Abstract
Migration is a common strategy used by birds that breed in seasonal environments. Selection for greater migration efficiency is likely to be stronger for terrestrial species whose migration strategies require non-stop transoceanic crossings. If multiple species use the same transoceanic flyway, then we expect the migration strategies of these species to converge geographically towards the most optimal solution. We test this by examining population-level migration trajectories within the Western Hemisphere for 118 migratory species using occurrence information from eBird. Geographical convergence of migration strategies was evident within specific terrestrial regions where geomorphological features such as mountains or isthmuses constrained overland migration. Convergence was also evident for transoceanic migrants that crossed the Gulf of Mexico or Atlantic Ocean. Here, annual population-level movements were characterized by clockwise looped trajectories, which resulted in faster but more circuitous journeys in the spring and more direct journeys in the autumn. These findings suggest that the unique constraints and requirements associated with transoceanic migration have promoted the spatial convergence of migration strategies. The combination of seasonal atmospheric and environmental conditions that has facilitated the use of similar broad-scale migration strategies may be especially prone to disruption under climate and land-use change.
Keywords: avian migration, geographical barriers, looped migration, migration speed, transoceanic migration, Western Hemisphere
1. Introduction
Migration is a common strategy used by organisms to take advantage of seasonal changes in resources and environmental conditions, and is typically defined as directed movements between breeding grounds and non-breeding or wintering grounds [1]. The migration of birds is a well-studied example, with ca 19% of extant bird species identified as migratory [2]. The hazards associated with migration can have negative effects on the survival and reproductive success of bird populations [3,4]. The resulting selection pressures have generated a unique set of morphological, physiological, and behavioural adaptations [5–7], whose quality varies based on the rigors of the migratory journey. For example, individuals that migrate longer distances tend to have adaptations that improve the efficiency of migratory flight [8–10]. The presence of geographical barriers to migration is an additional factor that may affect the quality of these adaptations. Geographical barriers for migratory birds are regions of inhospitable terrain containing few or no opportunities for stopping and refuelling, thus requiring long periods of continuous flight.
Oceans are one of the most common geographical barriers encountered by birds during migration. In contrast to overland migration, transoceanic crossings of the same distance carry significantly greater risk. For example, factors that interfere with migration efficiency such as navigational errors [11] or encounters with unfavourable atmospheric conditions [12] may have fatal consequences during transoceanic crossings. As a consequence, migrants that undertake transoceanic crossings display adaptations in migratory behaviour [13], physiology, and morphology that support more efficient migratory flight [14,15]. In some cases, migrants have been observed returning to their point of origin (reverse migration), suggesting that migrants are more cautious when initiating transoceanic crossings [16]. Transoceanic crossings may also carry some potential benefits; for example, encounters with pathogens and predators are substantially reduced [17]. Nevertheless, associating with favourable atmospheric conditions, specifically supportive winds, is considered essential for successful transoceanic crossings [18–20]. We would therefore expect that, for species using the same transoceanic flyway, the more stringent selection pressures should result in the broad-scale convergence of migration strategies towards the most optimal solution. Specifically, based on the seasonal atmospheric and environmental conditions that exist within the region, we would expect populations of migratory birds to converge on similar migration trajectories that minimize time, energy, and risk [21,22].
One broad-scale migratory feature often shared among transoceanic migrants, especially pelagic species, is looped migration strategies [22]. Evidence of looped migration strategies has been documented with terrestrial bird species, including a large number that migrate within the Western Hemisphere [23]. Seasonal variation in atmospheric conditions [19,24] and seasonal changes in ecological productivity [25] have been identified as likely drivers for these strategies. For species that conduct transoceanic migration within the Western Hemisphere, a migration flyway has been identified within the eastern portion of North America [24]. Migration strategies within this eastern flyway are characterized by clockwise looped migration trajectories where potentially longer spring migration routes occur west of autumn migration routes [23,24]. A seasonal atmospheric feature likely promoting this looped strategy is the Great Plains low-level jet stream, whose southerly winds may assist northward movements over the Gulf of Mexico in the spring [24]. In the autumn, the prevailing winds do not support southward migration and migrants tend to time migratory flight to coincide with synoptic meteorological events (passage of low-pressure systems) that provide periods of favourable atmospheric conditions [26–30].
Here, we document migration strategies at a population level [23] for a diverse collection of terrestrial migratory bird species within the Western Hemisphere (excluding Western Europe and Africa) to test for evidence of the geographical convergence of migration strategies. We expect spatial convergence to be most pronounced for transoceanic migrants and characterized, based on previous findings [23,24], by clockwise looped migration trajectories. When contrasted with other Western Hemisphere migrants, we anticipate that population-level migration speeds for transoceanic migrants will be faster owing to the use of non-stop flights over longer distances. We propose that faster migration speeds (through specialization enabling non-stop flight) and the use of clockwise looped migration trajectories (through heightened exploitation of seasonal atmospheric conditions) will provide evidence of increased adaptation and convergence of migration strategies.
To test our predictions, we estimate the geographical location of migration trajectories within the Western Hemisphere for 118 long-distance migratory bird species. We conduct the analysis at a daily temporal resolution using occurrence information from the eBird citizen-science database [31] for the combined period 2002–2014. Using these migration trajectories, we estimate population-level migration distance and speed, and we classify species as following either looped (clockwise or anticlockwise) or repeated migration trajectories. We further classified species as transoceanic or terrestrial migrants based on the location of their migration trajectories relative to the Gulf of Mexico and Atlantic Ocean. Through this analysis, our goal is to improve our understanding of the many factors that govern large-scale migration strategies within the Western Hemisphere [32], with the objective of better informing full life-cycle management and conservation efforts within the region [33,34].
2. Material and methods
(a). Data acquisition and preparation
We compiled avian occurrence information from the eBird citizen-science database [31] for the period 2002–2014 within the region of the Western Hemisphere between 170° to 30° W longitude and 60° S and 84° N latitude. eBird data are organized into lists of observed species (checklists) with the time and location (longitude and latitude) of the sampling event. Our data contained more than 6.1 million eBird checklists. We excluded from checklists invalidated observations and species that associate primarily with pelagic environments and species that are accidental within the Western Hemisphere. In the end, occurrence information was available for a total of 4 288 bird species. For each species and day, we grouped observations across checklists within equal-area hexagon cells (49 811 km2) of a global icosahedron (electronic supplementary material, figure S1) [35,36]. This information was subsequently aggregated across years for each species and day.
(b). Migration trajectory estimation
To estimate hemispheric-wide migration trajectories, we first converted the geodetic coordinates (longitude and latitude) of the geographical midpoints of the icosahedron cells to Earth-centred Earth-fixed (ECEF) Cartesian coordinates. ECEF coordinates are defined as three-dimensional vectors originating at the centre of the Earth and terminating at the terrestrial midpoint of each icosahedron cell. ECEF takes into consideration the precise shape and size of the Earth, here using the WGS84 reference datum. For our analysis, we rounded to the nearest kilometre the ECEF coordinates of each cell's geographical midpoint.
We used generalized additive models for location, scale, and shape (GAMLSS) [37] to estimate the daily location of the geographical midpoint of each species' population within the Western Hemisphere while accounting for spatio-temporal variation in sampling effort. GAMLSS is a flexible procedure that simultaneously models the mean and standard deviation using smooth linear predictors. The geographical breadth of observations provided by eBird (see the electronic supplementary material, figure S1) allowed GAMLSS to effectively estimate geographical midpoints of species' populations within the Western Hemisphere over both terrestrial and marine environments (see the electronic supplementary material, figure S2 for examples). The daily locations of the geographical midpoints of each species' population were modelled as the expected values of the three ECEF coordinates based on the locations of the icosahedron cells where eBird searches took place. The daily location of each of the coordinates was modelled separately using a Gaussian two-parameter distribution to describe the error variance. The mean of each coordinate was allowed to vary smoothly as a function of day of the year using a cyclic penalized B-spline, so the model smoothly joined daily location estimates made for 31 December and 1 January. To account for spatio-temporal variation in sampling effort, the standard deviation was allowed to vary smoothly as a function of the total number of checklists submitted within each cell, also specified as a penalized B-spline. To capture the spatial variation of each species' distribution on a given day, the icosahedron cell coordinates were weighted by the proportion of checklists where the species was observed in that cell on that day.
After fitting the GAMLSS models to each of the three ECEF coordinates for each species, we calculated the daily predicted values for the ECEF coordinates for days of the year containing occurrence information for that species. We converted these daily ECEF coordinates to geodetic coordinates (longitude and latitude) using methods described in [38]. In total, the GAMLSS procedure estimated the daily location of the geographical midpoint of each species' population within the Western Hemisphere while accounting for spatio-temporal variation in sampling effort. We defined the resulting product for each species as their migration trajectory.
After this method was applied to the data from all 4 288 species, we selected 118 migratory species (electronic supplementary material, table S1) for further consideration based on the following three criteria. First, the species had a maximum latitudinal geographical separation within its migration trajectory that was greater than 2 000 km. Second, the species contained geographically distinct breeding and non-breeding distributions (BirdLife International, IUCN Red List for birds, http://www.birdlife.org, accessed 16 June 2015) whose geographical centres were connected by the migration trajectories. Third, the migration trajectory followed a linear path with no longitudinal variability between the breeding and non-breeding grounds during spring and autumn migration. These criteria allowed us to focus our analysis on migratory species having evidence of geographically significant and distinct population-level movements that could be reliably estimated using our population-level methods. The 118 species considered in the study had geographical midpoints estimated for a mean of 343 days of the annual cycle (range = 138–365).
We used a bootstrap approach applied to the GAMLSS analysis described above to estimate the uncertainty associated with each species' daily geographical midpoint estimates. The bootstrap was implemented separately for each species and consisted of sampling with replacement checklists that contained observations for that species. These checklists were then aggregated within the icosahedron cells, and the GAMLSS analysis was repeated and the predicted daily geographical midpoints regenerated. This procedure was implemented 1 000 times for each species, resulting in 1 000 individual bootstrap migration trajectories. From the 1 000 bootstrap trajectories, we estimated longitudinal uncertainty for each day using the daily 2.5% and 97.5% longitudinal quantiles.
(c). Migration trajectory analysis
We described migration trajectories using several migration metrics. First, we calculated the great circle (orthodromic) distance between pairs of sequential daily geographical midpoints (see the electronic supplementary material, figure S2 for examples). The great circle distance was measured using the Vincenty ellipsoid method [39] with the WGS84 reference datum. With these measurements, we estimated population-level migration speed and total migration distance. Population-level migration speed, which should not be confused with the flight speed of an individual bird, is measured daily and is defined as the distance between the geographical midpoints of a species' population between sequential days (km day−1). The migration distance is defined as the sum of these distances calculated separately for spring and autumn migration using the daily geographical midpoints with the minimum and maximum latitudes to delineate the start and end of the two seasons. Following methods described in [23], we also calculated the maximum or peak daily migration speed occurring during spring and autumn migration based on the median of the five fastest speeds documented during each season. To determine how far the spring and autumn migration trajectories deviated from the great circle path between each species' maximum and minimum latitudes, we calculated the cross track distance for each species' spring and autumn migration trajectories. The cross track distance is defined as the distance between a location on the current path and the nearest point on the intended path. We estimated cross track distance using the great circle distance from the great circle path to each geographical midpoint within the migration trajectory averaged separately for spring and autumn migration. Higher values of cross track distance indicate that the migration trajectory deviates more substantially from the shortest geographical path between the centre of the breeding and non-breeding ranges. All speed and distance estimates took into consideration days with missing occurrence information, and were log-transformed before analysis to improve their distributional properties. To further account for heterogeneity of variance and non-normality in the speed and distance estimates, we used high breakdown and high efficiency robust three-way ANOVA in our analysis of these metrics among migration categories (defined below). We report the robust F-tests and t-tests from these analyses.
To determine the form and spatial correspondence of the migration trajectories for the 118 species, we summarized the location of species' geographical midpoints within 1° latitudinal bands across the full annual cycle. We first split the annual cycle for each species into a spring component and an autumn component separated at the dates at which the daily geographical midpoints were at their minimum and maximum latitudes. We then averaged within the 1° latitudinal bands for the spring and autumn component separately the longitude of the predicted geographical midpoints and associated bootstrap 95% confidence intervals (CIs). We then classified the 1° latitudinal bands for each species into one of three migration trajectory categories (clockwise, anticlockwise, or repeated) based on the longitudes of the spring and autumn geographical midpoints within each band and whether the spring and autumn bootstrap 95% CIs overlapped within that band. Specifically, the latitudinal band was classified as clockwise if the spring geographical midpoint was located west of the autumn geographical midpoint and the bootstrap 95% CIs did not overlap; the band was classified as anticlockwise if the spring geographical midpoint was located east of the autumn geographical midpoint and the bootstrap 95% CIs did not overlap; and the band was classified as repeated if the bootstrap 95% CIs overlapped. We used the category that was in the majority across the latitudinal bands to classify species' primary migration strategy. We further classified species as transoceanic or terrestrial migrants based on the location of their spring and autumn migration trajectories. Species whose migration trajectories crossed any portion of the Atlantic Ocean in the spring or autumn were classified as transoceanic, and any species whose migration trajectory crossed the central or eastern portion of the Gulf of Mexico in the spring or autumn were classified as transoceanic (see the electronic supplementary material, figure S2 for examples). This procedure resulted in a total of six mutually exclusive migration categories that considered both the shape and location of each migration trajectory: (i) clockwise oceanic, (ii) clockwise terrestrial, (iii) anticlockwise oceanic, (iv) anticlockwise terrestrial, (v) repeated oceanic, and (vi) repeated terrestrial.
We used R, v. 3.2.0 to conduct all analyses [40]. We used the gamlss library for GAMLSS [41] and the gamm4 library for GAMM [42]. We used the geosphere library to estimate the great circle distance using the distVincentyEllipsoid function, the great circle path using the gcIntermediate function, and the cross track distance using the dist2gc function [43]. We used the lmRob function in the robust library to implement the robust three-way ANOVA [44].
3. Results
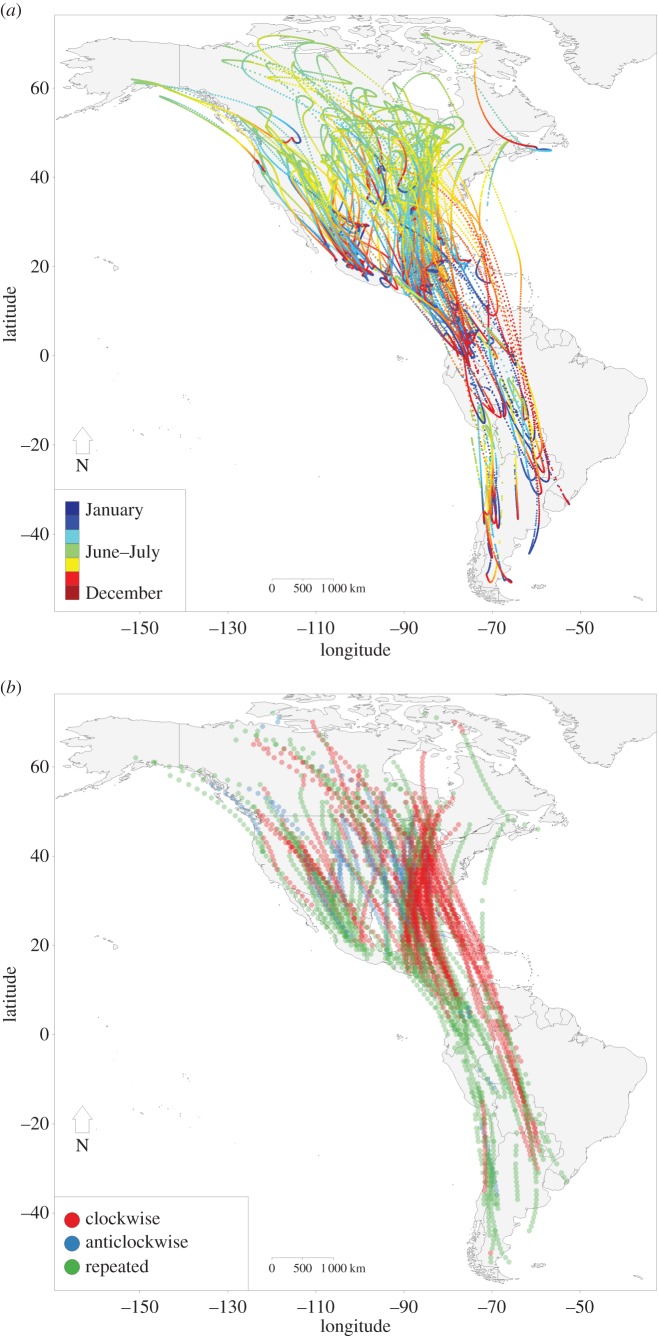
Population-level migration trajectories for the 118 long-distance migratory bird species were concentrated primarily within the Northern Hemisphere (figure 1a). When the migration trajectories were classified based on their shape, 53 species were identified as following clockwise, 14 anticlockwise, and 51 repeated migration trajectories (figure 1b; see the electronic supplementary material, table S1). The spatial distribution of species classified as following clockwise, anticlockwise, or repeated trajectories differed across the Western Hemisphere (figure 1b). The most diverse concentration of the three categories occurred within the central portion of North America; this region also contained the majority of the anticlockwise trajectories (figure 1b). The greatest concentration of repeated trajectories occurred within Central and South America, and the greatest concentration of clockwise trajectories occurred over the Gulf of Mexico and Atlantic Ocean (figure 1b).
Figure 1.
(a) Population-level migration trajectories within the Western Hemisphere at a daily temporal resolution for 118 migratory bird species for the combined period 2002–2014. (b) Migration trajectory classification within 1° latitudinal bands for the 118 species with each point defining species' annual centroid within that band (note the use of transparent points). From the 118 species, 53 were classified as clockwise, 14 as anticlockwise, and 51 as repeated.
When the 118 species were further classified as transoceanic or terrestrial migrants (see the electronic supplementary material, table S1), the distribution of these two classes among the three categories of migration trajectories was not uniform (χ2 = 20.33, d.f. = 2, p < 0.001). The majority of species following clockwise trajectories were classified as transoceanic migrants (χ2 = 11.79, d.f. = 1, p = 0.001), and the majority of species following repeated trajectories were classified as terrestrial migrants (χ2 = 8.65, d.f. = 1, p = 0.003). Species that followed anticlockwise trajectories were classified equally as transoceanic or terrestrial migrants.
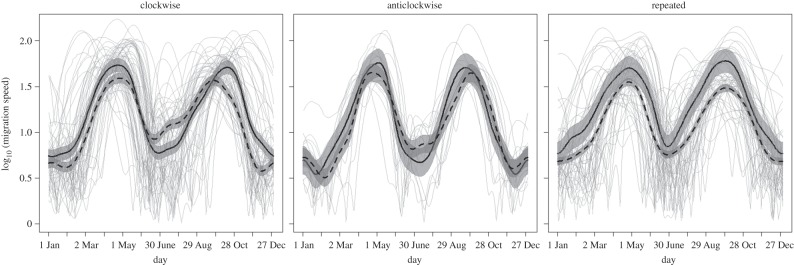
Population-level daily migration speeds peaked on average in a similar fashion for the three categories of migration trajectories during spring and autumn migration (figure 2). For species within each migration category whose migration trajectories included transoceanic crossings, daily migration speeds tended to be higher on average, an outcome that was most pronounced for species following clockwise and repeated migration trajectories (figure 2).
Figure 2.
Migration speeds for 118 bird species within the Western Hemisphere for the combined period 2002–2014 summarized within six migration categories. The grey lines are the migration speeds estimated for individual species, and the fitted lines and 95% confidence bands are from generalized additive mixed models with species as a random effect. The solid fitted lines are transoceanic migrants (n = 39, 7, and 15, respectively). The dashed fitted lines are terrestrial migrants (n = 14, 7, and 36, respectively).
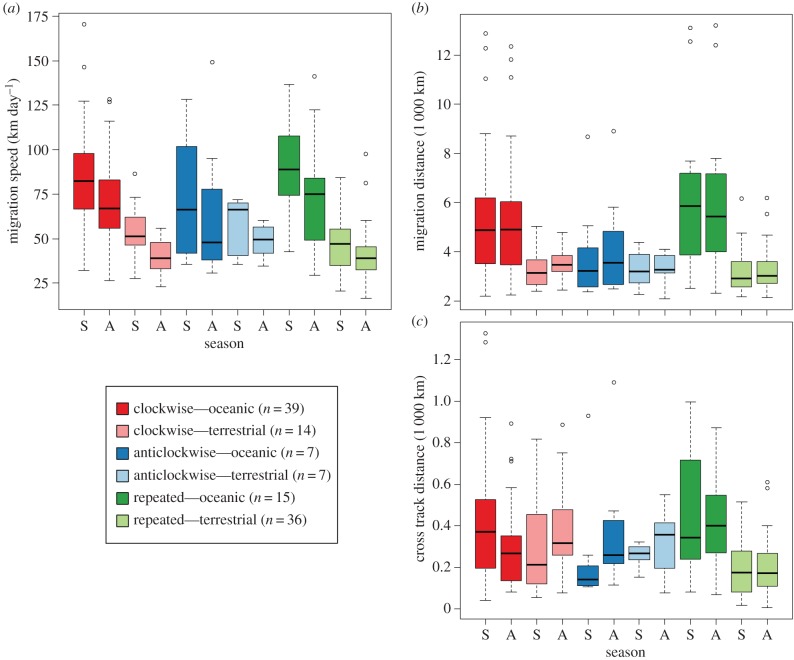
Peak population-level daily migration speeds differed on average among migration categories based on the shape of the trajectory, being greatest for species with clockwise migration trajectories (F2,224 = 14.32, p < 0.001; figure 3a). Peak daily migration speeds were greater on average for species whose migration trajectories included transoceanic crossings (F1,224 = 70.02, p < 0.001), which was most pronounced for species with clockwise and especially repeated migration trajectories (F2,224 = 11.23, p < 0.001; figure 3a). Across the six migration categories, peak daily migration speeds were consistently faster during spring migration (F1,224 = 11.62, p < 0.001; figure 3a).
Figure 3.
The distribution of estimates of (a) peak migration speed, (b) total migration distance, and (c) cross track distance for 118 bird species distributed among six migration categories. The metrics were derived from population-level migration trajectories compiled within the Western Hemisphere at a daily temporal resolution for the combined period 2002–2014.
Migration distances differed on average among migration categories based on the shape of the trajectory, being greatest for species with clockwise and repeated migration trajectories (F2,224 = 12.19, p < 0.001; figure 3b). These distances were greater on average for species whose migration trajectories included transoceanic crossings (F1,224 = 34.71, p < 0.001), which was most pronounced for species with clockwise and especially repeated migration trajectories (F2,224 = 10.91, p < 0.001; figure 3b). There was no evidence for differences on average between spring and autumn migration distances (F1,224 = 0.62, p = 0.423; figure 3b).
The cross track distance differed among migration categories based on the shape of the trajectory, with species exhibiting clockwise and anticlockwise trajectories having the largest cross track distances on average (F2,224 = 4.03, p = 0.041; figure 3c). Thus, looped migration trajectories, both clockwise and anticlockwise, resulted in migrants travelling longer distances than the minimum distances needed to travel between the breeding and non-breeding grounds. Species making transoceanic crossings in particular travelled greater distances east or west from the shortest path, having significantly greater cross track distances on average (F1,224 = 8.76, p = 0.003), especially so for species that followed repeated migration trajectories (F2,224 = 10.17, p = 0.001; figure 3c). There was no evidence for differences in the average cross track distance between spring and autumn migration (F1,224 = 0.077, p = 0.777; figure 3c). However, unlike migration speed (figure 3a) or migration distance (figure 3b), seasonal variation in cross track distance among the six migration categories was greater on average (F2,224 = 3.76, p = 0.048; figure 3c). When each of the six migration categories was examined individually, only clockwise transoceanic migrants presented average seasonal differences in cross track distance, with migrants more closely following the great circle path during autumn migration (t = 2.06, d.f. = 76, p = 0.043; figure 3c; see electronic supplementary material, figure S3).
4. Discussion
Following our expectations, the geographical convergence of migration strategies for Western Hemisphere migrants was evident for species that conducted transoceanic migration. In this case, the majority of migrants that crossed portions of the Gulf of Mexico or Atlantic Ocean shared clockwise looped migration trajectories [23,24]. These findings suggest that, when examined at the population level [23], the more stringent requirements and greater risks arising from transoceanic migration, in combination with the seasonal environmental and atmospheric constraints occurring within the region, resulted in species sharing similar broad-scale migration strategies. We also found evidence of convergence of migration strategies for species migrating within terrestrial regions of Central and South America. Here, migration trajectories that followed the same path in the spring and autumn were in the majority, and the mechanism likely responsible was not greater risk, but the presence of narrow migration corridors defined by the region's unique geomorphology. Specifically, the prevalence of north–south trending mountains and the presence of an extremely narrow isthmus connecting North and South America. The interior of the North American continent, in contrast, was dominated by a mixture of migration strategies, suggesting migrants responded differentially to the unique selection pressures occurring within the region, which are likely not as stringent as those associated with transoceanic crossings or as narrowly defined as those associated with following terrestrial migration corridors.
Our second expectation was that transoceanic migrants should have inherently faster population-level migration speeds owing to the need for non-stop flights over long distances. This outcome was supported when examining daily and peak migration speeds for clockwise, anticlockwise, and repeated migration trajectories. In agreement with previous findings [23], peak migration speed in the spring exceeded autumn peak migration speed for all six categories of migrants, highlighting the importance given to arriving on the breeding grounds in a timely manner in the spring, especially for males [45], and the influence of more variable migratory behaviour in the autumn, especially through the presence of juveniles [46,47].
Transoceanic migrants also tended to have longer overall migration distances, suggesting that the use of transoceanic strategies within the Western Hemisphere is most common for migrants travelling the longest distances. Within the northern portion of our study area, migration speed and migration distance have been shown to be positively correlated when measured at the population level [23]. This same relationship was evident within the Western Hemisphere for the 118 species examined in this study (see electronic supplementary material, figure S4). The presence of this relationship suggests that the faster migration speeds documented in this study for transoceanic migrants is due to both the need for non-stop transoceanic flights and the need to complete longer total migration journeys.
Our findings also indicate that migration trajectories for transoceanic migrants deviate more substantially from the shortest geographical path between the breeding and non-breeding grounds. This was the case for clockwise transoceanic migrants and especially transoceanic migrants that followed the same trajectory in the spring and autumn. There was also evidence, in agreement with our expectations [24], that the autumn migration trajectories for clockwise transoceanic migrants followed a more direct geographical path between the breeding and non-breeding grounds. Thus, clockwise transoceanic migrants had broadly defined looped migration trajectories that deviated from the shortest geographical path, especially in the spring. Unexpectedly, transoceanic migrants that followed repeated trajectories had migration trajectories that also deviated strongly from the shortest geographical path. Unlike the clockwise looped trajectories, this deviation resulted in repeated trajectories where the same path was followed in the spring and autumn. Transoceanic crossing for these species was confined primarily to the Gulf of Mexico, with the Bicknell's thrush (Catharus bicknelli) being an exception whose spring and autumn migration trajectories followed the same path between New England and the Caribbean (figure 1). Further work is needed to determine the factors that have promoted the use of these contrasting strategies by long-distance transoceanic migrants.
There is broad evidence that migratory birds time transoceanic departures to coincide with specific meteorological events that provide favourable atmospheric conditions when crossing the Atlantic Ocean [26–30,48] or Gulf of Mexico [49,50]. Therefore, to minimize the energetic costs and risks associated with transoceanic crossings, migrants must identify and respond to the occurrence of specific meteorological events. For example, if migratory flight is not timed correctly, the prevailing westerlies in the North Atlantic would direct autumn migrants out to sea and away from their winter destinations. The strength of these westerly winds increases at higher altitudes through the influence of the polar front jet stream and the subtropical jet stream [51,52]; thus, their effect cannot be easily avoided by moving to higher migration altitudes. As transatlantic migrants travel out of the temperate zone and into the tropics during autumn migration, the prevailing westerlies transition to the easterly trade winds, which are thought to direct migrants back towards their non-breeding grounds in the Caribbean or South America [27].
Our method for estimating migration trajectories at the population level provides a macro-scale summary of the movements of migratory bird populations. By design, variation in migration timing and routes occurring within species is integrated into this broader perspective [23]. The approach used in this study has the potential to be applied to other regions of the globe as the eBird database grows in geographical breadth and density (see electronic supplementary material, figure S1). Within regions of the globe that are currently well surveyed by eBird, more refined population-level summaries can be generated, resulting in inferences with greater temporal and spatial detail [53].
Our population-level estimates of migration trajectories relied primarily on terrestrial occurrence information. This constraint may have affected the quality of our estimates of the location and uncertainty of migration trajectories, especially for transatlantic migrants (see electronic supplementary material, figures S1 and S2). Improving our understanding on where individual migrants occur during transatlantic crossings would help clarify the quality of these population-level summaries.
For migratory species in general and for small-bodied migrants in particular [54], additional effort is needed to estimate [25] and model [55,56] spatial associations with seasonal atmospheric conditions. Research of this kind contains particular relevance when considering the implications of global climate change [57]. For example, the effect of climate change on seasonal patterns of atmospheric circulation over the Gulf of Mexico [58] or North Atlantic [59] may create additional costs or hazards during transoceanic migration. Climate change may also alter the quality of ecological resources on the breeding grounds through the influence of mid-latitude climate extremes [60] or on the wintering grounds through the influence of global warming [61].
In summary, our findings indicate that the geographical convergence of migration strategies within the Western Hemisphere can occur through two distinct mechanisms. The first is based on the presence of geomorphological features that provide geographically restricted terrestrial migration corridors. The second is based on the presence of transoceanic crossings. Convergence in the former represents the influence of simple geographical constraints where the need for behavioural control to optimize success is likely to be less stringent. Convergence with the latter, in contrast, likely represents the influence of more stringent selection pressures that operate within a well-delineated set of seasonal atmospheric and environmental conditions. Here, behaviour decisions related to where and when to initiate transoceanic crossings and the selection of flight speed, altitude, and bearing during different stages of the crossing are all likely to strongly influence the chances of success. In the end, how the balance between atmospheric constraints and behavioural requirements has promoted the evolution and maintenance of looped migration trajectories for transoceanic migrants remains poorly understood and requires further study. Nevertheless, it is likely this balance can be easily disrupted through climate or land-use change if the specific range of atmospheric and environmental conditions that exist during each stage of the migration journey are altered.
Supplementary Material
Supplementary Material
Acknowledgements
We thank T. Fredericks and K. Webb for assistance with data acquisition and preparation, R. Boyd, N. Bruns, and J. Ward for valuable discussions, the eBird team for their support, the eBird participants for their contributions, and two anonymous reviewers for constructive suggestions.
Authors' contributions
F.A.L. conceived the study; F.A.L. and D.F. designed the analysis; F.A.L. conducted the analysis; F.A.L. wrote the first draft of the manuscript, and all authors contributed suggestions and text to subsequent drafts.
Competing interests
We have no competing interests.
Funding
This work was supported by The Leon Levy Foundation, The Wolf Creek Foundation, and the following National Science Foundation awards: ABI sustaining: DBI-1356308, CDI: IIS-1125098, ITR: EF-0427914, ABI: DBI-0542868, and SEI+II: IIS-0612031 with computing support from CNS-1059284.
References
- 1.Ramenofsky M, Wingfield JC. 2007. Regulation of migration. Bioscience 57, 135–143. ( 10.1641/b570208) [DOI] [Google Scholar]
- 2.Kirby JS, Stattersfield AJ, Butchart SHM, Evans MI, Grimmett RFA, Jones VR, O'Sullivan J, Tucker GM, Newton I. 2008. Key conservation issues for migratory land- and waterbird species on the world's major flyways. Bird Conserv. Int. 18, S49–S73. ( 10.1017/s0959270908000439) [DOI] [Google Scholar]
- 3.Sillett TS, Holmes RT. 2002. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 71, 296–308. ( 10.1046/j.1365-2656.2002.00599.x) [DOI] [Google Scholar]
- 4.Klaassen RHG, Hake M, Strandberg R, Koks BJ, Trierweiler C, Exo K-M, Bairlein F, Alerstam T. 2014. When and where does mortality occur in migratory birds? Direct evidence from long-term satellite tracking of raptors. J. Anim. Ecol. 83, 176–184. ( 10.1111/1365-2656.12135) [DOI] [PubMed] [Google Scholar]
- 5.Lack D. 1968. Bird migration and natural selection. Oikos 19, 1–9. ( 10.2307/3564725) [DOI] [Google Scholar]
- 6.Zink RM. 2011. The evolution of avian migration. Biol. J. Linn. Soc. 104, 237–250. ( 10.1111/j.1095-8312.2011.01752.x) [DOI] [Google Scholar]
- 7.Alerstam T, Hedenström A, Åkesson S. 2003. Long-distance migration: evolution and determinants. Oikos 103, 247–260. ( 10.1034/j.1600-0706.2003.12559.x) [DOI] [Google Scholar]
- 8.Winkler H, Leisler B. 1992. On the ecomorphology of migrants. Ibis 134, 21–28. ( 10.1111/j.1474-919X.1992.tb04747.x) [DOI] [Google Scholar]
- 9.Fiedler W. 2006. Ecomorphology of the external flight apparatus of Blackcaps (Sylvia atricapilla) with different migration behavior. Ann. N.Y. Acad. Sci. 1046, 253–263. ( 10.1196/annals.1343.022) [DOI] [PubMed] [Google Scholar]
- 10.Marchetti K, Price T, Richman A. 1995. Correlates of wing morphology with foraging behaviour and migration distance in the genus Phylloscopus. J. Avian Biol. 26, 177–181. ( 10.2307/3677316) [DOI] [Google Scholar]
- 11.Thorup K, Ortvad T, Holland R, Rabøl J, Kristensen M, Wikelski M. 2012. Orientation of vagrant birds on the Faroe Islands in the Atlantic Ocean. J. Ornithol. 153, 1261–1265. ( 10.1007/s10336-012-0883-6) [DOI] [Google Scholar]
- 12.Butler RW. 2000. Stormy seas for some North American songbirds: are declines related to severe storms during migration? Auk 117, 518–522. ( 10.1642/0004-8038(2000)117%5B0518:ssfsna%5D2.0.co;2) [DOI] [Google Scholar]
- 13.Dierschke V, Delingat J. 2001. Stopover behaviour and departure decision of northern wheatears, Oenanthe oenanthe, facing different onward non-stop flight distances. Behav. Ecol. Sociobiol. 50, 535–545. ( 10.1007/s002650100397) [DOI] [Google Scholar]
- 14.Corman A-M, Bairlein F, Schmaljohann H. 2014. The nature of the migration route shapes physiological traits and aerodynamic properties in a migratory songbird. Behav. Ecol. Sociobiol. 68, 391–402. ( 10.1007/s00265-013-1653-z) [DOI] [Google Scholar]
- 15.Piersma T. 1998. phenotypic flexibility during migration: optimization of organ size contingent on the risks and rewards of fueling and flight? J. Avian Biol. 29, 511–520. ( 10.2307/3677170) [DOI] [Google Scholar]
- 16.Bruderer B, Liechti F. 1998. Flight behaviour of nocturnally migrating birds in coastal areas: crossing or coasting. J. Avian Biol. 29, 499–507. ( 10.2307/3677169) [DOI] [Google Scholar]
- 17.Gill RE, et al. 2009. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc. R. Soc. B 276, 447–457. ( 10.1098/rspb.2008.1142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liechti F. 2006. Birds: blowin’ by the wind? J. Ornithol. 147, 202–211. ( 10.1007/s10336-006-0061-9) [DOI] [Google Scholar]
- 19.Gill RE Jr, Douglas DC, Handel CM, Tibbitts TL, Hufford G, Piersma T. 2014. Hemispheric-scale wind selection facilitates bar-tailed godwit circum-migration of the Pacific. Anim. Behav. 90, 117–130. ( 10.1016/j.anbehav.2014.01.020) [DOI] [Google Scholar]
- 20.Mellone U, López-López P, Limiñana R, Urios V. 2011. Weather conditions promote route flexibility during open ocean crossing in a long-distance migratory raptor. Int. J. Biometeorol. 55, 463–468. ( 10.1007/s00484-010-0368-3) [DOI] [PubMed] [Google Scholar]
- 21.Liechti F, Bruderer B. 1998. The relevance of wind for optimal migration theory. J. Avian Biol. 29, 561–568. ( 10.2307/3677176) [DOI] [Google Scholar]
- 22.Alerstam T. 2011. Optimal bird migration revisited. J. Ornithol. 152, S5–S23. ( 10.1007/s10336-011-0694-1) [DOI] [Google Scholar]
- 23.La Sorte FA, Fink D, Hochachka WM, DeLong JP, Kelling S. 2013. Population-level scaling of avian migration speed with body size and migration distance for powered fliers. Ecology 94, 1839–1847. ( 10.1890/12-1768.1) [DOI] [PubMed] [Google Scholar]
- 24.La Sorte FA, et al. 2014. The role of atmospheric conditions in the seasonal dynamics of North American migration flyways. J. Biogeogr. 41, 1685–1696. ( 10.1111/jbi.12328) [DOI] [Google Scholar]
- 25.La Sorte FA, Fink D, Hochachka WM, DeLong JP, Kelling S. 2014. Spring phenology of ecological productivity contributes to the use of looped migration strategies by birds. Proc. R. Soc. B 281, 20140984 ( 10.1098/rspb.2014.0984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Able KP. 1973. The role of weather variables and flight direction in determining the magnitude of nocturnal bird migration. Ecology 54, 1031–1041. ( 10.2307/1935569) [DOI] [Google Scholar]
- 27.Williams TC, Williams JM. 1978. An oceanic mass migration of land birds. Sci. Am. 239, 166–176. ( 10.1038/scientificamerican1078-166) [DOI] [Google Scholar]
- 28.Larkin R, Griffin D, Torre-Bueno J, Teal J. 1979. Radar observations of bird migration over the Western North Atlantic Ocean. Behav. Ecol. Sociobiol. 4, 225–264. ( 10.1007/bf00297646) [DOI] [Google Scholar]
- 29.Richardson WJ. 1990. Timing of bird migration in relation to weather: updated review. In Bird migration: physiology and ecophysiology (ed. Gwinner E.), pp. 78–101. Berlin, Germany: Springer. [Google Scholar]
- 30.Alerstam T. 1990. Bird migration, 420 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 31.Sullivan BL, et al. 2014. The eBird enterprise: an integrated approach to development and application of citizen science. Biol. Conserv. 169, 31–40. ( 10.1016/j.biocon.2013.11.003) [DOI] [Google Scholar]
- 32.Faaborg J, et al. 2010. Recent advances in understanding migration systems of New World land birds. Ecol. Monogr. 80, 3–48. ( 10.1890/09-0395.1) [DOI] [Google Scholar]
- 33.La Sorte FA, et al. 2015. Documenting stewardship responsibilities across the annual cycle for birds on U.S. public lands. Ecol. Appl. 25, 39–51. ( 10.1890/14-0702.1) [DOI] [PubMed] [Google Scholar]
- 34.Faaborg J, et al. 2010. Conserving migratory land birds in the New World: do we know enough? Ecol. Appl. 20, 398–418. ( 10.1890/09-0397.1) [DOI] [PubMed] [Google Scholar]
- 35.Sahr K. 2011. Hexagonal discrete global grid systems for geospatial computing. Arch. Photogr. Cartogr. Remote Sensing 22, 363–376. [Google Scholar]
- 36.Sahr K, White D, Kimerling AJ. 2003. Geodesic discrete global grid systems. Cartogr. Geogr. Inf. Sci. 30, 121–134. ( 10.1559/152304003100011090) [DOI] [Google Scholar]
- 37.Rigby RA, Stasinopoulos DM. 2005. Generalized additive models for location, scale and shape. J. R. Stat. Soc. C Appl. Stat. 54, 507–554. ( 10.1111/j.1467-9876.2005.00510.x) [DOI] [Google Scholar]
- 38.Bowring BR. 1985. The accuracy of geodetic latitude and height equations. Surv. Rev. 28, 202–206. ( 10.1179/sre.1985.28.218.202) [DOI] [Google Scholar]
- 39.Vincenty T. 1975. Direct and inverse solutions of geodesics on the ellipsoid with application of nested equations. Surv. Rev. 23, 88–93. ( 10.1179/sre.1975.23.176.88) [DOI] [Google Scholar]
- 40.R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 41.Stasinopoulos DM, Rigby RA. 2007. Generalized additive models for location scale and shape (GAMLSS) in R. J. Stat. Softw. 23, 1–46. ( 10.18637/jss.v023.i07) [DOI] [Google Scholar]
- 42.Wood SN, Scheipl F. 2014. gamm4: generalized additive mixed models using mgcv and lme4. R package version 0.2-3.
- 43.Hijmans RJ. 2015. geosphere: spherical trigonometry. R package version 1.3-13. See http://CRAN.R-project.org/package=geosphere.
- 44.Wang J, et al. 2014. robust: robust library. R package version 0.4-16. See http://CRAN.R-project.org/package=robust.
- 45.Kokko H. 1999. Competition for early arrival in migratory birds. J. Anim. Ecol. 68, 940–950. ( 10.1046/j.1365-2656.1999.00343.x) [DOI] [Google Scholar]
- 46.Sergio F, Tanferna A, De Stephanis R, Jimenez LL, Blas J, Tavecchia G, Preatoni D, Hiraldo F. 2014. Individual improvements and selective mortality shape lifelong migratory performance. Nature 515, 410–413. ( 10.1038/nature13696) [DOI] [PubMed] [Google Scholar]
- 47.Mitchell GW, Woodworth BK, Taylor PD, Norris DR. 2015. Automated telemetry reveals age specific differences in flight duration and speed are driven by wind conditions in a migratory songbird. Mov. Ecol. 3, 19 ( 10.1186/s40462-015-0046-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.La Sorte FA, Hochachka WM, Farnsworth A, Sheldon D, Fink D, Geevarghese J, Winner K, Van Doren BM, Kelling S. 2015. Migration timing and its determinants for nocturnal migratory birds during autumn migration. J. Anim. Ecol. 84, 1202–1212. ( 10.1111/1365-2656.12376) [DOI] [PubMed] [Google Scholar]
- 49.Able KP. 1972. Fall migration in coastal Louisiana and evolution of migration patterns in Gulf region. Wilson Bull. 84, 231–242. [Google Scholar]
- 50.Rappole JH, Ramos MA. 1994. Factors affecting migratory bird routes over the Gulf of Mexico. Bird Conserv. Int. 4, 251–262. ( 10.1017/S095927090000280X) [DOI] [Google Scholar]
- 51.Archer CL, Caldeira K. 2008. Historical trends in the jet streams. Geophys. Res. Lett. 35, L08803 ( 10.1029/2008gl033614) [DOI] [Google Scholar]
- 52.Pena-Ortiz C, Gallego D, Ribera P, Ordonez P, Alvarez-Castro MDC. 2013. Observed trends in the global jet stream characteristics during the second half of the 20th century. J. Geophys. Res. Atmos. 118, 2702–2713. ( 10.1002/jgrd.50305) [DOI] [Google Scholar]
- 53.Supp SR, Sorte FAL, Cormier TA, Lim MCW, Powers DR, Wethington SM, Goetz S, Graham CH. 2015. Citizen-science data provides new insight into annual and seasonal variation in migration patterns. Ecosphere 6, part15. ( 10.1890/es14-00290.1) [DOI] [Google Scholar]
- 54.DeLuca WV, Woodworth BK, Rimmer CC, Marra PP, Taylor PD, McFarland KP, Mackenzie SA, Norris DR. 2015. Transoceanic migration by a 12 g songbird. Biol. Lett. 11, 20141045 ( 10.1098/rsbl.2014.1045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoddard PK, Marsden JE, Williams TC. 1983. Computer-simulation of autumnal bird migration over the western north Atlantic. Anim. Behav. 31, 173–180. ( 10.1016/s0003-3472(83)80186-9n) [DOI] [Google Scholar]
- 56.Kranstauber B, Weinzierl R, Wikelski M, Safi K. 2015. Global aerial flyways allow efficient travelling. Ecol. Lett. 18, 1338–1345. ( 10.1111/ele.12528) [DOI] [PubMed] [Google Scholar]
- 57.IPCC. 2013. Climate change 2013: the physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker TF et al.), 1535 Cambridge, UK. [Google Scholar]
- 58.Cook KH, Vizy EK, Launer ZS, Patricola CM. 2008. Springtime intensification of the great plains low-level jet and Midwest precipitation in GCM simulations of the twenty-first century. J. Clim. 21, 6321–6340. ( 10.1175/2008jcli2355.1) [DOI] [Google Scholar]
- 59.Vallis GK, Zurita-Gotor P, Cairns C, Kidston J. 2015. Response of the large-scale structure of the atmosphere to global warming. Q. J. R. Meteorol. Soc. 141, 1479–1501. ( 10.1002/qj.2456) [DOI] [Google Scholar]
- 60.La Sorte FA, Hochachka WM, Farnsworth A, Dhondt AA, Sheldon D. In press The implications of mid-latitude climate extremes for North American migratory bird populations. Ecosphere. [Google Scholar]
- 61.Mora C, Caldwell IR, Caldwell JM, Fisher MR, Genco BM, Running SW. 2015. Suitable days for plant growth disappear under projected climate change: potential human and biotic vulnerability. PLoS Biol. 13, e1002167 ( 10.1371/journal.pbio.1002167) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.