek C. 2011. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 480, 516–519. ( 10.1038/nature10650) [DOI] [PubMed] [Google Scholar]
- 53.Bellard C, Leclerc C, Courchamp F. 2015. Combined impacts of global changes on biodiversity across the USA. Sci. Rep. 5, 11828 ( 10.1038/srep11828) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.d4p98.