Abstract
Background
Diabetes mellitus a common metabolic disorder with hyperglycemia, is caused by the interaction of genetic and environmental factors. Approximately 12~20% of diabetic patients have risk of colorectal cancer. Recent studies revealed that the insulin-like growth factor system (IGFs) plays an important role in tumor occurrence. This study thus investigated the relationship between IGFs-related proteins in diabetic patients and the incidence of colorectal carcinoma.
Material/Methods
A retrospective study was performed in a total of 206 individuals, including 85 diagnosed with diabetes. The incidence of colorectal cancer was tracked, along with the detection of IGFs expression in serum. During the surgical resection, tumor tissues and adjacent tissues were collected and quantified for IGFs expression level.
Results
We found no significant difference in age or sex between the diabetic and control groups. Diabetic patients, however, had elevated body weight and higher incidence of colorectal cancer compared to non-diabetic controls (p<0.05). The diabetic group also had higher IGF-I and IGF-IR mRNA levels in serum, while IGFBP-6 expression was down-regulated. In comparison to adjacent healthy tissues, tumor tissue had higher levels of IGF-I and IGF-IR but lower levels of IGFBP-6 (p<0.05).
Conclusions
Our study showed higher incidence of colorectal cancer in diabetics compared to non-diabetics. The occurrence of colorectal cancer in diabetic patients may be associated with elevated IGFs-related protein expression level.
MeSH Keywords: Colorectal Neoplasms; Diabetes Insipidus, Nephrogenic; Insulin Lispro
Background
As a common malignant tumor in the digestive tract, colorectal cancer occurs in both the colon and rectum [1]. Nearly 1.5 million people are diagnosed with colorectal carcinoma each year, making it the second most common cancer in industrial countries [2]. The incidence of colorectal cancer in China is higher in urban than non-urban areas [3], possibly due to lifestyle transitions [4]. In urban areas, the incidence and mortality of colorectal cancer stay at relatively high levels, affecting human health [5]. It is estimated that with economic development and change of lifestyles, the incidence rate will continue to rise.
Multiple factors underlie the occurrence of colorectal cancer, including genetic influences, over-intake of fatty acids or calories, and lack of exercise. The exact mechanism of pathogenesis, however, remains unclear [6]. Recent studies have revealed a correlation between colorectal cancer and diabetes, which is a metabolic disorder featuring hyperglycemia, and can be caused by the interaction of genetic and environmental factors. Recently, the incidence of type 2 diabetes mellitus (T2DB), complicated with malignant tumors, especially the digestive tract cancers, has been increasing. Surveys have proved that about 12~20% of diabetic patients are at risk of colorectal cancer, and the incidence rate is more than twice as much as that in other populations [7,8]. These results suggest that certain unique biological molecules in diabetic patients might facilitate the occurrence of colorectal cancer.
Evidence has demonstrated a close correlation between insulin-like growth factor system (IGFs) and colorectal cancer pathogenesis and progression, as suggested by abnormal expression of IGFs proteins in diabetic patients’ blood samples [9]. IGFs include IGF-I, IGF-II, relevant receptors IGF-IR and IGF-IIR, and IGF-binding proteins (IGFBPs), all of which exert crucial roles in anti-apoptosis and facilitating cell proliferation [10]. We thus performed a case-control study to investigate the difference of colorectal cancer incidence between diabetic and non-diabetic people. The expression levels of IGFs-related genes were further tested and the role of IGFs in pathogenesis of colorectal cancer was investigated.
Material and Methods
Research objects
A total of 206 individuals who had undergone primary screening for colorectal cancer from January 2013 to December 2013 were recruited in the First Hospital of Hebei Medical University. Of these 206, 85 were diagnosed with diabetes, while the other 121 people were non-diabetics recruited as the control group. The diagnostic criteria for diabetes were: (1) Clinical symptoms of diabetes mellitus; (2) Fasted blood glucose ≥7.0 mmol/L or normal blood glucose ≥11.1 mmol/L or 2-h-OGTT test result ≥11.1 mmol/L. We collected 5-mL peripheral blood samples for separation of plasma by cold centrifugation.
Among the 85 diabetic patients, 11 were diagnosed with colorectal cancer; 5 patients eventually had surgical resection of tumors. Five out of 121 non-diabetics had colorectal cancer and underwent surgery. Tumor and adjacent tissue samples were collected from those 10 patients during the surgery. This study was approved by the ethics committee of the First Hospital of Hebei Medical University.
Plasma RNA extraction
Total RNA was extracted from plasma samples using an extraction kit (Invitrogen, USA), following the manufacturer’s instructions. In brief, 0.5 mL of plasma was mixed with an equal volume of 2× solution buffer. After iced incubation for 5 min, phenol-chloroform was added to extract RNA, which included vortex (1 min), room temperature incubation (5 min), and centrifugation (12 000 g, 10 min). The upper aqueous phase was transferred to a new tube containing 1 mL of absolute ethanol. The mixture was loaded onto the column, followed by 12 000 g centrifugation to precipitate RNA. The column was further washed with washing buffer I and II. RNA was eluted in 0.1 mL RNAse-free H2O and kept at −80ºC.
Real-time PCR
Extracted RNA was used as the template for synthesizing cDNA. The total system was 20 μL, containing RNA, buffer 2× mix, and reverse transcriptase. In vitro reverse transcription was performed with incubation at 65ºC for 5 min, followed by 37ºC for 15 min and 98ºC denaturing (5 min). A real-time PCR system containing cDNA, specific primers (sequence as Table 1), SYBR Green mixture (Toyobo, Japan), and sterilized water, was performed in a PCR cycler (Model VIIA7, ABI, USA). Reaction conditions were: 95ºC for 5 min, followed by 40 cycles of 95ºC denature for 15 s, 60ºC annealing for 45 s, and 72ºC elongation for 15 s. Relative DNA level was determined by 2−ΔΔCt method.
Table 1.
Specific primer sequence for real-time PCR.
| Target gene | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) |
|---|---|---|
| β-actin | GAGGGAAATCGTGCGTGAC | CTGGAAGGTGGACAGTGAG |
| IGF-I | TGGAGTTGGTAGATTGCTGTTG | TATGAAGGGAGGTGGTGGGTAT |
| IGF-II | GAAAAGAAGGACCCCAGAAATC | TGCTGTGTGTTGTGTGTGTGTC |
| IGF-IR | AAGGGTGTGAAAGATGAAC | GAACTTATTGGCGTTGAGGTATG |
| IGF-IIR | CGGCTGAGGTAGTAGATTGT | GTCGTATCCAGTGCAGGGTCCGAGGTATTC |
| IGFBP-2 | GGTGTGTGAACCCCAACA | GCCAACACCAACACTCTTTC |
| IGFBP-6 | CGAGGGGCTCAAACACTCTAC | GCCAACACCAACACTCTTTC |
Western blotting
About 1 g of tumor or adjacent tissues was homogenized on ice, along with 1 mL RIPA and proteinase inhibitor. The mixture was incubated on ice for 30 min and centrifuged at 12 000 g for 10 min. Protein concentration was quantified by Coomassie brilliant blue kit. Proteins were then separated by SDS-PAGE and were transferred to NC membrane under 300-mA electrical fields. The membrane was first blocked in 5% defatted milk powder for 2 h, and then rinsed in PBST buffer. Primary antibodies against IGF-I, IGF-IR, IGFBP-5, and β-actin (Abcam, USA) were applied at 1:1000 dilution for overnight incubation. On the next day, secondary antibody was added at 1:10 000 for 1-h incubation. After rinsing in PBST 3 times, the membrane was mixed with chromogenic substrates for exposure in the dark (90 s). The optical density (OD) values of each protein band were recorded using the GIS-2020D system (Tanon, China) for calculating relative expression levels of proteins.
Statistical analysis
The SPSS 16.0 software package (IBM, US) was used to process all collected data, and quantitative results are presented as mean ± standard deviation (SD). The t test or analysis of variance (ANOVA) was used to compare means between groups, as appropriate. Enumeration data are presented as percentages and were analyzed by the chi-square test. The correlation analysis was performed by Spearman method. Statistical significance was defined when p<0.05.
Results
General information of patients
In a total of 206 individuals recruited, there were 85 diabetic patients (47 males and 38 females, average age=57.2 years) and 121 non-diabetic controls (67 males and 54 females, average age=59.3 years). No significant difference was identified in the age or sex ratio between these 2 groups (t test or chi-square test, both p>0.05, Table 2), suggesting the comparability and homogeneity of the 2 groups. In the diabetes group, a total of 11 colorectal cancer patients were discovered, while only 5 people in the control group were found to have cancer. The incidence of colorectal carcinoma was significantly higher in diabetics (chi-square test, p<0.05). We also found that the diabetic group had higher body weight (p<0.05, Table 2).
Table 2.
General information between diabetic and control group.
| Index | Control group | Diabetic group | Statistics value | P value |
|---|---|---|---|---|
| N | 121 | 85 | ||
| Sex | ||||
| Male | 67 | 47 | 0.001 | 0.991 |
| Female | 54 | 38 | ||
| Age (year) | 59.3±13.1 | 57.2±12.3 | 1.161 | 0.124 |
| Body weight (kg) | 58.28±12.07 | 65.71±13.46 | 4.15 | <0.001 |
| Colorectal cancer | ||||
| Yes | 5 | 11 | 5.408 | 0.02 |
| No | 116 | 74 | ||
Plasma IGFs expression
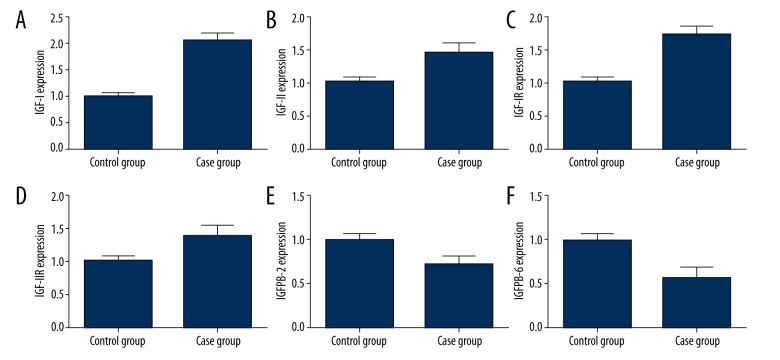
Real-time PCR was used to test the expression of IGFs in patient plasma. As shown in Figure 1, the level of IGF-I in plasma was elevated to 2.06-fold in the diabetic group compared to that in the control group (Figure 1A). IGF-II, IGF-IR, and IGF-IIR levels increased by 46%, 75%, and 38%, respectively (Figure 2B–2D), but IGFBP-2 and IGFBP-6 decreased to only 72% and 58% of the control level (Figure 2E, 2F).
Figure 1.
Plasma IGFs expression. (A) IGF-I; (B) IGF-II; (C) IGF-IR; (D) IGF-IIR; (E) IGFBP-2; (F) IGFBP-6. All values are as compared to the control group.
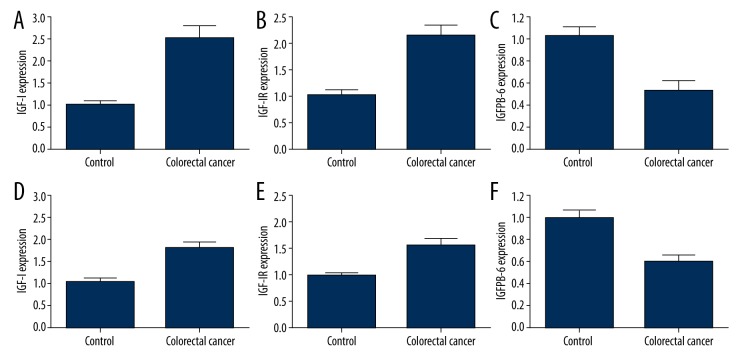
Figure 2.
IGFs expression level in tumor tissue and adjacent tissues. (A and D), IGF-I; (B and E), IGF-IR; (C and F), IGFBP-6. A–C, comparison between tumor and tumor adjacent tissues. D–F, comparison between control and diabetic patients.
IGFs expression in diabetic patients with colorectal carcinoma
In all 10 patients who had undergone surgical resection for colorectal carcinoma (5 from the diabetic group and 5 from the control group), we studied the expression of IGF-I, IGF-IR, and IGFBP-6 in tumors and adjacent tissues. As shown in Figure 2, the mRNA expressions of IGF-I and IGF-IR increased by 1.61-fold and 1.15-fold in tumor tissues compared to adjacent tissues (Figure 2A, 2B), while IGFBP-6 level decreased by 48% (Figure 2C). We further compared the expression level of these 3 proteins between diabetic and control groups, finding that IGF-I and IGF-IR were up regulated by 71% and 57%, respectively (Figure 2D, 2E) while IGFBP-6 was down regulated by 39% (Figure 2F).
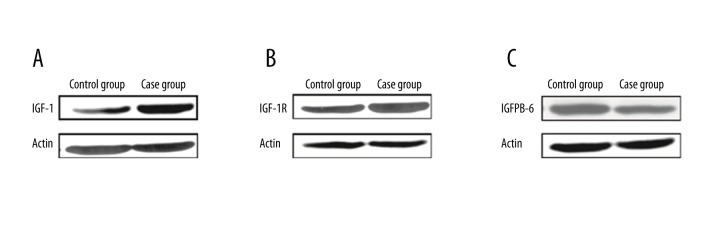
Western blot analysis obtained consistent results, showing that IGF-I and IGF-IR were up-regulated in tumor tissues, while IGFBP-6 was down-regulated (Figure 3). These results collectively suggest a correlation between IGFs-related protein expression and the occurrence of colorectal carcinoma.
Figure 3.
IGFs protein in colorectal cancer patients with diabetes. (A) IGF-I; (B) IGF-IR; (C) IGFBP-6.
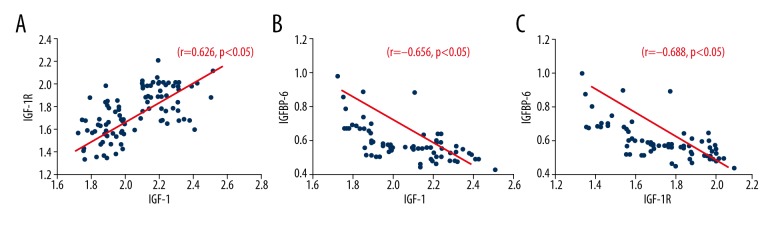
Correlation between IGF-I, IGF-IR and IGFBP-6 expression levels
To investigate the role of IGFs in pathogenesis of colorectal cancer, we tested the levels of IGFs-related factors in plasma in patients to analyze their correlation. As shown in Figure 4, there was a positive correlation between IGF-I and IGF-IR level (r=0.626, p<0.05, Figure 4A), while IGFBP-6 was negatively correlated with both IGF-I and IGF-IR (r=−0.656 and −0.688, p<0.05, Figure 4B, 4C).
Figure 4.
Correlations between plasma IGFs. (A) IGF-I and IGF-IR; (B) IGF-I and IGFBP-6; (C) IGF-IR and IGFBP-6.
Correlation between IGF-I, IGF-IR and IGFBP-6 expression levels
Based on previous studies, IGFs-related proteins expressions were closely correlated with the occurrence of colorectal cancer, and the cancer incidence in diabetic patients was significantly higher than that in non-diabetic individuals. To further prove the correlation between plasma IGFs expression and occurrence of colorectal cancer, we investigated levels of IGF-I, IGF-IR, and IGFBP-6 in blood of all 16 patients (11 from the diabetic group and 5 from the control group). Results showed that, compared to non-diabetic people, diabetic patients with colorectal carcinoma had higher IGF-I and IGF-IR levels in plasma, and reduced IGFBP-6 levels (p<0.05 in all cases, Table 3).
Table 3.
Plasma IGFs expression in diabetic and non-diabetic people with colorectal cancer.
| Non-diabetic | Diabetic | Statistic | P value | |||
|---|---|---|---|---|---|---|
| N | Mean value | N | Mean value | |||
| IGF-I | 5 | 0.842±0.105 | 11 | 0.956±0.113 | 1.908 | 0.038 |
| IGF-IR | 5 | 0.795±0.091 | 11 | 0.913±0.105 | 2.162 | 0.024 |
| IGFBP-6 | 5 | 0.621±0.108 | 11 | 0.503±0.101 | 2.123 | 0.036 |
IGFs expression level was presented as the relative value against actin.
Discussion
Our study illustrates the higher incidence of colorectal cancer in diabetes patients and shows the critical role of alternation of IGFs in occurrence of colorectal tumors among the diabetes group. As the most common malignant tumor in the digestive tract, the incidence and mortality rate of colorectal cancer remain high. Previous surveys showed a regional difference of tumor incidence, as industrial countries and more developed regions had higher incidence of colorectal cancer compared to developing countries and under-developed regions [11]. In China, tumor incidence is higher in urban than in rural areas. With economic development and lifestyle transition, it is predicted that the incidence rate of colorectal cancer will continue to rise. Current knowledge of pathogenesis mechanism underlying colorectal cancer mainly includes economic conditions, environmental pollution, lifestyle and diet. Recently, the relationship between diabetes and colorectal cancer has become a research focus [12]. Studies have revealed that about 12% to 20% of diabetic patients are at risk of colorectal cancer, but the underlying reason remains unclear. Some studies suggested the possible involvement of unhealthy lifestyle-induced compensatory insulinemia and T2DM in occurrence of colorectal cancer.
IGFs include IGF-I, IGF-II, IGF-IR, IGF-IIR, and IGFBPs, all of which exert critical roles in anti-apoptosis and facilitating cell proliferation [13]. IGF-I and IGF-II are polypeptides containing 70 or 67 amino acid residues, and are modulated by IGF-IR and IGFBPs [14]. Via specific binding onto membranes, glycosylated proteins, IGF-IR, and IGF-I can activate multiple intracellular pathways mediating cell growth, proliferation, and apoptosis [15,16]. Previous studies have shown the up-regulation of IGF-I in multiple tumors, including hepatocyte carcinoma [17], pancreatic cancer [18], and colorectal cancer [19,20]. It can also inhibit the expression of FAS/FASL gene and induce anti-apoptotic gene Bcl-2 expression, thus favoring tumor growth [21,22].
Our retrospective study found no statistically significant difference in sex and age between the diabetic and control groups, suggesting their comparability and homogeneity. The incidence of colorectal cancer was higher in diabetic patients compared to controls, in agreement with a previous study [23]. Subsequent studies shed light on the abnormal expression of IGFs in diabetics. Further study of tumor tissues also found elevated IGF-I and IGF-IR, and decreased IGFBP-6 compared to adjacent tissues. Consistent patterns also occurred in colorectal cancer complicated with diabetes in comparison to cancer patients without diabetes, indicating an important role of IGFs in the transformation from normal tissues into tumors. Based on these data, we further analyzed the relationship between those factors and found a positive correlation between IGF-I and IGF-IR, and a negative relationship between IGFBP-6 and IGF-I or IGF-IR. We thus postulated that IGF-I and IGF-IR might exert a synergistic effect to facilitate the tumor growth in colorectal tissues, while IGFBP-6 can antagonize such favorable effects. It has been demonstrated that active Rho GTPase Cdc42 can greatly enhance colorectal cancer cell SW480 metastasis, migration, and invasion showing a contributory role in colorectal cancer metastasis [24]. Similarly, early research has shown that the FAS/FASL or Bcl-2 pathway are activated in colorectal carcinoma cells by IGF-I/IGF-IR axis, indicating the regulatory role in the transcription and translation of related genes, and demonstrating its involvement in inhibiting cell apoptosis and affecting tumor growth [21,22]. It is thus possible to prevent or treat colorectal cancer by modulating the intracellular expression of IGF-I, IGF-IR, and IGFBP-6.
Conclusions
Our study demonstrated a high incidence of colorectal cancer in diabetic patients. The alteration of IGFs expressions in diabetic patients increases the risk of the occurrence of colorectal cancer. Our finding highlights the importance of early diagnosis for the prevention of colorectal cancer in diabetic patients and may provide evidence useful in the development of anti-colorectal cancer drugs, by using RNAi or chemical synthesis ways, which aim to target such IGFs.
Footnotes
Source of support: Departmental sources
References
- 1.Shibutani M, Maeda K, Nagahara H, et al. Significance of markers of systemic inflammation for predicting survival and chemotherapeutic outcomes and monitoring tumor progression in patients with unresectable metastatic colorectal cancer. Anticancer Res. 2015;35(9):5037–46. [PubMed] [Google Scholar]
- 2.Keku TO, Vidal A, Oliver S, et al. Genetic variants in IGF-I, IGF-II, IGFBP-3, and adiponectin genes and colon cancer risk in African Americans and Whites. Cancer Causes Control. 2012;23(7):1127–38. doi: 10.1007/s10552-012-9981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Starr MD, Brady JC, et al. Biomarker signatures correlate with clinical outcome in refractory metastatic colorectal cancer patients receiving bevacizumab and everolimus. Mol Cancer Ther. 2015;14(4):1048–56. doi: 10.1158/1535-7163.MCT-14-0923-T. [DOI] [PubMed] [Google Scholar]
- 4.Printz C. Vegetarian diet associated with lower risk of colorectal cancer. Cancer. 2015;121(16):2667. doi: 10.1002/cncr.29582. [DOI] [PubMed] [Google Scholar]
- 5.Yan XD, Yao M, Wang L, et al. Overexpression of insulin-like growth factor-I receptor as a pertinent biomarker for hepatocytes malignant transformation. World J Gastroenterol. 2013;19(36):6084–92. doi: 10.3748/wjg.v19.i36.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanilov NS, Karakolev IA, Deliysky TS, et al. Association of insulin-like growth factor-I receptor polymorphism with colorectal cancer development. Mol Biol Rep. 2014;41(12):8099–106. doi: 10.1007/s11033-014-3708-2. [DOI] [PubMed] [Google Scholar]
- 7.Muc-Wierzgoń M, Nowakowska-Zajdel E, Dzięgielewska-Gęsiak S, et al. Specific metabolic biomarkers as risk and prognostic factors in colorectal cancer. World J Gastroenterol. 2014;20(29):9759–74. doi: 10.3748/wjg.v20.i29.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao H, Jin C, Huang D, et al. Changes in serum IGF-1 level and tumor VEGF expression in mice with colorectal cancer under hyperglycemic conditions. Mol Med Rep. 2013;7(4):1361–65. doi: 10.3892/mmr.2013.1339. [DOI] [PubMed] [Google Scholar]
- 9.Kushlinskii NE, Gershtein ES, Nikolaev AA, et al. Insulin-like growth factors (IGF), IGF-binding proteins (IGFBP), and vascular endothelial growth factor (VEGF) in blood serum of patients with colorectal cancer. Bull Exp Biol Med. 2014;156(5):684–88. doi: 10.1007/s10517-014-2425-0. [DOI] [PubMed] [Google Scholar]
- 10.Sharon C, Baranwal S, Patel NJ, et al. Inhibition of insulin-like growth factor receptor/AKT/mammalian target of rapamycin axis targets colorectal cancer stem cells by attenuating mevalonate-isoprenoid pathway in vitro and in vivo. Oncotarget. 2015;6(17):15332–47. doi: 10.18632/oncotarget.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanella ER, Galimi F, Sassi F, et al. IGF2 is an actionable target that identifies a distinct subpopulation of colorectal cancer patients with marginal response to anti-EGFR therapies. Sci Transl Med. 2015;7(272):272ra12. doi: 10.1126/scitranslmed.3010445. [DOI] [PubMed] [Google Scholar]
- 12.Roep BO, Kleijwegt FS, van Halteren AG, et al. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol. 2010;159(3):338–43. doi: 10.1111/j.1365-2249.2009.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters G, Gongoll S, Langner C, et al. IGF-1R, IGF-1 and IGF-2 expression as potential prognostic and predictive markers in colorectal-cancer. Virchows Arch. 2003;443(2):139–45. doi: 10.1007/s00428-003-0856-5. [DOI] [PubMed] [Google Scholar]
- 14.Pankaj J, Kumari JR, Kim W, Lee SA. Insulin-like growth factor-1, IGF-binding protein-3, C-peptide and colorectal cancer: A case-control study. Asian Pac J Cancer Prev. 2015;16(9):3735–40. doi: 10.7314/apjcp.2015.16.9.3735. [DOI] [PubMed] [Google Scholar]
- 15.Peiró G, Lohse P, Mayr D, Diebold J. Insulin-like growth factor-I receptor and PTEN protein expression in endometrial carcinoma. Correlation with bax and bcl-2 expression, microsatellite instability status, and outcome. Am J Clin Pathol. 2003;120(1):78–85. doi: 10.1309/C1KA-H1PR-L1UB-W798. [DOI] [PubMed] [Google Scholar]
- 16.Kasprzak A, Szaflarski W, Szmeja J, et al. Differential expression of IGF-1 mRNA isoforms in colorectal carcinoma and normal colon tissue. Int J Oncol. 2013;42(1):305–16. doi: 10.3892/ijo.2012.1706. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q, Mao YQ, Jiang WD, et al. Development of IGF signaling antibody arrays for the identification of hepatocellular carcinoma biomarkers. PLoS One. 2012;7(10):e46851. doi: 10.1371/journal.pone.0046851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaquish DV, Yu PT, Shields DJ, et al. IGF1-R signals through the RON receptor to mediate pancreatic cancer cell migration. Carcinogenesis. 2011;32(8):1151–56. doi: 10.1093/carcin/bgr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abajo A, Bitarte N, Zarate R, et al. Identification of colorectal cancer metastasis markers by an angiogenesis-related cytokine-antibody array. World J Gastroenterol. 2012;18(7):637–45. doi: 10.3748/wjg.v18.i7.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang F, Xu LA, Khambata-Ford S. Correlation between gene expression of IGF-1R pathway markers and cetuximab benefit in metastatic colorectal cancer. Clin Cancer Res. 2012;18(4):1156–66. doi: 10.1158/1078-0432.CCR-11-1135. [DOI] [PubMed] [Google Scholar]
- 21.Yang C, Liu HZ, Fu ZX. PEG-liposomal oxaliplatin induces apoptosis in human colorectal cancer cells via Fas/FasL and caspase-8. Cell Biol Int. 2012;36(3):289–96. doi: 10.1042/CBI20100825. [DOI] [PubMed] [Google Scholar]
- 22.Parrizas M, LeRoith D. Insulin-like growth factor-1 inhibition of apoptosis is associated with increased expression of the bcl-xL gene product. Endocrinology. 1997;138(3):1355–58. doi: 10.1210/endo.138.3.5103. [DOI] [PubMed] [Google Scholar]
- 23.Xue M, Cao X, Zhong Y, et al. Insulin-like growth factor-1 receptor (IGF-1R) kinase inhibitors in cancer therapy: advances and perspectives. Curr Pharm Des. 2012;18(20):2901–13. doi: 10.2174/138161212800672723. [DOI] [PubMed] [Google Scholar]
- 24.Gao L, Bai L, Nan Q. Activation of Rho GTPase Cdc42 promotes adhesion and invasion in colorectal cancer cells. Med Sci Monit Basic Res. 2013;19:201–7. doi: 10.12659/MSMBR.883983. [DOI] [PMC free article] [PubMed] [Google Scholar]