Abstract
Background
The current study was performed to investigate the effect of adenosine monophosphate (AMP) – activated protein kinase (AMPK) activation on the extracellular matrix (ECM) remodeling of pulmonary arteries in pulmonary arterial hypertension (PAH) and to address its potential mechanisms.
Material/Methods
PAH was induced by a single intraperitoneal injection of monocrotaline (MCT) into Sprague-Dawley rats. Metformin (MET) was administered to activate AMPK. Immunoblotting was used to determine the phosphorylation and expression of AMPK and expression of tissue inhibitor of metalloproteinase-1 (TIMP-1). Gelatin zymography was performed to determine the activity of matrix metalloproteinase-2 (MMP-2) and MMP-9.
Results
Activation of AMPK by MET significantly reduced the right ventricle systolic pressure and the right ventricular hypertrophy in MCT-induced rat PAH model, and partially inhibited the ECM remodeling of pulmonary arteries. These effects were coupled with the decrease of MMP-2/9 activity and TIMP-1 expression.
Conclusions
This study suggests that activation of AMPK benefits PAH by inhibiting ECM remodeling of pulmonary arteries. Enhancing AMPK activity might have potential value in clinical treatment of PAH.
MeSH Keywords: AMP-Activated Protein Kinases, Extracellular Matrix, Hypertension, Matrix Metalloproteinase 2, Matrix Metalloproteinase 9, Pulmonary Artery
Background
Pulmonary arterial hypertension (PAH) is a devastating disease characterized by progressive increase in pulmonary vascular resistance and pulmonary arterial pressure resulting from persistent vasoconstriction, vascular remodeling, and thrombosis [1,2]. Eventually, the increase in pulmonary arterial pressure leads to right ventricular heart failure and premature death. It has been demonstrated that pulmonary vascular remodeling characterized by thickening of all layers of vascular wall is extremely critical in the progression of pulmonary hypertension [3]. Pathologic changes leading to vascular remodeling include endothelial dysfunction, increased migration/proliferation of pulmonary arterial smooth muscle cells (PASMCs), adventitial fibroblast proliferation, and abnormal deposition of extracellular matrix (ECM) [4,5].
ECM is a highly dynamic composition of vasculature [6]. Collagen is the most abundant protein in the ECM [7]. It has been shown that accumulation of ECM is one of the most notable pathologic changes across the vascular wall in pulmonary hypertension [8]. Matrix metalloproteinases (MMPs) are the most significant modifying enzymes in ECM remodeling; they are largely produced by vascular smooth muscle cells, endothelial cells, and fibroblasts in the vascular wall [9–11]. Studies have shown that elevated MMP activity, especially MMP-2/9, leading to accelerated turnover of ECM, plays an important role in the initial remodeling of ECM in pulmonary vessels in both patients with PAH and animal models of PAH [12]. The function of MMPs is tightly controlled by a group of endogenous inhibitors called tissue inhibitors of metalloproteinases (TIMPs) to prevent excessive degradation of ECM [13]. Loss of balance between MMPs and TIMPs has been shown to induce ECM remodeling in patients with idiopathic PAH [14].
Adenosine monophosphate-activated protein kinase (AMPK) is a sensor of energy status that regulates cellular and whole-body energy balance [15]. A previous study has shown that activation of AMPK by metformin (MET) inhibits the development of PAH in animal models through suppressing vascular remodeling [16]. Our group has recently demonstrated that activation of AMPK by MET inhibits PASMCs proliferation [17]. However, it is still unclear whether activation of AMPK by MET also modulates MMP-TIMP balance, thus ECM remodeling to confer its overall beneficial effects on pulmonary hypertension. In the current study, we used rat models of monocrotaline (MCT)-induced PAH to evaluate the effects of activation of AMPK by MET on the ECM remodeling of the pulmonary artery and to further explore its potential molecular mechanisms.
Material and Methods
Material
Polyclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH), horseradish peroxidase-conjugated secondary antibodies, gelatin, and MCT were purchased from Sigma-Aldrich (St Louis, MO, USA). Metformin HCl tablets were obtained from Bristol-Myers Squibb (Shanghai, China). Tissue protein extraction buffer was supplied by Wolsen (Beijing, China). Monoclonal antibodies against total-AMPK and phosphor-AMPK were purchased from Cell Signaling Technology (Danvers, MA, USA). Polyclonal antibody against TIMP-1 was provided by Bioworld Technology (St Louis Park, MN, USA). All other reagents used in this study were of analytical grade.
Animals
Male Sprague-Dawley rats weighing 150 to 200 g used in the current study were obtained from the Laboratory Animal Center of Xi’an Jiaotong University. All animal experiments were approved by the Laboratory Animal Care Committee of Xi’an Jiaotong University and performed in accordance with the Guidelines for Animal Experimentation of Xi’an Jiaotong University. The rats were randomly divided into 3 groups: control group (Con, n=10), MCT-treated group (MCT, n=12), MCT and MET-treated group (MCT+MET, n=10). All animals were kept on a 12-h light/12-h dark cycle at 22±2ºC, with 50% relative humidity and atmospheric pressure.
Generation of PAH models and drug treatment
MCT was dissolved in 0.1 mol/L HCl, and the pH was adjusted to 7.4 with 0.1 mol/L NaOH. Intraperitoneal (IP) injection of MCT (60 mg/kg) was performed to induce the PAH model on day 1. Metformin HCl tablets were dissolved in 0.9% NaCl solution with the final concentration of 50 mg/mL. Metformin (150 mg/kg) was administrated to the rats daily by IP injection throughout the experiment period. The healthy control rats received an equal volume of 0.9% NaCl solution by IP injection.
Measurement of RVSP and RVH
Twenty-eight days after MCT injection, all surviving rats were anesthetized with an IP injection of 10% chloral hydrate (3 mL/kg). Hemodynamics were measured as described previously [18]. Briefly, right ventricle systolic pressure (RVSP) was measured using a Grass polygraph (Power Lab, Australia). The hearts and lungs were harvested after completion of hemodynamic measurements. The right ventricle (RV) was dissected from the left ventricle (LV) plus interventricular septum (S), and both parts were weighed separately to assess for right ventricular hypertrophy (RVH). Then the ratio of weight of the RV to the LV plus S [RV/(LV+S)] was calculated to determine the index of RVH.
Histologic analysis
After harvesting, marginal right lower pulmonary lobes were further fixed with 10% (v/v) buffered formalin for 4 h and then embedded with paraffin. Tissue blocks were then sectioned to 5 μm in thickness and stained with Masson’s trichrome staining. The stained sections were observed using an optical microscope at ×400 magnification. To evaluate the magnitude of collagen accumulation surrounding pulmonary vessels, perivascular collagen area (PVCA) was calculated using Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA). And PVCA was determined as the ratio of the perivascular fibrotic area (blue area) to the total vessel wall area.
Western blot analysis
Harvested peripheral lung tissues were homogenized in RIPA lysis buffer containing the phosphatase inhibitor and protease inhibitor on ice. Equal amounts of protein from each sample were denatured and separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels. The separated proteins were then transferred onto nitrocellulose membranes. The membranes were blocked with 5% bovine serum albumin in Tris-buffered saline with Tween for 1 h at room temperature and incubated overnight at 4ºC with primary antibodies (1:1000 dilution) against total-AMPK, phosphor-AMPK, TIMP-1, and GAPDH. Membranes were next washed and incubated with an appropriate horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. Finally, blots were developed using the ECL reagent kit (Millipore). The signal intensity of the immunoblots on the autoradiogram was quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
MMP activity determination by gelatin zymography
Gelatin zymography was performed to determine the enzymatic activity of MMP-2/9. Lung tissues were homogenized in Tris-HCl buffer containing 50 mmol/L Tris-HCl pH 7.6, 150 mmol/L NaCl, 5 mmol/L CaCl2, and 0.1% Triton X-100. Equal amounts of protein were mixed with Tris-glycine SDS sample buffer in the absence of reducing agents, separated on gelatin zymography gel by electrophoresis. The gel was then washed twice for 40 min in 2.5% Triton X-100 at room temperature, incubated for 42 h in development buffer containing 50 mmol/L Tris-HCl pH 7.6, 5 mmol/L CaCl2, and 1 μmol/L ZnCl2 at 37ºC. After incubation, the gel was stained with 0.5% Coomassie Blue R-250 for at least 3 h, and then destained in destaining buffer containing 30% MeOH and 10% acetic acid. The resulting bands were visualized and analyzed by Gel Doc™ XR (Bio-Rad).
Statistical analysis
Values are presented as mean plus or minus standard deviation. Differences among groups were analyzed using one-way analysis of variance followed by Tukey post hoc test. P<0.05 was considered statistically significant.
Results
Metformin prevents the increase of MCT-induced RVSP and RVH
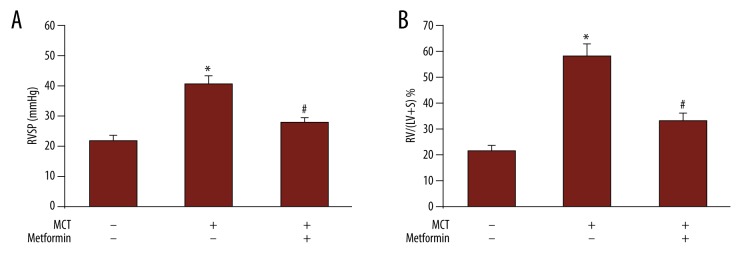
As shown in Figure 1A, the RVSP in MCT-treated rats was 40.6±2.7 mm Hg, which was significantly higher than that in control rats (21.8±1.6 mm Hg; P<0.05), suggesting that PAH was successfully induced in rats. Administration of MET in MCT-treated rats dramatically reduced RVSP to 27.7±1.8 mm Hg (P<0.05 vs. MCT-treated group). Similar changes were observed in RVH (Figure 1B). The RV/(LV+S) ratio increased dramatically in MCT-treated rats (58.3±4.8%) vs. control rats (21.4±2.3%; P<0.05). After the use of MET, the ratio declined to 33.1±3.2% (P<0.05 vs. MCT-treated rats), indicating that MET prevented the development of PAH.
Figure 1.
Metformin prevented MCT-induced pulmonary artery hypertension. (A) The right ventricular systolic pressure in different groups. (B) The right ventricle hypertrophy index in different groups. * P<0.05 vs. control group; # P<0.05 vs. MCT group.
Metformin prevents the MCT-induced excessive collagen deposition
To evaluate the accumulation of ECM components in pulmonary vessels in PAH, collagen deposition was determined by Masson’s staining, in which mature collagen fibers were stained blue. As shown in Figure 2, collagen deposition in pulmonary vessels was significantly increased in the lung tissues of MCT-treated rats compared with control rats. However, after administration of MET, deposition of collagen in pulmonary vessels in MCT-treated rats was dramatically reduced.
Figure 2.
Metformin prevented MCT-induced collagen deposition. Collagen deposition in small pulmonary arteries has been investigated by Masson’s staining. (A) Masson’s staining of lung tissues in different groups. (B) Perivascular collagen area values in different groups. Con – control. * P<0.05 vs. control group; # P<0.05 vs. MCT group.
Metformin prevents collagen deposition via AMPK activation
To determine whether activation of AMPK was involved in the inhibitory effect of MET on collagen deposition, phosphorylation of AMPK in lung tissue lysates was examined by Western blot. Figure 3 shows that the phosphorylation of AMPK was decreased in the MCT-induced PAH model, which was 0.58-fold over control (P<0.05), treatment with MET increased phosphorylation of AMPK in MCT-induced PAH rats (0.91-fold over control, P<0.05 vs. MCT-treated group).
Figure 3.

Effects of MCT and MET on the phosphorylation of AMPK in rat lung tissues. AMPK expression and phosphorylation have been analyzed by Western blot in lung tissues from different groups. A representative blot and quantification of bands are shown (n=3, each group). * P<0.05 vs. control group; # P<0.05 vs. MCT group.
Activation of AMPK inhibits activity of MMP-2/9 and expression of TIMP-1
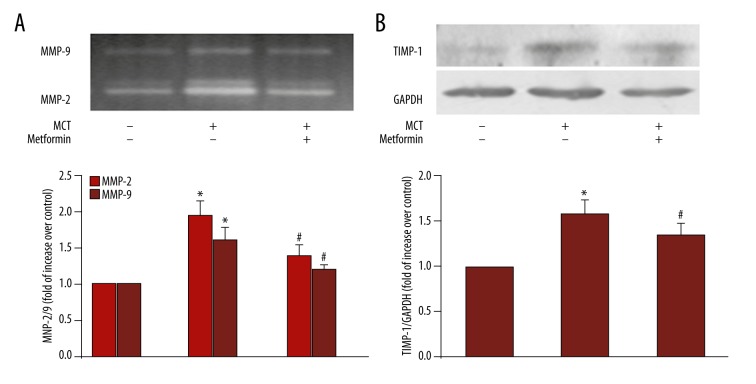
To further investigate the mechanisms of activation of AMPK by MET inhibition of MCT-induced collagen deposition, the activity of MMP-2/9 and the expression of TIMP-1 were examined in tissue lysates of rat lung. Figure 4A shows that MCT induced 1.96-fold increase in MMP-2 activity (P<0.05 vs. control) and 1.62-fold increase in MMP-9 activity (P<0.05 vs. control). Administration of MET decreased MMP-2/9 activity in MCT-treated rats to 1.41-/1.22-fold over control rats (P<0.05 vs. MCT-treated group). Figure 4B indicates that TIMP-1 protein level increased to 1.58-fold over control in the MCT-treated rats (P<0.05 vs. control group), whereas TIMP-1 level decreased to 1.35-fold over control rats in MCT+MET-treated rats (P<0.05 vs. MCT-treated group).
Figure 4.
Effects of MCT and MET on the activity of MMP-2 and MMP-9 and expression of TIMP-1 in rat lungs. Activity of MMP-2 and MMP-9 has been determined by gelatin zymography, and the expression of TIMP-1 has been determined by Western blot in lung tissues from different groups. (A) The activity of MMP-2 and MMP-9 in different groups. (B) The expression of TIMP-1 in different groups. GAPDH served as loading control. A representative blot and quantification of bands are shown (n=3 each group). * P<0.05 vs. control group; # P<0.05 vs. MCT group.
Discussion
The current study has indicated that activation of AMPK by MET dramatically reduces elevated RVSP and RVH in MCT-induced PAH, and these are accompanied by the inhibition of ECM remodeling in pulmonary arteries. The mechanisms underlying AMPK suppression of ECM remodeling in pulmonary arteries is coupled to decreased MMP-2/9 activity and TIMP-1 expression. Our study suggests that by enhancing AMPK activity, the strategy might prevent PAH partially by modulation of ECM remodeling in pulmonary arteries.
Elevated MMP activity and consequent ECM remodeling in pulmonary vasculature have been shown to be associated with the development of PAH in both experimental models and patients [12,14]. Injection of MCT causes inflammatory response in lungs, endothelial cell injury, and subsequent proliferation of vascular smooth muscle cells leading to the development of severe PAH, associated RVH, and pulmonary vascular lesions. Injured endothelial cells and inflammatory cells secrete more MMPs [19,20]. Consistent with previous studies, enhanced ECM accumulation in pulmonary arteries, accompanied by increased enzymatic activity of MMP-2/9 in lungs, has been found in MCT-induced PAH rat models in this study. MMP activation can initiate the ECM remodeling in pulmonary vasculature through a vicious circle in which ECM degradation promotes ECM protein synthesis. The degradation of ECM by MMPs and the subsequent release of bioactive ECM fragments and growth factors sequestered within the ECM promote proliferation of endothelial and smooth muscle cells and trans-differentiation of fibroblast, which in turn synthesizes and secretes more ECM components. The dysregulated ECM metabolism finally leads to abnormal ECM accumulation [12,21]. Further, it has been demonstrated that MMPs can directly and indirectly regulate proliferation, migration, invasion, and apoptosis of endothelial cell and smooth muscle cell [12]. All these findings suggest that MMPs play an important role in the development of PAH. In support, MMP inhibitor has been found to protect against the development of MCT-induced PAH in rats [22].
The function of MMPs is modulated by TIMPs. In the current study, we have found that expression of TIMP-1 in lungs of MCT-induced PAH rats is also upregulated, which implies a dynamic, balanced relationship between MMP and its specific tissue inhibitors [23].
AMPK is a crucial energy sensor that regulates cellular and whole-body energy balance. Although best known for its effects on metabolism, AMPK has many other functions, including regulation of autophagy, cell proliferation, and ECM synthesis and degradation [24,25]. Goncharov et al reported that AMPK phosphorylation is reduced in PASMCs of idiopathic PAH [26]. In the current study, we have found AMPK phosphorylation is also reduced in the lungs of rat models of MCT-induced PAH. A previous study has shown that activation of AMPK plays a protective role in the development of PAH in animal models by suppressing vascular remodeling [16]. Our group has recently demonstrated that activation of AMPK inhibits the proliferation of PASMCs by decrease of S phase kinase-associated protein 2 and increase of p27 [17]. ECM accumulation has also been shown to be an important component of the pathologic changes contributing to vascular remodeling in PAH and remodeling of valves [8,27]. The inactivation of AMPK has been demonstrated to mediate high phosphate-induced ECM accumulation in immortalized human mesangial cells [28]. AMPK activation by 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) has been shown to prevent renal fibrosis in the rat model of renal ablation and also in experimental diabetic nephropathy [29–31]. In the current study, we have found activation of AMPK by MET dramatically inhibits accumulation of ECM in pulmonary arteries induced by MCT. This effect is accompanied by a decrease in MMP-2/9 activity. The inhibition of expression and activity of MMP-2/9 by activation of AMPK has been shown in previous studies conducted on different types of cells [32–34]. In addition, studies have suggested that AMPK signaling pathway might regulate expression of MMPs by modulating the activation of nuclear factor-κB [34–36]. Based on these results, we speculate that the mechanisms underlying AMPK suppression of ECM accumulation in pulmonary arteries might involve the inhibition of MMP-2/9 activity. Further in vitro experiments are warranted to verify this finding.
PAH is a progressive disease with high mortality; however, no medication is available that effectively and safely treats this disease. MET is an in vitro synthetic AMPK agonist that has been used to treat type 2 diabetes for nearly a century with wide clinical experience and safety record [37]. Studies conducted by this group as well as others have indicated that activation of AMPK by MET prevents the development of MCT-induced PAH model. The suppression of ECM remodeling of pulmonary arteries and inhibition of PASMCs proliferation by activation of AMPK may contribute to its beneficial effects. However, the safety and efficacy of this therapeutic strategy in patients with PAH should be further investigated.
The current study has 2 limitations. First, animal models of MCT-induced PAH are characterized predominantly by pulmonary arterial medial hypertrophy rather than by the endothelial cell-mediated angio-obliteration observed in humans. Second, because pharmacologic modulation of AMPK is not entirely specific for AMPK, we cannot completely exclude the potential roles of other mediators modulated by MET during this process.
Conclusions
In conclusion, our study demonstrated in a MCT-induced PAH rat model that treatment with MET may prevent the development of PAH by reducing ECM remodeling of pulmonary arteries. The underlying mechanisms are associated with the activation of AMPK, which induces a decrease in the MMP-2/9 activity. Our results suggest that the strategy of activation of AMPK may have potential value in clinical treatment of PAH.
Footnotes
Source of support: National Natural Science Foundation of China (Grant No. 81070045) and the Key Clinical Project for Affiliated Hospital of Ministry of Public Health of China (Grant No. 111)
Conflicts of interest
None of the authors have any conflict of interest to declare.
References
- 1.Miura Y, Fukumoto Y, Sugimura K, et al. Identification of new prognostic factors of pulmonary hypertension. Circ J. 2010;74:1965–71. doi: 10.1253/circj.cj-10-0270. [DOI] [PubMed] [Google Scholar]
- 2.Vaillancourt M, Ruffenach G, Meloche J, Bonnet S. Adaptation and remodelling of the pulmonary circulation in pulmonary hypertension. Can J Cardiol. 2015;31:407–15. doi: 10.1016/j.cjca.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Sakao S, Tatsumi K, Voelkel NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2010;43:629–34. doi: 10.1165/rcmb.2009-0389TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research “Work in progress”. Circulation. 2000;102:2781–91. doi: 10.1161/01.cir.102.22.2781. [DOI] [PubMed] [Google Scholar]
- 6.Eble JA, Niland S. The extracellular matrix of blood vessels. Curr Pharm Des. 2009;15:1385–400. doi: 10.2174/138161209787846757. [DOI] [PubMed] [Google Scholar]
- 7.Nita M, Michalska-Malecka K, Mazurek U, et al. Influence of ranibizumab treatment on the extracellular matrix in patients with neovascular age-related macular degeneration. Med Sci Monit. 2014;20:875–83. doi: 10.12659/MSM.890031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case D, Irwin D, Ivester C, et al. Mice deficient in galectin-1 exhibit attenuated physiological responses to chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;292:L154–64. doi: 10.1152/ajplung.00192.2006. [DOI] [PubMed] [Google Scholar]
- 9.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: Potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 10.Hassoun PM. Deciphering the “matrix” in pulmonary vascular remodelling. Eur Respir J. 2005;25:778–79. doi: 10.1183/09031936.05.00027305. [DOI] [PubMed] [Google Scholar]
- 11.Nita M, Strzalka-Mrozik B, Grzybowski A, et al. Age-related macular degeneration and changes in the extracellular matrix. Med Sci Monit. 2014;20:1003–16. doi: 10.12659/MSM.889887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chelladurai P, Seeger W, Pullamsetti SS. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur Respir J. 2012;40:766–82. doi: 10.1183/09031936.00209911. [DOI] [PubMed] [Google Scholar]
- 13.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Lepetit H, Eddahibi S, Fadel E, et al. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension. Eur Respir J. 2005;25:834–42. doi: 10.1183/09031936.05.00072504. [DOI] [PubMed] [Google Scholar]
- 15.Grahame Hardie D. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J Intern Med. 2014;276:543–59. doi: 10.1111/joim.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agard C, Rolli-Derkinderen M, Dumas-de-La-Roque E, et al. Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br J Pharmacol. 2009;158:1285–94. doi: 10.1111/j.1476-5381.2009.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Liu L, Zhang Y, et al. Activation of AMPK inhibits pulmonary arterial smooth muscle cells proliferation. Exp Lung Res. 2014;40:251–58. doi: 10.3109/01902148.2014.913092. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Xie X, Zhu Y, et al. Inhibition of Notch3 prevents monocrotaline-induced pulmonary arterial hypertension. Exp Lung Res. 2015;41:435–43. doi: 10.3109/01902148.2015.1060545. [DOI] [PubMed] [Google Scholar]
- 19.Firth AL, Choi IW, Park WS. Animal models of pulmonary hypertension: Rho kinase inhibition. Prog Biophys Mol Biol. 2012;109:67–75. doi: 10.1016/j.pbiomolbio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Li XQ, Wang HM, Yang CG, et al. Fluoxetine inhibited extracellular matrix of pulmonary artery and inflammation of lungs in monocrotaline-treated rats. Acta Pharmacol Sin. 2011;32:217–22. doi: 10.1038/aps.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang D, Xu C, Li Z, et al. Protective action of hepatocyte growth factor on transforming growth factor beta-1-induced alpha-smooth muscle actin and extracellular matrix in cultured human peritoneal fibroblasts. Med Sci Monit. 2010;16(8):BR250–54. [PubMed] [Google Scholar]
- 22.Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest. 2000;105:21–34. doi: 10.1172/JCI6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XM, Shi K, Li JJ, et al. Effects of angiotensin II intervention on MMP-2, MMP-9, TIMP-1, and collagen expression in rats with pulmonary hypertension. Genet Mol Res. 2015;14:1707–17. doi: 10.4238/2015.March.6.17. [DOI] [PubMed] [Google Scholar]
- 24.Hardie DG. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan M, Tang G, Rui H. Adiponectin attenuates Ang-induced TGFbeta1 production in human mesangial cells via an AMPK-dependent pathway. Biotechnol Appl Biochem. 2015;62:848–54. doi: 10.1002/bab.1323. [DOI] [PubMed] [Google Scholar]
- 26.Goncharov DA, Kudryashova TV, Ziai H, et al. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation. 2014;129:864–74. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloch O, Erdbrugger W, Volker W, et al. Extracellular matrix in deoxycholic acid decellularized aortic heart valves. Med Sci Monit. 2012;18:BR487–92. doi: 10.12659/MSM.883618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadimitriou A, Peixoto EB, Silva KC, et al. Inactivation of AMPK mediates high phosphate-induced extracellular matrix accumulation via NOX4/TGFss-1 signaling in human mesangial cells. Cell Physiol Biochem. 2014;34:1260–72. doi: 10.1159/000366336. [DOI] [PubMed] [Google Scholar]
- 29.Satriano J, Sharma K, Blantz RC, Deng A. Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am J Physiol Renal Physiol. 2013;305:F727–33. doi: 10.1152/ajprenal.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dugan LL, You YH, Ali SS, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123:4888–99. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MJ, Feliers D, Mariappan MM, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292:F617–27. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 32.Esfahanian N, Shakiba Y, Nikbin B, et al. Effect of metformin on the proliferation, migration, and MMP-2 and -9 expression of human umbilical vein endothelial cells. Mol Med Rep. 2012;5:1068–74. doi: 10.3892/mmr.2012.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao X, Cao X, Song G, et al. Metformin rescues the MG63 osteoblasts against the effect of high glucose on proliferation. J Diabetes Res. 2014;2014:453940. doi: 10.1155/2014/453940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morizane Y, Thanos A, Takeuchi K, et al. AMP-activated protein kinase suppresses matrix metalloproteinase-9 expression in mouse embryonic fibroblasts. J Biol Chem. 2011;286:16030–38. doi: 10.1074/jbc.M110.199398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Essick EE, Ouchi N, Wilson RM, et al. Adiponectin mediates cardioprotection in oxidative stress-induced cardiac myocyte remodeling. Am J Physiol Heart Circ Physiol. 2011;301:H984–93. doi: 10.1152/ajpheart.00428.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai SC, Tsai MH, Chiu CF, et al. AMPK-dependent signaling modulates the suppression of invasion and migration by fenofibrate in CAL 27 oral cancer cells through NF-kappaB pathway. Environ Toxicol. 2014 doi: 10.1002/tox.22097. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.He L, Wondisford FE. Metformin action: concentrations matter. Cell Metab. 2015;21:159–62. doi: 10.1016/j.cmet.2015.01.003. [DOI] [PubMed] [Google Scholar]