Abstract
Reproduction requires resources that cannot be allocated to other functions resulting in direct reproductive costs (i.e. trade-offs between current reproduction and subsequent survival/reproduction). In wild vertebrates, direct reproductive costs have been widely described in females, but their occurrence in males remains to be explored. To fill this gap, we gathered 53 studies on 48 species testing direct reproductive costs in male vertebrates. We found a trade-off between current reproduction and subsequent performances in 29% of the species and in every clade. As 73% of the studied species are birds, we focused on that clade to investigate whether such trade-offs are associated with (i) levels of paternal care, (ii) polygyny or (iii) pace of life. More precisely for this third question, it is expected that fast species (i.e. short lifespan, early maturity, high fecundity) pay a cost in terms of survival, whereas slow species (with opposite characteristics) do so in terms of fecundity. Our findings tend to support this hypothesis. Finally, we pointed out the potential confounding effects that should be accounted for when investigating reproductive costs in males and strongly encourage the investigation of such costs in more clades to understand to what extent our results are relevant for other vertebrates.
Keywords: costs of reproduction, generation time, life history, paternal care, polygyny, trade-off
1. Introduction
Trade-offs, resulting from constraints on the evolution of linked traits, play a central role in life-history theory [1]. According to the principle of allocation [2], individuals allocate their limited amount of resources to a function at the cost of other ones. Therefore, the maximum fitness an individual can reach is limited by trade-offs among fitness components. The theory about reproductive trade-offs, first formalized by Williams [3], suggested that, as reproduction is energy-demanding, individuals should trade current reproduction versus future reproduction via reduced future fecundity (i.e. fecundity costs of reproduction) and/or reduced future survival (i.e. survival costs of reproduction) [1,4]. However, as formulated by van Noordwijk & de Jong's model [5], even if two traits compete for the same resource at the individual level, such a negative trade-off can remain undetected at the population level, because individuals can differ in both resource acquisition and resource allocation to each trait depending on the quality of their habitat or on their own quality.
Nearly 50 years after Williams's publication [3], studies investigating costs of reproduction have flourished. Stearns reviewed studies investigating the effects of reproduction on growth, parental survival, late fecundity and longevity in a wide range of taxa and with different methods, including laboratory experiments, unmanipulated and manipulated field populations [1]. More recently, studies investigating specifically direct reproductive costs in wild mammals—the covariations between current reproduction (at time t) and subsequent reproduction and/or survival (at time t + 1)—have been reviewed [6]. Interestingly, most of the studies gathered in this review focused on females [6]. There have in fact been considerably fewer attempts to assess trade-offs among fitness components in wild males. This bias may be in part explained by methodological problems, as it is often more difficult to assess reproductive effort in wild males than females [7]. Also, while it has become an accepted notion that reproduction is costly for males as well [8–10], studies dealing with costs of reproduction in males are often rooted in the theory of sexual selection and therefore refer to the cost of producing or maintaining sexual traits on future survival [8] (see [11] for a recent review on the relationship between strength of sexual selection and age-specific survival patterns across vertebrates). Investigating reproductive costs in males specifically through the covariations between life-history traits (also called direct fitness traits sensu Roff [4], i.e. fecundity and survival) can be important because such costs that are expressed at the individual level may also have implications at the population level [12]. Thus, in the perspective of the development of more realistic population models that include both females and males, a better understanding of constraints shaping fitness traits in males appears important.
In a broad evolutionary context, theory predicts that (i) the intensity of mating competition in polygynous species should translate into higher costs of reproduction for males compared to socially monogamous species [13]. At the same time, (ii) species characterized with high level of paternal care are expected to be the ones with the highest reproductive costs, as parental care is energy-demanding [3,14,15]. While a high level of polygyny or paternal care is expected to be costly for males, such costs of reproduction can also strongly depend upon the position of the species on the fast–slow continuum of life-history variation [16,17]. Indeed, the recent extension of van Noordwijk & de Jong's model [5] predicts that reproductive costs are closely linked to the position of a given species on the fast–slow continuum [6], which contrasts fast species with early maturity, high fecundity and short lifespan to slow species with opposite characteristics (see [18] for a recent review). Briefly, (iii) fast species characterized with high variance in survival and low variance in reproduction should exhibit survival costs of reproduction, whereas slow species should rather suffer from fecundity costs. While this evolutionary model has been first supported in female mammals [6], its validity for male vertebrates remains to be investigated.
In this review, we summarize the empirical tests of reproductive trade-off between two breeding seasons in males in unmanipulated wild terrestrial and seasonally reproducing birds, mammals, squamates and amphibians (electronic supplementary material, section S1 and figure S1). We choose to focus on these clades because most of the long-term individual-based field studies, essential in this context, are done on these species [19]. Experimental approaches have been a useful tool to study trade-offs and to show relationship of causality [20]. Thus, numerous studies have investigated costs of reproduction in males from experimental field populations, by assessing for instance the effect of brood size manipulation in birds (i.e. reduced or enlarged) on subsequent survival and/or reproduction (e.g. [21,22] for reviews of experimental studies). However, as we aim here to report tests of direct reproductive costs with potential demographic consequences, we focus our literature survey on males of unmanipulated wild populations. Then, we compare the occurrence of direct costs of reproduction between clades and species. As most of the gathered studies in our review use birds as case studies, we focus on that clade to question the link between direct costs of reproduction and (i) level of polygyny, (ii) level of paternal care and (iii) pace of life (i.e. the position on the fast–slow continuum). Moreover, we discuss possible biases of correlative studies in the wild with regard to identifying reproductive costs in males. Finally, we discuss the implication of our results for the other clades of terrestrial vertebrates and suggest some future lines of research. In particular, we encourage the inclusion of trade-offs between fitness-related traits in males into demographic models of population growth to better predict the fate of wild vertebrate populations.
2. Tests of direct costs of reproduction in males
We gathered 53 studies on 48 vertebrate species investigating the covariations between current (t) reproduction and subsequent (t + 1) reproduction/survival (search protocol in electronic supplementary material, section S2; results of the literature search in electronic supplementary material, table S1). It is noteworthy that the most represented clade is that of birds with 35 species for which such covariations have been investigated. We found only seven mammalian species, four squamate species and two amphibian species. In total, we reported 116 covariations (including one non-informative covariation) between reproductive trait at t and reproduction/survival at t + 1 (electronic supplementary material, tables S1 and S2; figure 1).
Figure 1.
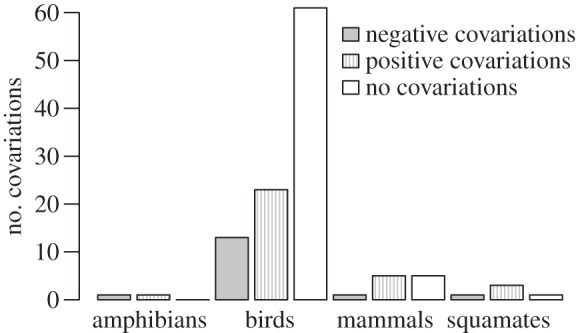
Distribution of the number of tested covariations between current reproduction and subsequent performances in male terrestrial vertebrates. Number of covariations between reproduction at time t and survival/reproduction at time t + 1 collected in the literature in amphibians, birds, mammals and squamates.
Because of high variability of traits considered at t in the reviewed studies (electronic supplementary material, table S1), we summarized them into six types of traits; namely, ‘number of young’ (e.g. when clutch size has been considered as a reproductive trait at t), ‘breeding status’ (e.g. comparison of subsequent performances of breeders versus non-breeders), ‘mating’ (e.g. number of matings), ‘paternal care’ (e.g. a measure of feeding rate), ‘timing’ (e.g. comparison of early versus late breeders) and ‘number of breeding’ (e.g. number of broods produced). In 74% of the covariations (i.e. 85/115), the ‘number of young’ produced or ‘breeding status’ was used to assess reproductive effort at time t (electronic supplementary material, table S2). The use of breeding status as a trait at t means that direct costs of reproduction are investigated through the comparison of subsequent performances of breeders (or successful breeders) with subsequent performances of non-breeders (or failed breeders) (electronic supplementary material, table S1). Reproductive traits linked to the level of paternal care, the timing of breeding, the number of matings or the number of breeding may be difficult to measure in the field and are specific to the ecology/reproductive cycle of each species. That can explain why these traits are less often used in the reviewed studies (electronic supplementary material, table S2). For example, in the black grouse (Tetrao tetrix), a lekking species for which there is no paternal care, the authors used the number of matings as a reproductive trait at time t [23].
Negative covariations between reproduction at t and survival or reproduction at time t + 1 were detected in 29% of the reported species (electronic supplementary material, table S1). Despite the low number of studies on some clades, it seems that males of all the clades have equal probability to exhibit direct costs of reproduction (figure 1; electronic supplementary material, table S3). More precisely, these costs correspond to 16 negative covariations between life-history traits among the 115 covariations reported (electronic supplementary material, tables S1 and S2). Interestingly, even if the traits ‘breeding status’ and ‘mating’ have a tendency to be more frequently involved in such negative covariations, there is no significant effect of the types of trait considered at t on the probability to detect reproductive costs (electronic supplementary material, tables S2 and S4). Therefore, breeding (or successfully breeding) in a given breeding season may negatively affect male survival to the next breeding season (this is the case for three species out of 21) and may also affect reproduction (for four out of 15 species) (electronic supplementary material, table S2). Similarly, for some species, a high number of young, of paternal care, of matings or of breeding reduces male survival or reproduction the next breeding season (electronic supplementary material, tables S1 and S2). Moreover, the probability to find a negative covariation in a study does not depend on the number of covariations tested (electronic supplementary material, table S5) or the number of types of traits tested neither (electronic supplementary material, table S6), suggesting that the differences in sample sizes between studies do not bias our results.
Regarding traits considered at t + 1, 57% of the studies investigated only survival costs, 34% both survival and fecundity costs, and only 9% investigated fecundity costs alone (electronic supplementary material, table S1). Interestingly, no study investigating both types of costs provided support for both. For instance, in the Laysan albatross (Phoebastria immutabilis) or Nazca booby (Sula granti), male breeders (or successful breeders) can pay a cost in terms of survival to the next breeding season but not in terms of fecundity (i.e. probability to reproduce given survival) [44,51]. In the king penguin (Aptenodytes patagonicus), southern giant petrel (Macronectes giganteus) and spotted owl (Strix occidentalis), the effect was opposite: breeding affected future breeding probability but not survival [41,52,57]. Similarly, in the great reed warbler (Acrocephalus arundinaceus), there is a cost of paternal care on future reproduction (timing of settlement) but no detectable cost on survival [32]. This stresses the importance of studing both fecundity costs of reproduction and survival costs of reproduction.
3. Reproductive costs in male birds and their link with paternal care, polygyny and pace of life
For some species, negative covariation between reproduction at time t and subsequent reproductive performance occurred, whereas for others species, the covariation was null or positive (figure 1). These differences among species may be explained by differences in mating systems, levels of paternal care or pace of life resulting in different life-history strategies. Indeed, we expect higher costs for males in polygynous mating systems, or when males invest more in paternal care. Moreover, as highlighted for female mammals, a close relationship between pace of life and the type of direct reproductive costs detected may be expected (i.e. fast species should be affected by survival costs of reproduction, whereas slow species by fecundity costs of reproduction [6]). Generation time provides a relevant measure to rank the species on the fast–slow continuum [17]. Therefore, we expect an increase of the probability to find a fecundity cost of reproduction compared with the probability to find a survival cost of reproduction with longer generation times.
We chose to test these predictions only for male birds because 73% of the species in the reviewed studies are birds. Moreover, the different bird species gathered in this review exhibit a wide range of avian life histories along the fast–slow continuum, and diverse mating systems and levels of paternal care, which are essential to test these three hypotheses. More precisely, 11 studies revealed direct costs of reproduction in males (seven found survival costs and four fecundity costs of reproduction) among the 35 different bird species we gathered (electronic supplementary material, table S1). For most species, a score of paternal care and polygyny was available from the literature [24] (see table 1 for details on score calculation) and, for each species, the generation time (in years) was retrieved from species-specific demographic studies (table 1). We found that the probability to observe a cost of reproduction in one species did not depend on the level of polygyny (electronic supplementary material, table S7.A) or the level of paternal care (electronic supplementary material, table S7.B). Thus, with the current dataset, there is no evidence that species suffering from direct reproductive costs are the ones with the highest levels of paternal care or polygyny. However, as expected, species suffering from fecundity costs of reproduction have longer generation times than species suffering from survival costs of reproduction, except for one outlier, the Laysan albatross [44] (figure 2). This result tends to support a relationship between the pace of life and reproductive costs [6]. It remains to see from studies in other clades whether this is a general pattern in vertebrates.
Table 1.
Details on the bird species for which direct reproductive costs have been tested in males. We present their order and family, and their associated scores of paternal care and polygyny. These scores were calculated by Olson et al. [24]. Briefly, the method to calculate the score of paternal care consists in scoring paternal investment in five different activities: nest building, incubation, brooding, chick feeding and chick defence. For each activity, the participation of males was scored on a 5-point scale: 0 (no male care), 1 (1–33% male care), 2 (34–66% male care), 3 (67–99% male care) or 4 (100% male care). Thus the maximum score for each species is 20. The score of polygyny represents the percentage of males exhibiting polygyny, as follows: 0 (no polygyny or less than 0.1% of individuals), 1 (rare polygyny: 0.1–1%), 2 (uncommon polygyny: 1–5%), 3 (moderate polygyny: 5–20%) and 4 (common polygyny, greater than 20%). Generation time (in years), a measure ranking the species on the fast–slow continuum, was extracted from Birdlife International database (http://www.birdlife.org/datazone/home). The column ‘negative covariation?’ indicates whether at least one negative covariation between reproduction at t and fecundity (‘F’) and/or survival (‘S’) at t + 1 was found for the considered species or not (‘N’).
| species | order | family | paternal care | polygyny | generation time | negative covariation? | references |
|---|---|---|---|---|---|---|---|
| barn swallow (Hirundo rustica) | Passeriformes | Hirundinidae | 7 | 1 | 3.9 | N | [25] |
| barnacle goose (Branta leucopsis) | Anseriformes | Anatidae | 6 | 0 | 10.5 | N | [26] |
| black grouse (Tetrao tetrix) | Galliformes | Phasianidae | 0 | 4 | 6.4 | N | [23] |
| blue tit (Cyanistes caeruleus) | Passeriformes | Paridae | 4 | 3 | 4.4 | N | [27] |
| brown thornbill (Acanthiza pusilla) | Passeriformes | Acanthizidae | 4a | n.a. | 5.7 | N | [28] |
| cliff swallow (Petrochelidon pyrrhonota) | Passeriformes | Hirundinidae | 10 | 0 | 4.3 | S | [29] |
| collared flycatcher (Ficedula albicollis) | Passeriformes | Muscicapidae | 4 | 3 | 3.9 | N | [30] |
| crested tit (Parus cristatus) | Passeriformes | Paridae | 2 | 0 | 4 | N | [31] |
| great reed warbler (Acrocephalus arundinaceus) | Passeriformes | Sylviidae | 6 | 2 | 4.2 | F | [32] |
| great tit (Parus major) | Passeriformes | Paridae | 4 | 1 | 4.3 | N | [33,34] |
| greater prairie-chicken (Tympanuchus cupido) | Galliformes | Phasianidae | 0 | 4 | 5.5 | N | [35] |
| green-rumped parrotlet (Forpus passerinus) | Psittaciformes | Psittacidae | 2 | 0 | 4.1 | N | [36] |
| Hawai'i ‘elepaio (Chasiempis sandwichensis sandwichensis) | Passeriformes | Monarchidae | n.a. | n.a. | 5.9 | N | [37] |
| house martin (Delichon urbica) | Passeriformes | Hirundinidae | 10 | 0 | 4.3 | N | [38] |
| indigo bunting (Passerina cyanea) | Passeriformes | Cardinalidae | 3 | 3 | 4.1 | N | [39] |
| jackdaw (Corvus monedula) | Passeriformes | Corvidae | n.a. | n.a. | 7.4 | N | [40] |
| king penguin (Aptenodytes patagonicus) | Ciconiiformes | Spheniscidae | 6 | 0 | 12.7 | F | [41] |
| kittiwake gull (Rissa tridactyla) | Ciconiiformes | Laridae | 10 | 0 | 12.9 | N | [42,43] |
| Laysan albatross (Phoebastria immutabilis) | Procellariiformes | Diomedeidae | n.a. | n.a. | 28.5 | S | [44] |
| long-tailed tit (Aegithalos caudatus) | Passeriformes | Aegithalidae | n.a. | n.a. | 4.2 | N | [45,46] |
| marsh tit (Parus palustris) | Passeriformes | Paridae | n.a. | n.a. | 4.2 | N | [47,48] |
| Monteiro's storm-petrel (Oceanodroma monteiroi) | Procellariiformes | Hydrobatidae | n.a. | n.a. | 16.5 | N | [49] |
| mountain white-crowned sparrow (Zonotrichia leucophrys oriantha) | Passeriformes | Fringillidae | 2 | 2 | 4.3 | S | [50] |
| nazca booby (Sula granti) | Suliformes | Sulidae | n.a. | n.a. | 10 | S | [51] |
| northern giant petrel (Macronectes halli) | Procellariiformes | Procellariidae | n.a. | n.a. | 17 | N | [52] |
| oystercatcher (Haematopus ostralegus) | Charadriiformes | Haematopodidae | 9 | 1 | 13.7 | N | [53] |
| savannah sparrow (Passerculus sandwichensis) | Passeriformes | Fringillidae | 4 | 4 | 3.4 | S N | [54] [55] |
| snowy plover (Charadrius nivosus) | Ciconiiformes | Charadriidae | n.a. | n.a. | 5 | N | [56] |
| southern giant petrel (Macronectes giganteus) | Procellariiformes | Procellariidae | n.a. | n.a. | 21.3 | F | [52] |
| spotted owl (Strix occidentalis) | Strigiformes | Strigidae | 4 | 0 | 10.1 | F | [57] |
| Tengmalm's owl (Aegolius funereus) | Strigiformes | Strigidae | 3 | 4 | 5.8 | N | [58] |
| tree swallow (Tachycineta bicolor) | Passeriformes | Hirundinidae | 3 | 2 | 4 | S | [59] |
| wheatear (Oenanthe oenanthe) | Passeriformes | Muscicapidae | 4a | n.a. | 4.1 | N | [60,61] |
| willow ptarmigan (Lagopus lagopus) | Galliformes | Phasianidae | 3 | 3 | 4.2 | N | [62] |
| willow tit (Parus montanus) | Passeriformes | Paridae | 3 | 0 | 4.6 | S N | [63] [31] |
aAndras Liker and Tamas Szekely 2015, personal communication.
Figure 2.
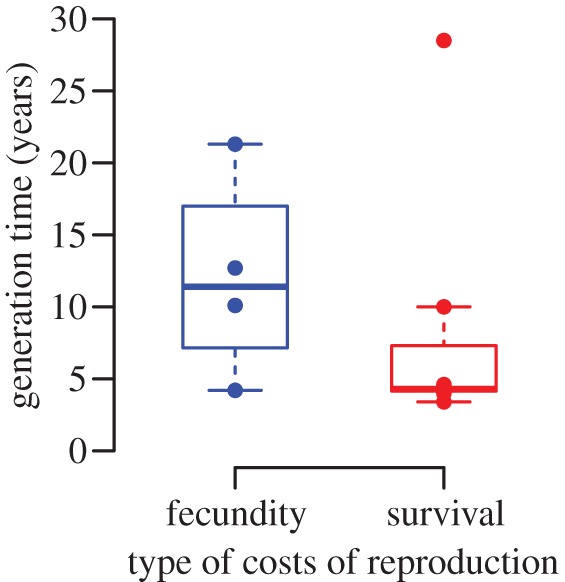
Types of direct costs of reproduction in male birds and pace of life. Differences in generation times (in years) between the bird species for which fecundity costs of reproduction (left) and survival costs of reproduction (right) have been reported for at least one reproductive trait. Dots represent the data points (table 1). (Online version in colour.)
The results of these statistical comparisons should be considered with some caution. First, the power of our tests may be low due to the number of studies that include data on costs of reproduction. Second, the levels of polygyny and paternal care and the generation times were calculated at the species level. However, it is true that some variation in these variables can exist among populations of the same species [64–66] and even among individuals within a population (e.g. for paternal care [67]). As a consequence, investigating the relationship between reproductive trade-offs and levels of paternal care, polygyny or pace of life measured at different levels of biological organization (such as at the population level) may be really interesting. It is noteworthy that in this review, we reported opposite results in terms of reproductive costs in two populations of the same species, the willow tit (Parus montanus). In one of them, survival costs of reproduction have been detected [31], but not in the other one [63]. Whether these two populations differ in their level of paternal care, polygyny or pace of life, and whether these differences are translated into different costs of reproduction, remain to be explored.
4. Negative, positive or null covariations between current reproduction and subsequent performances and the detection of reproductive costs
We reported 14% of negative covariations between current reproduction and subsequent performances, and also 58% of null and 28% of positive covariations (figure 1). The high proportions of null and positive covariations may partly explain why studies dealing with reproductive costs are often oriented towards females in the literature.
A negative covariation between current reproduction and subsequent performances may indicate that males trade their current reproduction versus their subsequent performances, and thus that males suffer from direct reproductive costs [3]. However, this pattern may be more complex. In mating systems where males and females interact, the investment of one sex in a current reproductive event may depend on the characteristics of the other sex. For instance, the theory of differential allocation predicts that a mate may invest more or less in reproduction when paired with their preferred mate (or with a high-quality mate; positive or negative differential allocation [68]). This pattern can create positive or negative correlations between life-history traits for males that are driven by females’ investment in reproduction and that are not linked to male reproductive costs. However, because positive differential allocation seems more frequent than negative differential allocation (at least in birds [69]), it should be rare to find a negative covariation between male life-history traits in absence of real reproductive costs for males.
A null or a positive covariation between current reproduction and subsequent performances may indicate the absence of direct reproductive costs in males. Again, interaction between the sexes may drive this pattern, for example if the absence of reproductive costs in males is correlated to the presence of such costs in females. Such correlations may depend on how strong the sexual conflict over parental investment is and how it is resolved in each species [24,70]. Therefore, it would be particularly interesting to compare both males and females in the same study. Moreover, it is noteworthy that males that do not exhibit direct reproductive costs may pay a cost later in life [71], or may suffer from other types of costs, such as inter-generational or cumulative costs of reproduction (see electronic supplementary material, section S1).
Remarkably, null or positive covariations between current reproduction and subsequent performances may be found even if direct reproductive costs are present. First, in certain cases, individuals that try to reproduce but do not succeed in siring offspring may pay quite similar fitness costs to successful breeders. This could be more important in males than females because typically males can invest large amount of energy prior to mating (e.g. actively searching for mates, trying to defend a territory, injuries during combat) without managing to successfully sire offspring [7]. Also, brood loss may happen late in the season or, at least, after most of the energy allocated for reproduction has already been invested in the current reproductive event. Therefore, in these cases, one will fail to detect any difference in terms of future survival and/or reproduction between breeders (or successful breeders) and non-breeders (or failed breeders) even if direct costs of reproduction occur. Second, the detection of reproductive costs may be masked by phenotypic differences in individual quality. Indeed, in case of high variance in resources acquisition, individuals able to acquire more resources (i.e. high-quality individuals) are also able to allocate more resources than other individuals to both current reproduction and future survival and/or reproduction, which can prevent the detection of costs of reproduction at the population level [5]. These limitations due to individual heterogeneity explain in part why experimental studies have been widely used to study trade-offs (e.g. [22]). However, studies of unmanipulated wild populations are still essential to understand the ecological consequences of life-history variations and to obtain realistic estimates of demographic parameters [19]. Thanks to the development of appropriate statistical tools, accounting for individual heterogeneity is possible, making the correlative studies more powerful [6,72]. Yet it is likely that among the high proportion of studies that reported no negative covariation between current reproduction and subsequent performances, some of them actually concluded an absence of direct costs of reproduction even if they occurred in the considered species.
More generally, it is also possible that some studies may fail to detect negative covariation between reproduction at time t and survival/fecundity at time t + 1, even if there are some real costs of reproduction, because appropriate cofactors are not taken into account. For example, differences in individual quality may be more pronounced in years with harsher environmental conditions when resources are more limited, resulting in annual variations in trade-off detection (e.g. [51,59]). Accounting for age effects may also be recommended while investigating direct reproductive costs. Indeed, reproductive performances may be age-specific, with for example lower reproductive output at old ages compared to younger ages due to senescence [73,74], or at the opposite lower reproductive output at young ages due to inexperience [75], possibly preventing the detection of reproductive costs. Carefully disentangling the age effects from the costs of previous reproduction appears crucial. Moreover, it is also important to keep in mind that the age of the individuals can mediate the trade-offs between current reproduction and subsequent reproduction and/or survival. In other words, reproductive costs themselves can be dependent on the age. For example, reproduction can be more costly in young individuals [76] or on the contrary more costly in old individuals [77], or appear more costly due to terminal investment [78]. Therefore, studies combining the information of age effects and life-history trade-offs should be developed to strengthen comparative studies and to improve our general understanding of such patterns.
Finally, in many of the studies included in the present review, it is assumed that the social and genetic father is the same. In birds for instance, the number of eggs/chicks present in the nests is used as an estimator of male reproductive success. However, thanks to molecular genetic tools, it is now accepted that, even in socially monogamous species, individuals can engage in extra-pair copulations [14,79]. In particular, males involved in such extra-pair copulations can increase their reproductive success without increasing their amount of paternal care. This means that some traits measured at time t may be more or less correlated to the reproductive success and the paternal investment of the males. For example, certainty of paternity has been shown to covary with paternal care in birds [80]. Thus, even if a male has a large clutch, its investment may be low if some chicks are sired by a different male. This is why the quantification of extra-pair parternity can allow more precise measurement of reproductive effort, which may allow highlighting different relationships between reproduction at time t and fitness-related trait at t + 1.
5. Conclusion and perspectives
In this review, we gathered studies exploring the covariations between current reproduction and subsequent reproduction and/or survival of wild unmanipulated terrestrial male birds, mammals, squamates and amphibians. It is noteworthy that our review reports some studies highlighting positive covariations between life-history traits, suggesting that the individual quality hypothesis is often supported in male vertebrates. But we also found empirical evidence of direct reproductive costs in several species belonging to all clades, even with the inherent difficulties of correlative studies. It is thus obvious that direct reproductive costs concern both males and females in wild populations.
We showed that the occurrence of reproductive costs in males is not correlated to polygyny and levels of paternal care but is associated with pace of life, in birds at least. Unfortunately, the small number of studies in the other clades did not allow us testing our evolutionary hypotheses in other terrestrial vertebrates. However, we are confident that our results, drawn from birds, may also be relevant to other clades. Indeed, after some evidence of a link between pace of life and costs of reproduction in female mammals [6], our review provides support for that life-history model in male birds. Yet exploring to what extent such a model can be generalized to all terrestrial vertebrates, and, in particular, unravelling the factors that may explain variations among clades, remain an exciting challenge. For example, in line with a comparative study that has shown that birds have a slower life history than mammals for the same body mass [81], one could expect different relationships between pace of life and costs of reproduction in males in these two clades. Another important difference within vertebrates is the mode of temperature regulation. Indeed, ectotherms can store energy more efficiently than endotherms like birds, and thus rely more often on stored resources to fuel reproduction (capital versus income breeders) [82]. Such different reproductive strategies may induce different reproductive costs. Thus, we strongly encourage further studies in more taxa with diverse mating systems and life-history strategies to be able to broaden these results.
Correlations between life-history traits and, in particular, reproductive trade-offs can have demographic consequences and can influence population dynamics [12]. It is true that most models in population dynamics are female-based and neglect males [83], but there is now growing evidence that males may markedly influence population dynamics as well (e.g. [84,85]). While methodological developments now provide the tools to integrate costs of reproduction into population models [12,86], a promising avenue of research could be to take into account reproductive costs in males as well as females into population dynamic models. Even if all models are approximations, capturing the fluctuation of demographic parameters and accounting for it into population models is the best way to provide sustainable and relevant management and conservation scenarios.
Supplementary Material
Acknowledgements
We thank Andras Liker and Tamas Szekely for sharing data on paternal care in birds. We warmly thank Jean-François Lemaître, Per Lundberg, Irja Ida Ratikainen and three anonymous referees for their helpful comments on a previous draft.
Data accessibility
The datasets supporting this article are in table 1 and electronic supplementary material, table S1.
Authors' contributions
J.B. and M.G. proposed the study and performed the literature search; J.B., M.G. and B.-E.S. wrote the paper.
Competing interests
We have no competing interests.
Funding
This work was supported by the European Research Council (grant STOCHPOP to B.-E.S.) and by the Research Council of Norway through its Centres of Excellence funding scheme, project no. 223257.
References
- 1.Stearns SC. 1992. The evolution of life histories. London, UK: Oxford University Press. [Google Scholar]
- 2.Cody ML. 1966. A general theory of clutch size. Evolution 20, 174–184. ( 10.2307/2406571) [DOI] [PubMed] [Google Scholar]
- 3.Williams GC. 1966. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690. ( 10.1086/282461) [DOI] [Google Scholar]
- 4.Roff DA. 2002. Life history evolution, 2nd edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 5.van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 6.Hamel S, Gaillard J-M, Yoccoz NG, Loison A, Bonenfant C, Descamps S. 2010. Fitness costs of reproduction depend on life speed: empirical evidence from mammalian populations. Ecol. Lett. 13, 915–935. ( 10.1111/j.1461-0248.2010.01478.x) [DOI] [PubMed] [Google Scholar]
- 7.Festa-Bianchet M. 2012. The cost of trying: weak interspecific correlations among life-history components in male ungulates. Can. J. Zool. 90, 1072–1085. ( 10.1139/Z2012-080) [DOI] [Google Scholar]
- 8.Kotiaho JS. 2001. Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol. Rev. Camb. Phil. Soc. 76, 365–376. ( 10.1017/S1464793101005711) [DOI] [PubMed] [Google Scholar]
- 9.Scharf I, Peter F, Martin OY. 2013. Reproductive trade-offs and direct costs for males in arthropods. Evol. Biol. 40, 169–184. ( 10.1007/s11692-012-9213-4) [DOI] [Google Scholar]
- 10.Bonduriansky R, Maklakov A, Zajitschek F, Brooks R. 2008. Sexual selection, sexual conflict and the evolution of ageing and life span. Funct. Ecol. 22, 443–453. ( 10.1111/j.1365-2435.2008.01417.x) [DOI] [Google Scholar]
- 11.Tidière M, Gaillard J-M, Müller D, Bingamam Lackay L, Gimenez O, Clauss M, Lemaître J-F. 2015. Does sexual selection shape sex-differences in longevity and senescence patterns across vertebrates ? A review and new insights from captive ruminants. Evolution 69, 3123–3140. ( 10.1111/evo.12801) [DOI] [PubMed] [Google Scholar]
- 12.Proaktor G, Coulson T, Milner-Gulland EJ. 2008. The demographic consequences of the cost of reproduction in ungulates. Ecology 89, 2604–2611. ( 10.1890/07-0833.1) [DOI] [PubMed] [Google Scholar]
- 13.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man, 1871-1971, pp. 136–179. Chicago, IL: Aldine-Atherton. [Google Scholar]
- 15.Bennett P, Owens I. 2002. Evolutionary ecology of birds: life histories, mating systems, and extinction. Oxford Series in Ecology and Evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Sæther B-E, Bakke Ø. 2000. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81, 642–653. ( 10.1890/0012-9658(2000)081%5B0642:ALHVAC%5D2.0.CO;2) [DOI] [Google Scholar]
- 17.Gaillard J-M, Yoccoz NG, Lebreton J-D, Bonenfant C, Devillard S, Loison A, Pontier D, Allaine D. 2005. Generation time: a reliable metric to measure life-history variation among mammalian populations. Am. Nat. 166, 119–123. ( 10.1086/an.2005.166.issue-1) [DOI] [PubMed] [Google Scholar]
- 18.Gaillard J-M, Lemaître J-F, Berger V, Bonenfant C, Devillard S, Douhard M, Gamelon M, Plard F, Lebreton J-D. In press. Axes of variation in life histories. In Encyclopedia of evolutionary biology (ed. Coulson T.). Oxford, UK: Elsevier. [Google Scholar]
- 19.Clutton-Brock T, Sheldon BC. 2010. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 25, 562–573. ( 10.1016/j.tree.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 20.Zera AJ, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126. ( 10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 21.Murphy MT. 2000. Evolution of clutch size in the eastern kingbird: tests of alternative hypotheses. Ecol. Monogr. 70, 1–20. ( 10.2307/2657165) [DOI] [Google Scholar]
- 22.Santos ESA, Nakagawa S. 2012. The costs of parental care: a meta-analysis of the trade-off between parental effort and survival in birds. J. Evol. Biol. 25, 1911–1917. ( 10.1111/j.1420-9101.2012.02569.x) [DOI] [PubMed] [Google Scholar]
- 23.Alatalo RV, Hoglund J, Lundberg A. 1991. Lekking in the black grouse—a test of male viability. Nature 352, 155–156. ( 10.1038/352155a0) [DOI] [Google Scholar]
- 24.Olson VA, Liker A, Freckleton RP, Székely T. 2008. Parental conflict in birds: comparative analyses of offspring development, ecology and mating opportunities. Proc. R. Soc. B 275, 301–307. ( 10.1098/rspb.2007.1395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaub M, Von Hirschheydt J. 2009. Effect of current reproduction on apparent survival, breeding dispersal, and future reproduction in barn swallows assessed by multistate capture–recapture models. J. Anim. Ecol. 78, 625–635. ( 10.1111/j.1365-2656.2008.01508.x) [DOI] [PubMed] [Google Scholar]
- 26.Forslund P, Larsson K. 1992. Age-related reproductive success in the barnacle goose. J. Anim. Ecol. 61, 195–204. ( 10.2307/5522) [DOI] [Google Scholar]
- 27.Dhondt AA. 1987. Reproduction and survival of polygynous and monogamous blue tit Parus caeruleus. Ibis 129, 327–334. ( 10.1111/j.1474-919X.1987.tb03176.x) [DOI] [Google Scholar]
- 28.Green DJ. 2001. The influence of age on reproductive performance in the Brown Thornbill. J. Avian Biol. 32, 6–14. ( 10.1034/j.1600-048X.2001.320102.x) [DOI] [Google Scholar]
- 29.Brown CR, Brown MB. 1998. Fitness components associated with alternative reproductive tactics in cliff swallows. Behav. Ecol. 9, 158–171. ( 10.1093/beheco/9.2.158) [DOI] [Google Scholar]
- 30.Gustafsson L, Sutherland WJ. 1988. The costs of reproduction in the collared flycatcher Ficedula albicollis. Nature 335, 813–815. ( 10.1038/335813a0) [DOI] [Google Scholar]
- 31.Ekman J, Askenmo C. 1986. Reproductive cost, age-specific survival and a comparison of the reproductive strategy in two european tits (genus Parus). Evolution 40, 159–168. ( 10.2307/2408613) [DOI] [PubMed] [Google Scholar]
- 32.Urano E. 1992. Early settling the following spring: a long-term benefit of mate desertion by male great reed warblers Acrocephalus arundinaceus. Ibis 134, 83–86. ( 10.1111/j.1474-919X.1992.tb07235.x) [DOI] [Google Scholar]
- 33.Den Boer-Hazewinkel J. 1987. On the costs of reproduction—parental survival and production of second clutches in the great tit. Ardea 75, 99–110. [Google Scholar]
- 34.McCleery RH, Perrins CM. 1988. Lifetime reproductive success of the great tit, Parus major. In Reproductive success: studies of individual variation in contrasting breeding systems (ed. Clutton-Brock TH.), pp. 136–153. Chicago, IL: University of Chicago Press. [Google Scholar]
- 35.Nooker JK, Sandercock BK. 2008. Phenotypic correlates and survival consequences of male mating success in lek-mating greater prairie-chickens (Tympanuchus cupido). Behav. Ecol. Sociobiol. 62, 1377–1388. ( 10.1007/s00265-008-0566-8) [DOI] [Google Scholar]
- 36.Sandercock BK, Beissinger SR, Stoleson SH, Melland RR, Hughes CR. 2000. Survival rates of a neotropical parrot: implications for latitudinal comparisons of avian demography. Ecology 81, 1351–1370. ( 10.1890/0012-9658(2000)081%5B1351:SROANP%5D2.0.CO;2) [DOI] [Google Scholar]
- 37.Vanderwerf EA. 2008. Sources of variation in survival, recruitment, and natal dispersal of the Hawai'i ’Elepaio. Condor 110, 241–250. ( 10.1525/cond.2008.8476) [DOI] [Google Scholar]
- 38.Bryant DM. 1979. Reproductive costs in the house martin (Delichon urbica). J. Anim. Ecol. 48, 655–675. ( 10.2307/4185) [DOI] [Google Scholar]
- 39.Payne RB. 1989. Indigo bunting. In Lifetime reproduction in birds (ed. Newton I.), pp. 153–172. London, UK: Academic Press. [Google Scholar]
- 40.Verhulst S, Salomons HM. 2004. Why fight? Socially dominant jackdaws, Corvus monedula, have low fitness. Anim. Behav. 68, 777–783. ( 10.1016/j.anbehav.2003.12.020) [DOI] [Google Scholar]
- 41.Le Bohec C, Gauthier-Clerc M, Grémillet D, Pradel R, Béchet A, Gendner J-P, Le Maho Y. 2007. Population dynamics in a long-lived seabird: I. Impact of breeding activity on survival and breeding probability in unbanded king penguins. J. Anim. Ecol. 76, 1149–1160. ( 10.1111/j.1365-2656.2007.01268.x) [DOI] [PubMed] [Google Scholar]
- 42.Thomas CS, Coulson JC. 1988. Reproductive success of kittiwake gulls, Rissa tridactyla. In Reproductive success: studies of individual variation in contrasting breeding systems (ed. TH Clutton-Brock), pp. 251–262. Chicago, IL: University of Chicago Press. [Google Scholar]
- 43.Cam E, Hines JE, Monnat J-Y, Nichols JD, Danchin E. 1998. Are adult nonbreeders prudent parents? The kittiwake model. Ecology 79, 2917–2930. ( 10.1890/0012-9658(1998)079%5B2917:AANPPT%5D2.0.CO;2) [DOI] [Google Scholar]
- 44.Vanderwerf EA, Young LC. 2011. Estimating survival and life-stage transitions in the laysan albatross (Phoebastria immutabilis) using multistate mark-recapture models. Auk 128, 726–736. ( 10.1525/auk.2011.10285) [DOI] [Google Scholar]
- 45.MacColl ADC, Hatchwell BJ, Dunn P. 2003. Heritability of parental effort in a passerine bird. Evolution 57, 2191–2195. ( 10.1554/02-685) [DOI] [PubMed] [Google Scholar]
- 46.McGowan A, Hatchwell BJ, Woodburn RJW. 2003. The effect of helping behaviour on the survival of juvenile and adult long-tailed tits Aegithalos caudatus. J. Anim. Ecol. 72, 491–499. ( 10.1046/j.1365-2656.2003.00719.x) [DOI] [Google Scholar]
- 47.Smith HG. 1993. Parental age and reproduction in the marsh tit Paras palustris. Ibis 135, 196–201. ( 10.1111/j.1474-919X.1993.tb02832.x) [DOI] [Google Scholar]
- 48.Wesołowski T, Rowiński P. 2006. Is there a cost of reproduction for marsh tits Parus palustris in a primeval forest? Ibis 148, 126–132. ( 10.1111/j.1474-919X.2006.00491.x) [DOI] [Google Scholar]
- 49.Robert A, Paiva VH, Bolton M, Jiguet F, Bried J. 2012. The interaction between reproductive cost and individual quality is mediated by oceanic conditions in a long-lived bird. Ecology 93, 1944–1952. ( 10.1890/11-1840.1) [DOI] [PubMed] [Google Scholar]
- 50.Morton ML, Pereyra ME, Crandall JD, MacDougall-Shackleton EA, Hahn TP. 2004. Reproductive effort and return rates in the mountain white-crowned sparrow. Condor 106, 131–138. ( 10.1650/7304) [DOI] [Google Scholar]
- 51.Townsend HM, Anderson DJ. 2007. Assessment of costs of reproduction in a pelagic seabird using multistate mark-recapture models. Evolution 61, 1956–1968. ( 10.1111/j.1558-5646.2007.00169.x) [DOI] [PubMed] [Google Scholar]
- 52.Crossin GT, Phillips RA, Lattin CR, Romero LM, Williams TD. 2013. Corticosterone mediated costs of reproduction link current to future breeding. Gen. Comp. Endocrinol. 193, 112–120. ( 10.1016/j.ygcen.2013.07.011) [DOI] [PubMed] [Google Scholar]
- 53.Safriel UN, Harris MP, Brooke MDL, Britton CK. 1984. Survival of breeding oystercatchers Haematopus ostralegus. J. Anim. Ecol. 53, 867–877. ( 10.2307/4664) [DOI] [Google Scholar]
- 54.Wheelwright NT, Tice KA, Freeman-Gallant CR. 2003. Postfledging parental care in Savannah sparrows: sex, size and survival. Anim. Behav. 65, 435–443. ( 10.1006/anbe.2003.2086) [DOI] [Google Scholar]
- 55.Mitchell GW, Wheelwright NT, Guglielmo CG, Norris DR. 2012. Short- and long-term costs of reproduction in a migratory songbird. Ibis 154, 325–337. ( 10.1111/j.1474-919X.2012.01212.x) [DOI] [Google Scholar]
- 56.Colwell MA, Pearson WJ, Eberhart-Phillips LJ, Dinsmore SJ. 2013. Apparent survival of snowy plovers (Charadrius nivosus) varies with reproductive effort and year and between sexes. Auk 130, 725–732. ( 10.1525/auk.2013.13147) [DOI] [Google Scholar]
- 57.Stoelting RE, Gutiérrez RJ, Kendall WL, Peery MZ. 2014. Life-history tradeoffs and reproductive cycles in spotted owls. Auk 132, 46–64. ( 10.1642/AUK-14-98.1) [DOI] [Google Scholar]
- 58.Korpimaki E. 1988. Costs of reproduction and success of manipulated broods under varying food conditions in Tengmalm's owl. J. Anim. Ecol. 57, 1027–1039. ( 10.2307/5109) [DOI] [Google Scholar]
- 59.Wheelwright NT, Leary J, Fitzgerald C. 1991. The costs of reproduction in tree swallows (Tachycineta bicolor). Can. J. Zool. 69, 2540–2547. ( 10.1139/z91-358) [DOI] [Google Scholar]
- 60.Buchmann M, Helm B, Rothery P, Flinks H. 2009. Consequences of late breeding on moult and recovery rate of a long-distance migrant, the Weatear (Oenanthe oenanthe). Vogelwarte 47, 125–133. [Google Scholar]
- 61.Low M, Arlt D, Eggers S, Pärt T. 2010. Habitat-specific differences in adult survival rates and its links to parental workload and on-nest predation. J. Anim. Ecol. 79, 214–224. ( 10.1111/j.1365-2656.2009.01595.x) [DOI] [PubMed] [Google Scholar]
- 62.Hannon SJ, Dobush G. 1997. Pairing status of male willow ptarmigan: is polygyny costly to males? Anim. Behav. 53, 369–380. ( 10.1006/anbe.1996.0328) [DOI] [Google Scholar]
- 63.Orell M, Belda EJ. 2002. Delayed cost of reproduction and senescence in the willow tit Parus montanus. J. Anim. Ecol. 71, 55–64. ( 10.1046/j.0021-8790.2001.00575.x) [DOI] [Google Scholar]
- 64.Petrie M, Kempenaers B. 1998. Extra-pair paternity in birds: explaining variation between species and populations. Trends Ecol. Evol. 13, 52–58. ( 10.1016/S0169-5347(97)01232-9) [DOI] [PubMed] [Google Scholar]
- 65.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bears H, Martin K, White GC. 2009. Breeding in high-elevation habitat results in shift to slower life-history strategy within a single species. J. Anim. Ecol. 78, 365–375. ( 10.1111/j.1365-2656.2008.01491.x) [DOI] [PubMed] [Google Scholar]
- 67.Badyaev AV, Hill GE. 2002. Paternal care as a conditional strategy: distinct reproductive tactics associated with elaboration of plumage ornamentation in the house finch. Behav. Ecol. 13, 591–597. ( 10.1093/beheco/13.5.591) [DOI] [Google Scholar]
- 68.Ratikainen II, Kokko H. 2010. Differential allocation and compensation: who deserves the silver spoon? Behav. Ecol. 21, 195–200. ( 10.1093/beheco/arp168) [DOI] [Google Scholar]
- 69.Horváthová T, Nakagawa S, Uller T. 2012. Strategic female reproductive investment in response to male attractiveness in birds. Proc. R. Soc. B 279, 163–170. ( 10.1098/rspb.2011.0663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lessells CM. 2012. Sexual conflict. In The evolution of parental care (eds Royle NJ, Smiseth PT), pp. 150–170. Oxford, UK: Oxford University Press. [Google Scholar]
- 71.Lemaître J-F, Berger V, Bonenfant C, Douhard M, Gamelon M, Plard F, Gaillard J-M. 2015. Early-late life trade-offs and the evolution of ageing in the wild. Proc. R. Soc. B 282, 20150209 ( 10.1098/rspb.2015.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cam E, et al. 2013. Looking for a needle in a haystack: inference about individual fitness components in a heterogeneous population. Oikos 122, 739–753. ( 10.1111/j.1600-0706.2012.20532.x) [DOI] [Google Scholar]
- 73.Zhang H, Rebke M, Becker PH, Bouwhuis S. 2015. Fitness prospects: effects of age, sex and recruitment age on reproductive value in a long-lived seabird. J. Anim. Ecol. 84, 199–207. ( 10.1111/1365-2656.12259) [DOI] [PubMed] [Google Scholar]
- 74.Sæther B-E. 1990. Age-specific variation in reproductive performance. Curr. Ornithol. 7, 251–283. [Google Scholar]
- 75.Forslund P, Pärt T. 1995. Age and reproduction in birds—hypotheses and tests. Trends Ecol. Evol. 10, 374–378. ( 10.1016/S0169-5347(00)89141-7) [DOI] [PubMed] [Google Scholar]
- 76.Clinton WL, Le Boeuf BJ. 1993. Sexual selection's effects on male life history and the pattern of male mortality. Ecology 74, 1884–1892. ( 10.2307/1939945) [DOI] [Google Scholar]
- 77.Robinson MR, Mar KU, Lummaa V. 2012. Senescence and age-specific trade-offs between reproduction and survival in female Asian elephants. Ecol. Lett. 15, 260–266. ( 10.1111/j.1461-0248.2011.01735.x) [DOI] [PubMed] [Google Scholar]
- 78.Froy H, Phillips RA, Wood AG, Nussey DH, Lewis S. 2013. Age-related variation in reproductive traits in the wandering albatross: evidence for terminal improvement following senescence. Ecol. Lett. 16, 642–649. ( 10.1111/ele.12092) [DOI] [PubMed] [Google Scholar]
- 79.Griffith SC, Owens IPF, Thuman KA. 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212. ( 10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 80.Møller AP, Birkhead TR. 1993. Certainty of paternity covaries with paternal care in birds. Behav. Ecol. Sociobiol. 33, 261–268. ( 10.1007/BF02027123) [DOI] [Google Scholar]
- 81.Jones OR, et al. 2008. Senescence rates are determined by ranking on the fast–slow life-history continuum. Ecol. Lett. 11, 664–673. ( 10.1111/j.1461-0248.2008.01187.x) [DOI] [PubMed] [Google Scholar]
- 82.Bonnet X, Bradshaw D, Shine R. 1998. Capital versus income breeding: an ectothermic perspective. Oikos 83, 333–342. ( 10.2307/3546846) [DOI] [Google Scholar]
- 83.Caswell H. 2001. Matrix population models: construction, analysis, and interpretation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 84.Rankin DJ, Kokko H. 2007. Do males matter? The role of males in population dynamics. Oikos 116, 335–348. ( 10.1111/j.2006.0030-1299.15451.x) [DOI] [Google Scholar]
- 85.Engen S, Lande R, Sæther B-E. 2005. Effective size of a fluctuating age-structured population. Genetics 170, 941–954. ( 10.1534/genetics.104.028233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller TEX, Williams JL, Jongejans E, Brys R, Jacquemyn H. 2012. Evolutionary demography of iteroparous plants: incorporating non-lethal costs of reproduction into integral projection models. Proc. R. Soc. B 279, 2831–2840. ( 10.1098/rspb.2012.0326) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are in table 1 and electronic supplementary material, table S1.


