Abstract
Somatic mutations in the promoter region of telomerase reverse transcriptase (TERT) gene, mainly at positions c.−124 and c.−146 bp, are frequent in several human cancers; yet its presence in gastrointestinal stromal tumor (GIST) has not been reported to date. Herein, we searched for the presence and clinicopathological association of TERT promoter mutations in genomic DNA from 130 bona fide GISTs. We found TERT promoter mutations in 3.8% (5/130) of GISTs. The c.−124C>T mutation was the most common event, present in 2.3% (3/130), and the c.−146C>T mutation in 1.5% (2/130) of GISTs. No significant association was observed between TERT promoter mutation and patient's clinicopathological features. The present study establishes the low frequency (4%) of TERT promoter mutations in GISTs. Further studies are required to confirm our findings and to elucidate the hypothetical biological and clinical impact of TERT promoter mutation in GIST pathogenesis.
Introduction
The telomerase reverse transcriptase (TERT) gene encodes the catalytic subunit of telomerase that is crucial to maintenance and regulation of the telomeres.1,2 In normal somatic adult tissues, telomerase activity is restricted to stem cells, and telomerase reactivation was proposed to be one of cancer hallmarks.3 Recently, hotspot somatic mutations in the promoter region of TERT, located −124 and −146 bp upstream from the ATG start site (c.−124C>T and c.−146C>T) were reported in several human cancers, including bladder (~85% of mutated cases), gliomas (~50%), thyroid (~15%) and melanoma (22–85%).4, 5, 6, 7, 8, 9 It was proposed that both c.−124C>T and c.−146C>T mutations create new binding motif sites (GGAA) of ETS transcription factors leading to upregulation of TERT levels and protein activity.4,5
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor on the gastrointestinal tract characterized by hotspot mutations in KIT and PDGFRA genes, which are predictive of imatinib-based therapy response.10,11 Somatic BRAF mutations12, 13, 14 and germinative SDHx mutations were reported in a subset of KIT/PDGFRA wild-type GIST.15,16 Increased telomerase activity was reported in GISTs and was associated with poor prognosis.17 Yet, TERT promoter mutation has not been reported in GIST. Herein, we searched for the presence and clinicopathological association of the c.−124C>T and c.−146C>T TERT promoter mutations in a series of 130 bona fide GISTs.12,18, 19, 20
Materials and methods
One hundred and thirty cases of GIST were selected from the files of the Department of Pathology from Barretos Cancer Hospital, Brazil, Centro Hospitalar S. João and Garcia de Orta Hospital, Portugal. The cases were retrospectively re-evaluated and classified according to the WHO classification,21 and were assessed for the mean age, primary localization, tumor size, National Comprehensive Cancer Network (NCCN) risk classification,22 metastasis and overall survival. The mean age of the patients was 59.8 years, 52.3% were male and the tumors were located mainly in the stomach (50%) and the small intestine (32.7%). Most tumors had tumor size >5 cm, high malignancy risk and metastatic potential (Table 1).
Table 1. Association of TERT promoter mutation status and clinicopathological and molecular features of GISTs.
| Variable | TERT wild type | TERT mutated | P-value |
|---|---|---|---|
| Mean Age | 59.6 | 65.9 | 0.351 |
| Primary localization | |||
| Esophagus | 1 (0.9) | 0 | 0.132 |
| Stomach | 62 (53) | 3 (60) | |
| Small intestine | 37 (31.6) | 0 | |
| Large intestine | 2 (1.7) | 1 (20) | |
| Rectum | 4 (3.4) | 1 (20) | |
| Mesentery | 2 (1.7) | 0 | |
| Retroperitoneum | 6 (5.1) | 0 | |
| Esophagus/stomach | 1(0.9) | 0 | |
| Other | 2 (1.7) | 0 | |
| Tumor size | |||
| ≤5 cm | 41 (36.3) | 1 (20) | 0.654 |
| >5 cm | 72 (63.7) | 4 (80) | |
| NCCN risk classificationa | |||
| Benign | 2 (2.2) | 0 | 0.646 |
| Very low | 19 (21.3) | 0 | |
| Low | 18 (20.2) | 2 (40) | |
| Intermediate | 10 (11.2) | 0 | |
| High | 40 (44.9) | 3 (60) | |
| Metastasis | |||
| Absent | 78 (70.9) | 4 (80) | 1 |
| Present | 32 (29.1) | 1 (20) | |
| Overall survival | |||
| Dead | 23 (27.4) | 1 (20) | 0.398 |
| Alive | 61 (72.6) | 4 (80) | |
| KIT/PDGFRA/BRAF status | |||
| KIT mutated | 83 (66.4) | 2 (40) | 0.186 |
| PDGFRA mutated | 16 (12.8) | 0 | |
| Wild type | 26 (20.8) | 3 (60) | |
Abbreviations: NCCN, National Comprehensive Cancer Network.
Miettinen and Lasota.22
The characterization of the mutational status for KIT and PDGFRA was performed in all GISTs.12,18, 19, 20 In addition, the BRAF mutation status was evaluated in KIT/PDGFRA wild-type GISTs (n=9) from Barretos Cancer Hospital and the SDH genes status was evaluated in KIT/PDGFRA/BRAF wild-type GISTs (n=18) from Centro Hospitalar S. João.15,16
Tumor genomic DNA was extracted from formalin-fixed and paraffin-embedded tissues using the QIAamp DNA MicroKit (Qiagen, Hilden, Germany), following the manufacturer's instructions.19 A fragment of the TERT promoter was amplified with PCR using primers 5′-AGTGGATTCGCGGGCACAGA-3′ and 5′-CAGCGCTGCCTGAAACTC-3′, resulting in a PCR product of 235 bp, which contained the chr5.hg19:g.1295228C>T and Chr5.hg19:g.1295250C>T sites of mutations. Alternatively, gene mutations can be designated based on their upstream location to the ATG initiation codon of TERT, as c.−124C>T, and c.−146C>T, as previously described.7 PCR was performed with an initial denaturation at 95 °C for 15 min, followed by 40 cycles of 95 °C denaturation for 30 s, 64 °C annealing for 90 s and 72 °C elongation for 30 s and 72 °C final elongation for 7 min. Quality of PCR products was confirmed with gel electrophoresis. DNA sequencing of the PCR product was performed using the BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA) and an ABI PRISM 3500 xL Genetic Analyzer (Applied Biosystems). The chromatograms were compared with the reference sequence (GeneBank, TERT: ENST00000310581). The SPSS 19.0 software (IBM Corp, Armonk, NY, USA) was used for all statistical analysis. To assess the relationship between variables, we used the Fisher's exact test. The P-value established for the statistics significance was <0.05.
Results
We found TERT promoter mutations in 3.8% (5/130) of the GISTs (Table 2 and Figure 1). The identified mutations are described in the LOVD database (https://research.cchmc.org/LOVD2/home.php;patient IDs 819–823). The c.−124C>T mutation was the most common event, present in 2.3% (3/130), and the c.−146C>T mutation in 1.5% (2/130) of GISTs. The two mutations occur in a mutually exclusive manner. No statistical correlation was found between TERT mutation and GIST clinical or molecular features (Table 1). Yet, TERT mutations appeared in tumors of slightly older patients, and no TERT-mutated cases were detected in benign/very-low malignancy risk GISTs (Table 1).
Table 2. Clinicopathological and molecular data of the GISTs with TERT promoter mutation (clinicopathological and molecular features of TERT promoter-mutated GISTs).
| ID | Hospital | Age (years) | Gender | Primary localization | Tumor size (cm) | NCCN risk classificationa | Metastasis | Status at last follow-up | Follow-up time (years) | KIT/PDGFRA/BRAF mutation status | TERT promoter mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 44 | Barretos | 77 | Female | Stomach | 7 | Intermediate | Absent | Alive without cancer | 10.71 | KIT | c.−146C>T |
| Case 75 | Barretos | 47 | Male | Rectum | 4 | Intermediate | Present | Alive with cancer | 11.71 | KIT | c.−124C>T |
| Case 159 | S. João | 49 | Male | Stomach | 6.5 | High | Absent | Alive without cancer | 22 .63 | Wild type | c.−124C>T |
| Case 215 | S. João | 76 | Male | Stomach | 6 | Intermediate | Absent | Alive without cancer | 8.78 | Wild type | c.−124C>T |
| Case 216 | S. João | 81 | Male | Large intestine | 12.5 | High | Absent | Death due to cancer | 0.02 | Wild type | c.−146C>T |
Abbreviation: NCCN, National Comprehensive Cancer Network.
Miettinen and Lasota.22
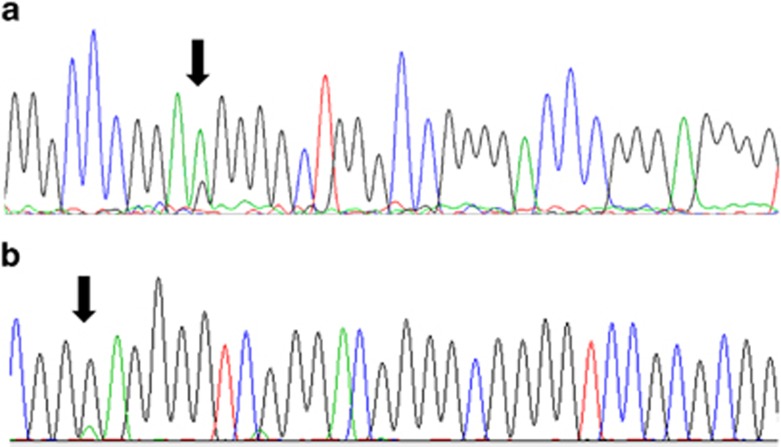
Figure 1.
Electropherogram of TERT promoter mutations. (a) Heterozigotic c.−124C>T mutation (arrow); (b) Heterozigotic c.−146C>T mutation (arrow).
Discussion
This study describes for the first time the occurrence of TERT promoter mutations (c.−124C>T and c.−146C>T) in GISTs, being present in ~4% of the cases. As paired blood or constitutive DNA of the tumors analyzed in the present study was not available, we cannot confirm the somatic nature of the c.−124 or c.−146 mutations identified. However, germline mutations at these hotspots were not reported in the various TERT studies that performed such paired (tumor versus normal) analysis.4, 5, 6, 7,23, 24, 25 In addition, in the COSMIC database,26 these mutations are described as somatic, and they are not present in the 1000 Genomes database.27 Therefore, we can almost certainty assume that the mutations observed in GISTs were somatically acquired.
Previously, we analyzed a series of 36 GISTs and did not identify any TERT promoter mutation.7 Likewise, Killela et al6 also analyzed nine GISTs and did not found any TERT promoter mutation. As identical methodologies were used in all studies, one plausible reason for this discrepancy is the small number of cases analyzed in the previous studies.6,7
TERT promoter mutations seem to be widespread in cancer, although showing tissue specificity. Killela et al6 suggested that cancers developing in tissues that are regularly self-renewing, such as in the gastrointestinal tract, skin and bone marrow, are not likely to harbor telomere-maintaining mutations, as telomerase is already epigenetically activated in their precursor cells. In contrast, cancers arising from cells that are not regularly self-renewing might harbor such mutations.6 GISTs fit to the second setting, as they are assumed to originate from the low-renewal Cajal cells or their precursors.28 GISTs are prone to exhibit a high risk of disease relapse and metastasis spreading to distant organs such as the liver, peritoneal surface and lung.10 Previous reports associate telomerase activity in GIST with higher tumor malignancy risk, metastasis and worse prognosis.29, 30, 31 We found a low frequency of TERT mutations in GIST, but any statistical association was found with tumor aggressiveness; however, most TERT-mutated GISTs displayed high recurrence risk features. Although our series is undersized to allow definitive conclusions, it would be of interest to further evaluate whether TERT promoter mutations associate with a higher expression of telomerase in GISTs, and to assess whether TERT promoter mutations associate with poor prognosis as reported in other cancers such as cancers of the thyroid,32 melanoma9 and brain.6
On the whole, our study establishes the presence of TERT promoter mutations in a subset of GISTs (4%). Future studies are required to validate our findings and to elucidate the potential biological and clinical impact of TERT promoter mutation in GIST pathogenesis.
Acknowledgments
This project was partially supported by Barretos Cancer Hospital internal research funds (PAIP) and CNPq Universal Grant (476192/2013-7) to RMR. NCC is a recipient of an FAPESP Doctoral Fellowship (2013/25787-3). Further funding from the project ‘Microenvironment, metabolism and cancer' that was partially supported by Programa Operacional Regional do Norte (ON.2—O Novo Norte) under the Quadro de Referência Estratégico Nacional (QREN) and the Fundo Europeu de Desenvolvimento Regional (FEDER). IPATIMUP is an Associate Laboratory of the Portuguese Ministry of Science, Technology and Higher Education that is partially supported by the FCT.
The authors declare no conflict of interest.
References
- Daniel M, Peek GW , Tollefsbol TO: Regulation of the human catalytic subunit of telomerase (hTERT). Gene 2012; 498: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich B, Rachakonda PS, Hemminki K, Kumar R: TERT promoter mutations in cancer development. Curr Opin Genet Dev 2013; 24C: 30–37. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Horn S, Figl A, Rachakonda PS et alTERT promoter mutations in familial and sporadic melanoma. Science 2013; 339: 959–961. [DOI] [PubMed] [Google Scholar]
- Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA: Highly recurrent TERT promoter mutations in human melanoma. Science 2013; 339: 957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killela PJ, Reitman ZJ , Jiao Y et alTERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA 2013; 110: 6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinagre J, Almeida A, Populo H et alFrequency of TERT promoter mutations in human cancers. Nat Commun 2013; 4: 2185. [DOI] [PubMed] [Google Scholar]
- Rachakonda PS, Hosen I , de Verdier PJ et alTERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci USA 2013; 110: 17426–17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pópulo H, Boaventura P, Vinagre J et al. TERT promoter mutations in skin cancer: the effects of sun exposure and irradiation. J Investig Dermatol 2014; 134: 2251–2257. [DOI] [PubMed] [Google Scholar]
- Campanella NC, de Oliveira AT, Scapulatempo-Neto C, Guimaraes DP, Reis RM: Biomarkers and novel therapeutic targets in gastrointestinal stromal tumors (GISTs). Recent Pat Anticancer Drug Discov 2013; 8: 288–297. [DOI] [PubMed] [Google Scholar]
- Joensuu H, Hohenberger P, Corless CL: Gastrointestinal stromal tumour. Lancet 2013; 382: 973–983. [DOI] [PubMed] [Google Scholar]
- Martinho O, Gouveia A , Viana-Pereira M et alLow frequency of MAP kinase pathway alterations in KIT and PDGFRA wild-type GISTs. Histopathology 2009; 55: 53–62. [DOI] [PubMed] [Google Scholar]
- Agaimy A, Terracciano LM, Dirnhofer S et alV600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours. J Clin Pathol 2009; 62: 613–616. [DOI] [PubMed] [Google Scholar]
- Agaram NP, Wong GC , Guo T et alNovel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer 2008; 47: 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celestino R, Lima J , Faustino A et alA novel germline SDHB mutation in a gastrointestinal stromal tumor patient without bona fide features of the Carney-Stratakis dyad. Fam Cancer 2012; 11: 189–194. [DOI] [PubMed] [Google Scholar]
- Celestino R, Lima J, Faustino A et alMolecular alterations and expression of succinate dehydrogenase complex in wild-type KIT/PDGFRA/BRAF gastrointestinal stromal tumors. Eur J Hum Genet 2013; 21: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabah M, Cummins R, Leader M, Kay E: Expression of human telomerase reverse transcriptase in gastrointestinal stromal tumors occurs preferentially in malignant neoplasms. Hum Pathol 2004; 35: 1231–1235. [DOI] [PubMed] [Google Scholar]
- de Oliveira AT, Reis RM, Afonso J et alLymphangiogenic VEGF-C and VEGFR-3 expression in genetically characterised gastrointestinal stromal tumours. Histol Histopathol 2011; 26: 1499–1507. [DOI] [PubMed] [Google Scholar]
- de Oliveira AT, Pinheiro C , Longatto-Filho A et alCo-expression of monocarboxylate transporter 1 (MCT1) and its chaperone (CD147) is associated with low survival in patients with gastrointestinal stromal tumors (GISTs). J Bioenerg Biomembr 2012; 44: 171–178. [DOI] [PubMed] [Google Scholar]
- Gomes AL, Gouveia A, Capelinha AF et alMolecular alterations of KIT and PDGFRA in GISTs: evaluation of a Portuguese series. J Clin Pathol 2008; 61: 203–208. [DOI] [PubMed] [Google Scholar]
- Bosman FT CF, Hruban RH , Theise ND: WHO Classification of Tumours of the Digestive System. Lyon: IARC, Vol 3, 2010. [Google Scholar]
- Miettinen M, Lasota J: Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006; 23: 70–83. [DOI] [PubMed] [Google Scholar]
- Griewank KG, Murali R , Schilling B et alTERT promoter mutations in ocular melanoma distinguish between conjunctival and uveal tumours. Br J Cancer 2013; 109: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa I, Ganly I , Chan TA et alFrequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab 2013; 98: E1562–E1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault JC, Mallet M, Pilati C et alHigh frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun 2013; 4: 2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmic Database Home. (http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/).
- Abecasis GR, Auton A, Brooks LD et alAn integrated map of genetic variation from 1,092 human genomes. Nature 2012; 491: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corless CL, Barnett CM , Heinrich MC: Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer 2011; 11: 865–878. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Fukayama M , Kaizaki Y et alTelomerase activity in gastrointestinal stromal tumors. Cancer 1998; 83: 2060–2066. [DOI] [PubMed] [Google Scholar]
- Kawai J, Kodera Y, Fujiwara M et alTelomerase activity as prognostic factor in gastrointestinal stromal tumors of the stomach. Hepatogastroenterology 2005; 52: 959–964. [PubMed] [Google Scholar]
- Wang Q, Kou YW: Study of the expressions of p53 and bcl-2 genes, the telomerase activity and apoptosis in GIST patients. World J Gastroenterol 2007; 13: 2626–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo M, Rocha AG, Vinagre J et alTERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab 2014; 99: E754–E765. [DOI] [PMC free article] [PubMed] [Google Scholar]