Supplemental Digital Content is Available in the Text.
Keywords: peripheral vestibular hypofunction, vestibular rehabilitation, clinical practice guideline
Abstract
Background:
Uncompensated vestibular hypofunction results in postural instability, visual blurring with head movement, and subjective complaints of dizziness and/or imbalance. We sought to answer the question, “Is vestibular exercise effective at enhancing recovery of function in people with peripheral (unilateral or bilateral) vestibular hypofunction?”
Methods:
A systematic review of the literature was performed in 5 databases published after 1985 and 5 additional sources for relevant publications were searched. Article types included meta-analyses, systematic reviews, randomized controlled trials, cohort studies, case control series, and case series for human subjects, published in English. One hundred thirty-five articles were identified as relevant to this clinical practice guideline.
Results/Discussion:
Based on strong evidence and a preponderance of benefit over harm, clinicians should offer vestibular rehabilitation to persons with unilateral and bilateral vestibular hypofunction with impairments and functional limitations related to the vestibular deficit. Based on strong evidence and a preponderance of harm over benefit, clinicians should not include voluntary saccadic or smooth-pursuit eye movements in isolation (ie, without head movement) as specific exercises for gaze stability. Based on moderate evidence, clinicians may offer specific exercise techniques to target identified impairments or functional limitations. Based on moderate evidence and in consideration of patient preference, clinicians may provide supervised vestibular rehabilitation. Based on expert opinion extrapolated from the evidence, clinicians may prescribe a minimum of 3 times per day for the performance of gaze stability exercises as 1 component of a home exercise program. Based on expert opinion extrapolated from the evidence (range of supervised visits: 2-38 weeks, mean = 10 weeks), clinicians may consider providing adequate supervised vestibular rehabilitation sessions for the patient to understand the goals of the program and how to manage and progress themselves independently. As a general guide, persons without significant comorbidities that affect mobility and with acute or subacute unilateral vestibular hypofunction may need once a week supervised sessions for 2 to 3 weeks; persons with chronic unilateral vestibular hypofunction may need once a week sessions for 4 to 6 weeks; and persons with bilateral vestibular hypofunction may need once a week sessions for 8 to 12 weeks. In addition to supervised sessions, patients are provided a daily home exercise program.
Disclaimer:
These recommendations are intended as a guide for physical therapists and clinicians to optimize rehabilitation outcomes for persons with peripheral vestibular hypofunction undergoing vestibular rehabilitation.
Video Abstract available for more insights from the author (see Video, Supplemental Digital Content 1, http://links.lww.com/JNPT/A124).
TABLE OF CONTENTS
INTRODUCTION AND METHODS
Levels of Evidence and Grades of Recommendations................................................. 127
Summary of Action Statements.................................................................................. 128
Introduction................................................................................................................. 129
Methods....................................................................................................................... 130
VESTIBULAR REHABILITATION RECOMMENDATIONS
Action Statements....................................................................................................... 137
Guideline Implementation Recommendations............................................................ 151
Summary of Research Recommendations.................................................................. 152
ACKNOWLEDGEMENTS AND REFERENCES
Acknowledgements..................................................................................................... 153
References................................................................................................................... 153
TABLES AND FIGURES
Table 1: Levels of Evidence........................................................................................ 127
Table 2: Grades of Recommendations for Action Statements.................................... 127
Table 3: Literature Search Query................................................................................ 131
Table 4: Brief Core Set for Vertigo............................................................................. 133
Table 5: Recommended Outcome Measures............................................................... 135
Figure 1: Literature Search Flowchart........................................................................ 132
TABLE 1. Level of Evidencea.
| I | Evidence obtained from high-quality (≥50% critical appraisal score) diagnostic studies, prospective studies, or randomized controlled trials |
| II | Evidence obtained from lesser quality (<50% critical appraisal score) diagnostic studies, prospective studies, or randomized controlled trials |
| III | Case-controlled studies or retrospective studies |
| IV | Case study or case series |
| V | Expert opinion |
aBased on information from the Centre for Evidence Based Medicine website: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
TABLE 2. Grades of Recommendationsa.
| GRADE | RECOMMENDATION | STRENGTH OF RECOMMENDATION |
|---|---|---|
| A | Strong evidence | A preponderance of level I and/or level II studies supports the recommendation. This must include at least 1 level I study |
| B | Moderate evidence | A single high-quality randomized controlled trial or a preponderance of level II evidence supports the recommendation |
| C | Weak evidence | A single level II study or a preponderance of level III and IV studies supports the recommendation |
| D | Expert opinion | Best practice based on the clinical experience of the guideline development team and guided by the evidence, which may be conflicting. Where higher quality studies disagree with respect to their conclusions, it may be possible to come to agreement on certain aspects of intervention (eg, variations in treatment/diagnostic test, population, or setting that may account for conflict) |
aEach Action Statement is preceded by a bolded letter grade (A-D) indicating the strength of the recommendation.
TABLE 3. Search Query Combined Terms From the Following Concept Sets (Patient Population, Intervention, Outcome) to Retrieve All Articles That Included at Least One Term From Each Set (ie, Patient Population and Intervention and Outcome).
| Concept Sets |
| Patient population set |
| Peripheral vestibular (hypofunction or loss) |
| Vestibular system |
| Vestibular labyrinth |
| Vestibular nervous system |
| Vestibular nerve |
| Vestibular nucleus |
| Vestibulocochlear nerve |
| Benign paroxysmal positional |
| Vertigo |
| Inner ear |
| Labyrinth disease |
| Vestibular disease |
| Labyrinth vestibule |
| Vestibulum auris |
| Ear vestibule |
| Vestibular apparatus |
| Oval window and ear |
| Saccule and utricle |
| Acoustic maculae |
| Vestibular aqueduct |
| Dizziness |
| Intervention set |
| Exercise |
| Visual-vestibular interaction |
| Adaptation exercises |
| Substitution exercises |
| Habituation exercises |
| Outcome set |
| Balance |
| Gait |
| Quality of life |
| Position |
| Falls |
TABLE 4. Components of the Brief Core Set for Vertigo, Which Is the Minimal Standard for Assessment and Description of Functioning and Disability, Are Listed and Categorized According to the International Classification of Functioning, Disability and Health (ICF) Modela.
| ICF CATEGORY | DESCRIPTION |
|---|---|
| Body functions | |
| Mental functions | |
| b152 | Emotional functions |
| b156 | Perceptual functions |
| Sensory functions and pain | |
| b210 | Seeing functions |
| b215 | Functions of structures adjoining the eye |
| b230 | Hearing functions |
| b235 | Vestibular functions |
| b240 | Sensations associated with hearing and vestibular function |
| b260 | Proprioceptive function |
| Neuromusculoskeletal and movement-related functions | |
| b770 | Gait pattern functions |
| Body Structure | |
| Nervous system | |
| s110 | Structure of brain |
| s120 | Spinal cord and related structures |
| The eye, ear, and related structures | |
| s260 | Structure of inner ear |
| Structures of the cardiovascular, immunological, and respiratory systems | |
| s410 | Structure of cardiovascular system |
| Activities and Participation | |
| General tasks and demands | |
| d230 | Carrying out daily routine |
| Mobility | |
| d410 | Changing the basic body position |
| d415 | Maintaining a body position |
| d450 | Walking |
| d455 | Moving around |
| d460 | Moving around in different locations |
| d469 | Walking and moving, other specified and unspecified |
| d475 | Driving |
| Domestic life | |
| d640 | Doing housework |
| Environmental Factors | |
| Products and technology | |
| e110 | Products or substances for personal consumption |
| e120 | Products and technology for personal indoor and outdoor mobility and transportation |
| Natural environment and human-made changes to environment | |
| e240 | Light |
| Support and relationships | |
| e310 | Immediate family |
| e355 | Health professionals |
| Services, systems, and policies | |
| e580 | Health services, systems, and policies |
Abbreviation: ICF, International Classification of Functioning, Disability and Health.
aCategories are denoted as follows: b for body functions, s for body structures, d for activities and participation, and e for environmental factors. The numbers refer to the World Health Organization's coding system for the specific domains.
Adapted from Grill et al36 with permission from IOS Press.
TABLE 5. Summary of Outcome Measures Recommended for Use by the Vestibular Evidence Database to Guide Effectiveness Task Force to Assess Patients With Vestibular Hypofunctiona.
| ICF LEVEL | MEASURE | WHAT IT MEASURES |
|---|---|---|
| Body structure/function | Dynamic Visual Acuity | Visual acuity during fixed head movement velocity with decreasing optotype size |
| Gaze Stabilization Test | Visual acuity during increasing head movement velocity with fixed optotype size | |
| Sharpened Romberg | Static stance with altered base of support (tandem) | |
| Sensory Organization Test | Computerized assessment of postural control by measuring sway under conditions in which visual/somatosensory feedback is altered | |
| Sensory Organization Test With Head Shake | Postural stability during head rotations compared to head still | |
| (Modified) Clinical Test of Sensory Interaction on Balance | Postural control under various sensory conditions, including eyes open and closed and firm and foam surfaces | |
| Visual Analog Scale | Symptoms are quantified on a 10-cm line corresponding to intensity | |
| Visual Vertigo Analog Scale | Intensity of visual vertigo in 9 challenging situations of visual motions using Visual Analog Scale | |
| Motion Sensitivity Quotient | Motion-provoked dizziness during a series of 16 quick changes to head or body positions | |
| Vertigo Symptoms Scale | Symptoms of balance disorder and somatic anxiety and autonomic arousal | |
| Activity/participation | Five Times Sit to Stand | Functional lower extremity strength with published norms in older adults |
| 30-Second Chair Stand | Functional lower extremity strength with published norms in older adults | |
| Functional Reach/Modified Functional Reach | Stability of the maximum forward reaching distance while standing in a fixed position. The modified version is performed sitting | |
| Gait Velocity (10-m Walk Test) | Walking at preferred speed | |
| Balance Evaluation Systems Test | Six different balance control systems | |
| Mini Balance Evaluation Systems Test | Shortened version of the Balance Evaluation Systems Test | |
| Berg Balance Scale | 14-item measure of static balance and fall risk during common activities | |
| Dynamic Gait Index | Postural stability during various walking tasks including change speed, turn head, walk over/around obstacles, and climb stairs | |
| Functional Gait Assessment | Postural stability during various walking tasks including tandem, backwards, and eyes closed | |
| Four-Square Step Test | Ability to step over objects forward, sideways, and backwards | |
| Unipedal Stance Test | Static stance on 1 leg | |
| Timed Up and Go | Mobility and fall risk | |
| Modified Timed Up and Go With Dual-Task | Mobility under dual-task conditions and fall risk | |
| Activities-Specific Balance Confidence Scale | Confidence in balance without falling or being unsteady across a continuum of activities | |
| Disability Rating Scale | Level of disability based on descriptions of symptoms and limited activities | |
| Dizziness Handicap Inventory | Perceived handicap as a result of dizziness | |
| UCLA Dizziness Questionnaire | Severity, frequency, and fear of dizziness and its effect on quality of life and activities of daily living | |
| Vertigo Handicap Questionnaire | Effects of vertigo on disability, handicap, and psychological distress | |
| Vestibular Activities and Participation | Effects of dizziness and/or balance problems on ability to perform activity and participation tasks | |
| Vestibular Disorders Activities of Daily Living Scale | Independence in everyday activities of daily living | |
| Vestibular Rehabilitation Benefit Questionnaire | Impact of symptoms on quality of life |
Abbreviation: ICF, International Classification of Functioning, Disability and Health.
aThe measures are organized on the basis of the ICF model. Details regarding recommendations are available online at http://www.neuropt.org/professional-resources/neurology-section-outcome-measures-recommendations/vestibular-disorders.
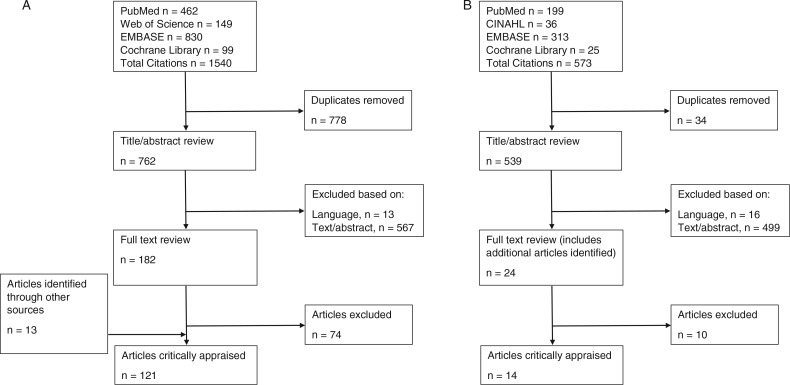
FIGURE 1.
(A) Flowchart of initial identification of relevant articles from 1985 through March 2013. (B) Flowchart of identification of additional relevant articles through February 2015.
LEVELS OF EVIDENCE AND GRADE OF RECOMMENDATIONS
This clinical practice guideline is intended to optimize rehabilitation outcomes for persons with vestibular hypofunction undergoing vestibular rehabilitation. As such, the intention of the recommendations is to provide guidance to clinicians providing vestibular rehabilitation. The clinician should interpret the guidelines in the context of their specific clinical practice, patient situation, and preference, as well as the potential for harm.
The methods of critical appraisal, assigning levels of evidence to the literature and assigning levels of strength to the recommendations, follow accepted international methodologies of evidence-based practice. The guideline is organized to present the definitions of the levels of evidence and grades for action statements (Tables 1 and 2), the summary of 10 action statements, followed by the description of each action statement with a standardized profile of information that meets the Institute of Medicine's criteria for transparent clinical practice guidelines. Recommendations for research are also made in the text.
Each individual research article was graded on the basis of criteria from the Centre for Evidence-Based Medicine from 2009 to determine the level of evidence of intervention studies (Table 1). Levels I and II differentiate stronger from weaker studies by using key questions, adapted from Fetters and Tilson1, that evaluate the research design, quality of study execution, and reporting. The criteria for the grades of recommendation assigned to each action statement are provided in Table 2. The grade reflects the overall strength of the evidence available to support the action statement. Throughout the guideline, each action statement is preceded by a letter grade indicating the strength of the recommendation, followed by the statement and summary of the supporting evidence.
SUMMARY OF ACTION STATEMENTS
Therapeutic Intervention for Persons With Peripheral Vestibular Hypofunction
A. Action Statement 1: EFFECTIVENESS OF VESTIBULAR REHABILITATION IN PERSONS WITH ACUTE AND SUBACUTE UNILATERAL VESTIBULAR HYPOFUNCTION. Clinicians should offer vestibular rehabilitation to patients with acute or subacute unilateral vestibular hypofunction. (Evidence quality: I; recommendation strength: strong)
A. Action Statement 2: EFFECTIVENESS OF VESTIBULAR REHABILITATION IN PERSONS WITH CHRONIC UNILATERAL VESTIBULAR HYPOFUNCTION. Clinicians should offer vestibular rehabilitation to patients with chronic unilateral vestibular hypofunction. (Evidence quality: I; recommendation strength: strong)
A. Action Statement 3: EFFECTIVENESS OF VESTIBULAR REHABILITATION IN PERSONS WITH BILATERAL VESTIBULAR HYPOFUNCTION. Clinicians should offer vestibular rehabilitation to patients with bilateral vestibular hypofunction. (Evidence quality: I; recommendation strength: strong)
A. Action Statement 4: EFFECTIVENESS OF SACCADIC OR SMOOTH-PURSUIT EXERCISES IN PERSONS WITH PERIPHERAL VESTIBULAR HYPOFUNCTION (UNILATERAL OR BILATERAL). Clinicians should not offer saccadic or smooth-pursuit exercises in isolation (ie, without head movement) as specific exercises for gaze stability to patients with unilateral or bilateral vestibular hypofunction. (Evidence quality: I; recommendation strength: strong)
B. Action Statement 5: EFFECTIVENESS OF DIFFERENT TYPES OF EXERCISES IN PERSONS WITH ACUTE OR CHRONIC UNILATERAL VESTIBULAR HYPOFUNCTION. Clinicians may provide targeted exercise techniques to accomplish specific goals appropriate to address identified impairments and functional limitations. (Evidence quality: II; recommendation strength: moderate)
B. Action Statement 6: EFFECTIVENESS OF SUPERVISED VESTIBULAR REHABILITATION. Clinicians may offer supervised vestibular rehabilitation to patients with unilateral or bilateral peripheral vestibular hypofunction. (Evidence quality: I-III; recommendation strength: moderate)
D. Action Statement 7: OPTIMAL EXERCISE DOSE OF TREATMENT IN PEOPLE WITH PERIPHERAL VESTIBULAR HYPOFUNCTION (UNILATERAL AND BILATERAL). Clinicians may prescribe a home exercise program of gaze stability exercises consisting of a minimum of 3 times per day for a total of at least 12 minutes per day for patients with acute/subacute vestibular hypofunction and at least 20 minutes per day for patients with chronic vestibular hypofunction. (Evidence quality: V; recommendation strength: expert opinion)
D. Action Statement 8: DECISION RULES FOR STOPPING VESTIBULAR REHABILITATION IN PERSONS WITH PERIPHERAL VESTIBULAR HYPOFUNCTION (UNILATERAL AND BILATERAL). Clinicians may use achievement of primary goals, resolution of symptoms, or plateau in progress as reasons for stopping rehabilitation. (Evidence quality: V; recommendation strength: expert opinion)
C. Action Statement 9: FACTORS THAT MODIFY REHABILITATION OUTCOMES. Clinicians may evaluate factors that could modify rehabilitation outcomes. (Evidence quality: I-III; recommendation strength: weak to strong)
A. Action Statement 10: THE HARM/BENEFIT RATIO FOR VESTIBULAR REHABILITATION IN TERMS OF QUALITY OF LIFE/PSYCHOLOGICAL STRESS. Clinicians should offer vestibular rehabilitation for persons with peripheral vestibular hypofunction. (Evidence quality: I-III; recommendation strength: strong)
These guidelines were issued in 2016 on the basis of the scientific literature published between January 1985 and February 2015. These guidelines will be considered for review in 2020, or sooner if new evidence becomes available. Any updates to the guidelines in the interim period will be noted on the Neurology Section of the APTA website: www.neuropt.org.
INTRODUCTION
Purpose of Clinical Practice Guidelines
The Neurology Section of the American Physical Therapy Association (APTA) supports the development of clinical practice guidelines (CPGs) to assist physical therapists with the treatment of persons with peripheral vestibular hypofunction to optimize rehabilitation outcomes. Generally, the purpose of CPGs is to help clinicians know who, what, how, and when to treat. Specifically, the purpose of this CPG for peripheral vestibular hypofunction is to describe the evidence supporting vestibular rehabilitation including interventions and discharge planning supported by current best evidence. Furthermore, this CPG identifies areas of research that are needed to improve the evidence base for clinical management of peripheral vestibular hypofunction.
This CPG seeks to answer the question of whether vestibular exercises are effective at enhancing recovery of function in people with peripheral vestibular hypofunction. The primary purpose of this CPG is to systematically assess the peer-reviewed literature and make recommendations on the basis of the quality of the research for the treatment of peripheral vestibular hypofunction. A secondary purpose of this CPG is to provide recommendations to reduce unwarranted variation in care and to ensure that exercise interventions provided by physical therapists and other clinicians for vestibular hypofunction are consistent with current best practice. Finally, it is hoped that this CPG will be helpful in developing collaborative relationships among health care providers and thus will serve to reduce unnecessary delays (>1 year in some cases) in referring appropriate patients with vestibular hypofunction for vestibular rehabilitation.2
Background and Need for a Clinical Practice Guideline on Vestibular Rehabilitation in Persons With Peripheral Vestibular Hypofunction
Uncompensated vestibular hypofunction results in postural instability, visual blurring with head movement, and subjective complaints of dizziness and/or imbalance. On the basis of data from the National Health and Nutrition Examination Survey for 2001 to 2004, it is estimated that 35.4% of adults in the United States have vestibular dysfunction requiring medical attention and the incidence increases with age.3 Appropriate treatment is critical because dizziness is a major risk factor for falls: the incidence of falls is greater in individuals with vestibular hypofunction than in healthy individuals of the same age living in the community.4 The direct and indirect medical costs of fall-related injuries are enormous.5,6
The precise incidence and prevalence of peripheral vestibular hypofunction is difficult to ascertain. The reported incidence of vestibular neuritis, a common etiology underlying vestibular hypofunction, is approximately 15 per 100,000 people.7,8 Based on a meta-analysis of published studies, Kroenke et al9 estimated that 9% of the approximately 7 million clinic visits (or 630,000 clinic visits) each year for dizziness are due to vestibular neuritis or labyrinthitis. However, this figure does not include etiologies such as vestibular schwannoma or bilateral vestibular loss and, therefore, underestimates the number of people with peripheral vestibular hypofunction. In the 2008 Balance and Dizziness Supplement to the US National Health Interview Survey, the prevalence of bilateral vestibular hypofunction was reported to be 28 per 100,000 US adults (or 64,046 Americans).10 Of the respondents with bilateral vestibular hypofunction, 44% had changed their driving habits, and approximately 55% reported reduced participation in social activities and difficulties with activities of daily living. Although vestibular dysfunction is less common in children with an estimated prevalence of 0.45%,11 20% to 70% of all children with sensorineural hearing loss also have vestibular loss that may be undiagnosed.12,13
The National Health and Nutrition Examination Survey trial revealed that vestibular dysfunction escalates with increasing age such that nearly 85% of people aged 80 years and more have vestibular dysfunction.3 According to Dillon et al,14 the prevalence of balance (vestibular and sensory loss in feet) impairment in persons older than 70 years is 75%. In addition, people with vestibular disorders were reported to have an 8-fold increase in their risk of falling, which is of concern because of the morbidity and mortality associated with falls.3,5
Persons with bilateral vestibular hypofunction had a 31-fold increase in the odds of falling compared with all respondents. In addition, 25% reported a recent fall-related injury.10 The Centers for Disease Control and Prevention reported the cost of falls in 2000 exceeded $19 billion, and that cost is projected to skyrocket to nearly $55 billion per year by the year 2020.15 Cost-effective treatments that can reduce the risk for falling, can therefore reduce overall health care costs as well as the cost to personal independence and functional decline of patients with vestibular dysfunction.
Therapeutic exercise interventions to address the signs, symptoms, and functional limitations secondary to vestibular deficits have been shown to decrease dizziness, improve postural stability thus reducing fall risk, and improve visual acuity during head movement in individuals with vestibular hypofunction.16–23 A newly-revised Cochrane Database Systematic Review published in 2015 concluded that there is moderate to strong evidence in support of vestibular rehabilitation in the management of patients with unilateral vestibular hypofunction, specifically for reducing symptoms and improving function.24 A recent systematic review concluded that there is moderate evidence to support the effectiveness of vestibular exercises in individuals with bilateral vestibular hypofunction for improving gaze and postural stability.25
At the time of submission, there are no clinical practice guidelines for the treatment of peripheral vestibular hypofunction. The 2015 Cochrane review24 of the treatment of vestibular hypofunction included etiologies such as benign paroxysmal positional vertigo, for which there are already 2 clinical practice guidelines from the American Academy of Neurology26 and the American Academy of Otolaryngology–Head and Neck Surgery Foundation.27 It was determined that a clinical practice guideline to address appropriate vestibular exercise options for use with patients with unilateral and bilateral peripheral vestibular hypofunction was appropriate.
Statement of Intent
This guideline is intended for clinicians, family members, educators, researchers, policy makers, and payers. It is not intended to be construed or to serve as a legal standard of care. As rehabilitation knowledge expands, clinical guidelines are promoted as syntheses of current research and provisional proposals of recommended actions under specific conditions. Standards of care are determined on the basis of all clinical data available for an individual patient/client and are subject to change as knowledge and technology advance, patterns of care evolve, and patient/family values are integrated. This clinical practice guideline is a summary of practice recommendations that are supported with current published literature that has been reviewed by expert practitioners and other stakeholders. These parameters of practice should be considered guidelines only, not mandates. Adherence to them will not ensure a successful outcome in every patient, nor should they be construed as including all proper methods of care or excluding other acceptable methods of care aimed at the same results. The ultimate decision regarding a particular clinical procedure or treatment plan must be made using the clinical data presented by the patient/client/family, the diagnostic and treatment options available, the patient's values, expectations, and preferences, and the clinician's scope of practice and expertise. However, we suggest that significant departures from accepted guidelines should be documented in patient records at the time the relevant clinical decisions are made.
METHODS
The vestibular guideline workgroup (CDH, SJH, SLW) proposed the topic to the APTA and Neurology Section. The topic was accepted and the workgroup attended the APTA Workshop on Developing Clinical Practice Guidelines in July 2012. The workgroup submitted and received 3-year grant funding from the APTA to support guideline development in October 2012. The workgroup solicited members to form an expert multidisciplinary (audiology, neurology, otolaryngology, patient representative, and physical therapy) Advisory Board of people who are actively involved in the management of patients with vestibular dysfunction. The first Advisory Board call took place in January 2013, and 5 subsequent conference calls occurred over the following 2 and a half years. The Advisory Board was intimately involved in the development of the content and scope of the guideline with key questions to be answered, determination of articles for inclusion, and writing/critical edits of the clinical practice guideline.
External Review Process by Stakeholders
Comments were solicited from the Practice Committee for the Neurology Section of the APTA and the public via email blasts to professional organizations (audiology, neurology, otolaryngology, and physical therapy) as well as postings on the Neurology Section and Vestibular Special Interest Group websites at 2 critical junctures during the guideline development. The first call for public comments on the Project Development Plan (the outline of the guideline authors, clinical questions to be answered, terms and databases to be searched, and project timeline) occurred in October 2013. The second call for comments on the complete draft of the clinical practice guideline occurred in April 2015. The second call included solicitation for feedback via email blasts to professional organizations as occurred with the first call. In addition, the second call included solicitation for feedback from consumers via postings on the Vestibular Disorders Association's (VEDA) website, Facebook page, and email blast to all VEDA members. Applicable comments have been incorporated into the final version of the guideline.
Literature Search
A systematic review of the literature was performed by the academic librarians from East Tennessee State University (Nakia Woodward, MSIS, AHIP; Richard Wallace, MSLS, EdD, AHIP), Emory University (Amy Allison, MLS, AHIP), and the University of Pittsburgh (Linda Hartman, MLS, AHIP) in collaboration with the workgroup (CDH, SJH, SLW). The original search included the following 4 databases: PubMed, EMBASE, Web of Science, and Cochrane Library. The subsequent search included the following 4 databases: PubMed, CINAHL, EMBASE, and Cochrane Library. The original PICO question was framed as, “Is exercise effective at enhancing recovery of function in people with peripheral vestibular hypofunction?” The search query in PubMed, CINAHL, EMBASE, and Web of Science combined terms from the concept sets of patient population (peripheral vestibular hypofunction), intervention (exercise), and outcomes (based on the International Classification of Functioning, Disability and Health model) to retrieve all article records that include at least 1 term from each set below (Table 3). The search query for the Cochrane Library included vertigo or vestibular and exercise.
In addition, websites of agencies and organizations that produce guidelines and/or systematic reviews on clinical medicine were searched for relevant publications. These included (1) Canada, Health Evidence; (2) UK, National Institute for Clinical Excellence; (3) United States, Agency for Healthcare Research and Quality; (4) National Guidelines Clearinghouse; and (5) ClinicalTrials.gov. The government agencies and websites produced only duplicates that were removed.
The study types included were meta-analyses, systematic reviews, randomized controlled trials, cohort studies, case control studies, and case series/studies. Inclusion criteria for articles included human subjects, published in English, and published after 1985. Exclusion criteria included superior canal dehiscence, blindness, primary diagnosis of benign paroxysmal positional vertigo, migraine, central vestibular disorder, or central nervous system pathology (Parkinson disease, multiple sclerosis, stroke, cerebellar ataxia).
The initial systematic search was performed in March 2013 and 1540 potential articles were identified (Figure 1A). Identification of relevant studies involved a 3-step process: (1) a title/abstract review during which obviously irrelevant articles were removed; (2) a full-text article review using the inclusion/exclusion criteria; and (3) review article reference lists searched for relevant, missed articles. After duplicates were removed (n = 778), 762 article titles and abstracts were each reviewed by 2 of the 3 members of the workgroup (CDH, SJH, SLW) to exclude obviously irrelevant ones. In the case of disagreement, the third member reviewed the article title and abstract to arbitrate. On the basis of the title and abstract, 13 articles were excluded because of language (not English) and 567 were excluded because of irrelevance to the topic; thus, 182 full-text articles were reviewed. In addition, review article reference lists were searched for relevant, missed articles by a graduate assistant and 13 additional articles were identified. Each full-text article was examined by 2 reviewers from the workgroup and Advisory Board using the inclusion/exclusion criteria. On the basis of the full-text article, 121 articles were identified as relevant to the CPG.
A follow-up literature search following the same strategy was performed in February of 2015, and 573 articles were identified. After duplicates were removed (n = 34), 539 article titles and abstracts were each reviewed by 2 members of the workgroup to exclude obviously irrelevant articles. On the basis of the title and abstract, 16 articles were excluded because of language (not English) and 499 were excluded because of irrelevance to the topic; thus, 24 full-text articles were reviewed. On the basis of the full-text article, 14 articles were identified as relevant to the CPG.
Critical Appraisal Process
Each intervention article was critically appraised using an electronic appraisal form based on key questions adapted from Fetters and Tilson.1 Critical appraisal scores based on these key questions regarding methodological rigor of the research design, study execution, and reporting have also been used by other groups in the development of clinical practice guidelines.28 Levels of evidence were determined using criteria from the Centre for Evidence-Based Medicine for intervention studies (Table 1), with the additional criteria that levels I and II are differentiated based on the critical appraisal score. Level I studies received a critical appraisal score of at least 50% and level II studies received critical appraisal scores less than 50%.
Volunteers were recruited from the Neurology Section and Vestibular Special Interest Group using an online “Call for Volunteers” to provide critical appraisals of the articles identified as being relevant to this clinical practice guideline. Two face-to-face training sessions (4 hours at the APTA Combined Section Meeting in 2013 and 2 hours at the Combined Section Meeting in 2014) were provided by the workgroup to the volunteers before performance of any critical appraisals. Selected intervention articles were critically appraised by the workgroup to establish the test standards. Volunteers performed 2 practice critical appraisals and were compared with scoring of the workgroup. Volunteers were considered to be qualified to review with 80% or more agreement with the workgroup. Critical appraisals and study characteristics extractions from each article were performed by 2 reviewers and the information entered into an electronic data extraction form. Discrepancies in scoring were discussed and resolved by the 2 reviewers. In situations that a score could not be agreed upon, the disagreement was resolved by consensus among the workgroup.
Diagnostic Considerations
The focus of this clinical practice guideline is on the treatment of peripheral vestibular hypofunction; thus, studies where the patient group involved primarily central involvement (eg, traumatic brain injury, concussion, multiple sclerosis, and Parkinson disease) were excluded. Studies in which the patient group involved primarily benign paroxysmal positional vertigo were excluded, whereas studies that included individuals with benign paroxysmal positional vertigo in addition to peripheral vestibular hypofunction were included. Specific diagnoses such as Meniere disease (for diagnostic criteria, see Lopez-Escamez et al29) or vestibular neuritis were included, but were not part of the search strategy because the patient population of interest was persons with peripheral vestibular hypofunction regardless of the etiology. For purposes of this guideline, acute is defined as the first 2 weeks after the onset of symptoms, subacute as after the first 2 weeks and up to 3 months after the onset of symptoms, and chronic as the presence of symptoms longer than 3 months.
Diagnostic Criteria for Vestibular Hypofunction
Diagnosis of peripheral vestibular hypofunction had to have been confirmed with vestibular function laboratory testing for an article to be included in this clinical practice guideline. Either caloric or rotational chair testing was used for diagnostic purposes. Unilateral vestibular hypofunction was determined by responses to bithermal air or water caloric irrigations, with at least 25% reduced vestibular responses on one side.30–32 Jongkees33 described the formula, which is typically used to calculate right-left asymmetry with caloric testing. Although caloric asymmetry is abnormal in persons with unilateral loss, saccades and smooth-pursuit eye movements are normal and therefore are not included in the diagnostic criteria.31 Rotational chair data on gain, asymmetry, and phase have been used to test the vestibulo-ocular system at higher frequencies up to 1.0 Hz and are utilized to diagnose bilateral vestibular hypofunction.22
Treatment Approach
The primary approach to the management of patients with peripheral vestibular hypofunction is exercise-based. Whereas management of the patient in the acute stage after vestibular neuritis or labyrinthitis may include medications, such as vestibular suppressants or antiemetics, the evidence does not support medication use for management of the chronic patient.21 A surgical or ablative approach is limited to patients who have recurrent vertigo or fluctuating vestibular function and symptoms that cannot be controlled by other methods, such as lifestyle modifications or medication. The goal of the ablative approach is to convert a fluctuating deficit into a stable deficit to facilitate central vestibular compensation for unilateral vestibular hypofunction.34
The original vestibular exercises were developed by Cawthorne and Cooksey in the 1940s.35 Cawthorne-Cooksey exercises are a general approach to vestibular rehabilitation and include a standardized series of exercises that involve a progression of eye movements only, head movements with eyes open or closed, bending over, sit-stand, tossing a ball, and walking.
Current vestibular rehabilitation is an exercise-based approach that typically includes a combination of 4 different exercise components to address the impairments and functional limitations identified during evaluation: (1) exercises to promote gaze stability (gaze stability exercises), (2) exercises to habituate symptoms (habituation exercises) including optokinetic exercises, (3) exercises to improve balance and gait (balance and gait training), and (4) walking for endurance.
Gaze stability exercises were developed on the basis of the concepts of vestibulo-ocular reflex adaptation and substitution (and are commonly referred to as adaptation exercises and substitution exercises). In the vestibular literature, adaptation has referred to long-term changes in the neuronal response to head movements with the goal of reducing symptoms and normalizing gaze and postural stability. Gaze stability exercises based on the assumption that they promote vestibular adaptation involve head movement while maintaining focus on a target, which may be stationary or moving. Gaze stability exercises based on the principles of substitution were developed with the goal of promoting alternative strategies (eg, smooth-pursuit eye movements or central pre-programming of eye movements) to substitute for missing vestibular function. For example, during active eye-head exercise between targets, a large eye movement to a target is made before the head moving to face the target, potentially facilitating use of preprogrammed eye movements. Both adaptation and substitution exercises are performed with head movements in the horizontal and vertical planes.
In the vestibular literature, habituation has referred to the reduction in a behavioral response to repeated exposure to a provocative stimulus, with the goal of reducing symptoms related to the vestibular system. Habituation exercises are chosen on the basis of particular movements or situations (eg, busy visual environments) that provoke symptoms. In this approach the individual performs several repetitions of the body or visual motions that cause mild to moderate symptoms. Habituation involves repeated exposure to the specific stimulus that provokes dizziness, and this systematic repetition of provocative movements leads to a reduction in symptoms over time. More recent approaches involve the use of optokinetic stimuli or virtual reality environments as habituation exercises. Optokinetic stimuli involve the use of repetitive moving patterns and virtual reality immerses patients in realistic, visually challenging environments; both are used to address visual motion sensitivity (also known as visual vertigo, space and motion discomfort, or visually-induced dizziness). Both approaches use stimuli that can be graded in intensity through manipulation of stimulus parameters such as velocity, direction of stimulus motion, size/color of stimulus, and instructions to the participant. The stimulus may be provided via high-tech equipment, such as optokinetic discs, moving rooms or virtual reality, or lower tech equipment, such as busy screen savers on a computer or videos of busy visual environments.
Balance and gait training under challenging sensory and dynamic conditions is typically included as part of vestibular rehabilitation. These exercises are intended to facilitate use of visual and/or somatosensory cues to substitute for missing vestibular function. Balance exercises include balancing under conditions of altered visual (eg, vision distracted or removed) and/or somatosensory input (eg, foam or moving surfaces) and may involve changes in the base of support (eg, Romberg, tandem, single-leg stance) to increase the challenge. Weight shifting in stance is used to improve center of gravity control and balance recovery. Gait exercises involve dynamic conditions and may include walking with head turns or performing a secondary task while walking. Equipment is available that can augment balance and gait training such as gaming technology, optokinetic drums, and virtual reality systems.
General conditioning, such as walking for endurance or aerobic exercise, is frequently an element of rehabilitation because people with peripheral vestibular dysfunction often limit physical activity to avoid symptom provocation. General conditioning exercise (eg, stationary bicycle) by itself has not been found to be beneficial in patients with vestibular hypofunction.21,22
Outcome Measures
A variety of outcome measures have been utilized to assess the impact of vestibular dysfunction; however, there is no consensus as to what aspects of function should be measured. An international group of investigators and health care providers developed a core set of key aspects of functioning that should be measured in the assessment of patients with vertigo, dizziness, and imbalance.36 The Brief Core Set is a short list of categories and is the minimal standard for assessment and description of functioning and disability. As such, there may be aspects of functioning that are relevant to a specific individual but are not included in the Brief Core Set. The Brief Core Set for vertigo includes both subjective complaints and physical function and has been organized on the basis of the International Classification of Functioning, Disability and Health (ICF) model (Table 4). The specific domains of the ICF model include (1) body function and structure (body level); (2) activity (individual level); and (3) participation (societal level). In addition, the ICF model considers personal and environmental contributions.
Recommendations for specific rehabilitation outcome measures to be used in the assessment of individuals with vestibular dysfunction have been made by the Vestibular Evidence Database to Guide Effectiveness task force. They used a modified Delphi process to identify and select recommended measures. The vestibular outcome measure recommendations are available online at http://www.neuropt.org/professional-resources/neurology-section-outcome-measures-recommendations/vestibular-disorders. We provide a summary of recommended measures categorized according to the ICF model (Table 5).
ACTION STATEMENTS AND RESEARCH RECOMMENDATIONS
Here, we present each action statement followed by a standardized information profile and then the supporting evidence for the statement. Recommendations for research are also included.
A. Action Statement 1: EFFECTIVENESS OF VESTIBULAR REHABILITATION IN PERSONS WITH ACUTE AND SUBACUTE UNILATERAL VESTIBULAR HYPOFUNCTION. Clinicians should offer vestibular rehabilitation to patients with acute or subacute unilateral vestibular hypofunction. (Evidence quality: I; recommendation strength: strong)
Action Statement Profile
Aggregate evidence quality: Level I. Based on 5 level I randomized controlled trials and 4 level II randomized controlled trials.
Benefits: Improved outcomes in patients receiving vestibular rehabilitation when compared with controls given either no exercise or sham exercises.
-
Risk, harm, and cost:
Increased cost and time spent traveling associated with supervised vestibular rehabilitation.
Increase in symptom intensity at the onset of treatment.
-
Benefit-harm assessment:
Preponderance of benefit.
-
Value judgments:
Early initiation of vestibular rehabilitation ensures shorter episodes of care, higher levels of recovery of balance function, reduced symptom complaints, improved functional recovery to activities of daily living, reduced fall risk, and improved quality of life.
-
Role of patient preferences:
Cost and availability of patient time and transportation may play a role.
-
Exclusions:
Individuals who have already compensated sufficiently to the vestibular loss and no longer experience symptoms or gait and balance impairments do not need formal vestibular rehabilitation. For example, people who resume their customary sporting or physical activities may compensate quickly so that they do not need vestibular rehabilitation and when evaluated by a physical therapist have normal test results.
Possible exclusions also include active Meniere disease or those with impairment of cognitive or general mobility function that precludes adequate learning and carryover or otherwise impedes meaningful application of therapy.
Supporting Evidence and Clinical Interpretation
Acute unilateral vestibular hypofunction is the most common cause of acute spontaneous vertigo.37,38 Acute unilateral vestibular hypofunction is most commonly due to vestibular neuritis but may also be due to trauma, surgical transection, ototoxic medication, Meniere disease, or other lesions of the vestibulocochlear nerve or labyrinth. The acute asymmetry results in imbalance in vestibular tone that manifests with vertigo, nausea, and unsteadiness of gait as well as spontaneous nystagmus with the fast component beating away from the dysfunctional ear. Although nystagmus and vertigo usually subside within hours to 14 days, imbalance and the sensation of dizziness, especially during head movement may persist for many months, or longer, resulting in a more chronic syndrome. Vestibular exercises have been used in recent years as a means of aiding patients to make a more speedy and thorough recovery.
Strong evidence indicates that vestibular rehabilitation provides clear and substantial benefit to patients with acute or subacute unilateral vestibular hypofunction, so, with the exception of extenuating circumstances, vestibular rehabilitation should be offered to patients who are still experiencing symptoms (eg, dizziness, dysequilibrium, motion sensitivity, and oscillopsia) or imbalance due to unilateral vestibular hypofunction. Two level I studies examined the effects of vestibular rehabilitation solely within the acute/subacute stage after resection of vestibular schwannoma. In the first level I study, patients scheduled for resection were randomly assigned to an exercise group (vestibular, n = 11, or control, n = 8).18 Exercises were started 3 days after resection of the vestibular schwannomas and continued until the patients were discharged from the hospital (average = postoperative day 6). The vestibular group performed gaze stabilization exercises for 1 minute each 5 times per day for a maximum of 10 to 20 minutes per day. The control group performed vertical and horizontal smooth-pursuit eye movements against a featureless background on the same schedule. Patients in both groups walked at least once each day. The vestibular group was older (mean age 59 years vs 48 years in controls, (P < 0.04), but otherwise the groups were similar. Both groups reported significantly more dizziness after surgery than before (P < 0.05) and had more postural sway on postoperative day 3 than preoperatively (P < 0.05). By days 5 and 6, the vestibular group reported less subjective disequilibrium compared with the control group (P < 0.05). Some posturographic measures improved more in the vestibular group compared with the control group on postoperative day 6, and more patients in the vestibular group were able to walk with head turns without staggering than in the control group. This study has several limitations: (1) no allocation concealment, (2) a relatively small number of subjects, and (3) it was assumed that patients developed acute unilateral vestibular hypofunction from surgery but this is not known. Some of the patients may have had a progressive loss of vestibular function over the years, with the growth of the tumor, and had adapted, and as such did not experience an acute loss postoperatively.
The second study examined the effectiveness of gaze stabilization exercises started after vestibular schwannoma surgery to reduce patients' perception of dizziness/imbalance.16 In this level I study, subjects were randomized into a vestibular exercise group who performed gaze stability and balance exercises (n = 30) or a control group who did not perform any exercises (n = 27). Patients were assigned to a group on the basis of a sequentially randomized design (the first part of the study was the control group, and the second part of the study was the vestibular exercise group). Patients in the vestibular exercise group performed gaze stabilization exercises starting on the third postoperative day. Each exercise was performed for 1 minute, 4 or 5 times each day. The exercises were initially performed while lying down or seated and were then performed while standing. Patients were reassessed for the first time at 2 to 3 weeks after surgery. The main finding was that there was less dizziness in the vestibular exercise group, based on the scores of the Dizziness Handicap Inventory, compared with the control group at 2 to 3 weeks, 6 to 7 weeks, and 10 to 12 weeks postoperatively. Secondary findings showed no difference between groups in spontaneous nystagmus, subjective complaints of vertigo, and vestibular asymmetry when measured over the 12-week course of the study.
Mruzek et al39 found that a course of vestibular exercises after unilateral vestibular ablation in patients with vestibular schwannoma or Meniere disease was beneficial in reducing symptom intensity and disability compared with a control group. In this level I study, they examined patients at postoperative day 5 and then 2, 5, and 7 weeks after surgery. Subjects were randomized into 3 groups: (1) vestibular exercises + social reinforcement, (2) vestibular exercises alone, and (3) a control group who performed range of motion exercises + social reinforcement. All interventions lasted 8 weeks. Vestibular exercises were initiated on postoperative day 5 and consisted of habituation exercises, based on the results of the Motion Sensitivity Test and Cawthorne-Cooksey exercises. The control group performed range of motion exercises. Social reinforcement consisted of periodic phone calls to urge adherence and encourage and praise the patients. They found that all patients improved in the Motion Sensitivity Test, computerized dynamic posturography, and Dizziness Handicap Inventory scores, but the patients who performed the vestibular exercises had significantly less motion sensitivity (groups 1 and 2) and had better (lower) scores on the physical subscale of the Dizziness Handicap Inventory (group 1) at 8 weeks after surgery than the control group (group 3).
Another study also started vestibular exercises in patients after vestibular schwannoma surgery 3 to 5 days postoperatively.40 In this level I study, patients were randomized (with allocation concealment) to 12 weeks of vestibular exercises (n = 16 younger, n = 15 older defined as older than 50 years) or to a control group (n = 11 younger, n = 11 older). There were no differences in tumor sizes or mean caloric asymmetry between the groups preoperatively. Vestibular exercises included supervised gaze stabilization exercises, walking, narrow-based walking with head turning, and treadmill training for a total of 4 sessions with a home exercise program 3 times per day. The control group was told to walk, read, and watch TV while in the hospital and then told to gradually increase their activity level once at home. There were no differences in balance measures between groups during the acute/subacute phase except for tandem gait, which was better in the vestibular exercise group. However, when only older subjects were considered, static balance, Timed Up and Go, and tandem gait were better in those who received vestibular exercises than in controls (P < 0.05). At 9 to 12 weeks, older subjects who received vestibular exercises were better on static balance, Timed Up and Go, tandem walk and the Dynamic Gait Index. This study found essentially no benefit in vestibular exercises compared with general instructions in those younger than 50 years. This study's limitations include a minimal period of supervised vestibular exercises (4 supervised sessions over 12 weeks).
In the final level I study, comparisons were made between patients with acute unilateral vestibular hypofunction treated with a course of Nintendo Wii Fit Balance Board balance exercises (n = 37) and a control group (n = 34).41 They examined patients on the second day after admission for vestibular neuritis and then randomly assigned the patients to the groups. The Wii exercise group performed a customized program of 5 to 6 exercises for a total of 45 minutes. The program consisted of 10 training sessions, partitioned in 2 daily sessions for 5 consecutive days. The control group performed only 1 session consisting of 2 exercises (the “1-leg figure” and the vendor-specific training test to calculate the “virtual fitness age”) for a total time of 5 minutes. Patients were reassessed on day 5 of treatment and after 10 weeks. Outcome measure included performance on 16 different exercises performed by the Wii group during the 5 days of the study, Sensory Organization Test, the Dizziness Handicap Inventory, Vertigo Symptom Scale, and the Falls Efficacy Scale. There were no differences in age, sex, or symptom duration between groups. Results showed that patients in the control group required 2.4 days (standard deviation = 0.4) longer hospitalization on average than patients after early rehabilitation with the Wii balance board. In addition, an absence of nystagmus was observed 2.1 days (standard deviation = 0.5) earlier in the exercise group than in the control group. At both day 5 and 10 weeks after exercise, the exercise group showed significantly better results in the Sensory Organization Test, Dizziness Handicap Inventory, Vertigo Symptom Scale, and Falls Efficacy Scale than the control group (P < 0.05). The authors concluded that the early use of a visual feedback system (Nintendo Wii Balance Board) for balance training facilitated recovery of balance and symptoms in patients with acute unilateral vestibular hypofunction. Although this study received a level I rating using our criteria, there are several limitations that temper this rating: (1) use of the same exercises performed by the exercise group as an outcome measure; (2) although the authors conclude that the Vertigo Symptom Scale improved only in the exercise group, they provided no data to support this; (3) a level of significance of alpha less than 0.05 was set, but no adjustment was made for multiple comparisons, so the potential for type I error is greater; (4) they do not account for all the subjects recruited or enrolled in the study.
Several level II studies also support the use of vestibular exercises in the treatment of patients with acute or subacute unilateral vestibular hypofunction. Strupp et al42 conducted a randomized controlled trial in which patients were randomized to a vestibular (n = 19) or a control group (n = 20). The control group was given no particular exercises; however, both groups were encouraged to engage in regular daily activities, such as walking to the bathroom and sitting up for meals. The vestibular group performed gaze stabilization exercises as well as static and dynamic balance exercises, which included head movement. The primary outcome was postural stability with eyes closed on foam as measured by sway path velocity. In general, both groups improved in postural stability across time; however, at the assessment 30 days after symptom onset, the vestibular group was significantly more stable compared with the control group (P < 0.001). They found no differences between groups in the recovery of signs and symptoms related to the tonic vestibular system (eg, ocular torsion and subjective visual vertical). This study shows that vestibular exercises administered early after onset of unilateral vestibular hypofunction result in improvement in sway and balance by day 30 after onset but that, as expected, problems that affect the tonic vestibular system recover with or without vestibular exercises.
A second level II study assessed 87 patients with at least 1 vertigo spell and 2 abnormal tests (Romberg, Fukuda Stepping Test, head shaking nystagmus, or spontaneous nystagmus) within 5 days of study enrollment.43 They excluded those with vestibular symptoms in the prior 6 months or those with benign paroxysmal positional vertigo. Patients were randomized and blinded to group: the vestibular group (n = 45) was given supervised gaze stability exercises performed with horizontal and vertical head movements for 1 minute 3 times per day for 21 days. The control group (n = 42) did gaze fixation without head movement while blinking their eyes 3 times per day for 21 days. By 10 days, the vestibular group showed significant improvement in Romberg, Fukuda Stepping Test, spontaneous nystagmus, and post head-shaking-induced nystagmus compared with the control group. Most patients in the vestibular group improved in the timeframe of 3 to 10 days compared with controls, but by 3 weeks the differences between the groups began to diminish.
A level II study by Marioni et al44 enrolled 30 patients starting 2 weeks after acute unilateral vestibular hypofunction: patients were randomized (no mention of allocation concealment) to posturography-assisted vestibular exercises + a home exercise program (n = 15) or to a control group (n = 15) that did no particular exercises. The vestibular group performed supervised vestibular exercises during 30-minute sessions once a week plus a home exercise program three times per day for 5 weeks. The vestibular group improved in static balance with eyes open on foam (P = 0.02) and eyes closed on foam conditions (P = 0.00004), whereas the control group only improved with eyes closed on foam conditions (P = 0.03). At 6 weeks, sway velocity with eyes open on foam (P = 0.03) and eyes closed on foam conditions (P = 0.000001) was better in treated than untreated subjects. This study demonstrates improvement in computerized posturography measures such as postural sway velocity when vestibular exercises are administered starting 2 weeks after a significant (defined as >50% asymmetry) unilateral vestibular hypofunction.
A level II study by Teggi et al45 examined the effect of vestibular exercises on patients hospitalized with acute vestibular neuritis. Patients were randomly assigned to either a vestibular or control group. The vestibular group (n = 20) underwent a total of 10 sessions of rehabilitation consisting of balance exercises on a force platform using both visual feedback and an optokinetic stimulus. They also performed gaze stability exercises and a subset of Cawthorne-Cooksey exercises. The control group was told only to “perform their daily activities.” Outcome measures included a sway path analysis of stance with eyes open and eyes closed, Dynamic Gait Index, Dizziness Handicap Inventory, and a Visual Analog Scale for anxiety, at baseline, and after 25 days. There was a significant difference in the Dizziness Handicap Inventory total scores (P < 0.002) and anxiety scores (P < 0.001) between the 2 groups, with the vestibular group showing more improvement than the control group; there was no significant difference in the Dynamic Gait Index scores between the groups.
Three level III retrospective studies introduced a new concept of rehabilitation for patients scheduled for vestibular ablation, either for vestibular schwannoma or Meniere disease.46–48 These studies advocate for treating the patients with a combination of intratympanic gentamicin to induce further loss of vestibular function and vestibular exercises to induce vestibular compensation before surgery. They report that patients undergoing this “pre-hab” had faster recovery of symptoms and balance after surgery. Further research is needed, however, to determine whether there is a significant difference in the rate and level of recovery with pre-hab compared with a control group who receives only postoperative rehabilitation.
R. Research Recommendation 1: Researchers should examine the concept of a critical period for optimal vestibular compensation through studies that examine early versus delayed intervention. Researchers should identify factors that predict which patients will recover without the benefit of vestibular rehabilitation and which patients will need vestibular rehabilitation to optimize outcomes.
A. Action Statement 2: EFFECTIVENESS OF VESTIBULAR REHABILITATION IN PERSONS WITH CHRONIC UNILATERAL VESTIBULAR HYPOFUNCTION. Clinicians should offer vestibular rehabilitation to patients with chronic unilateral vestibular hypofunction. (Evidence quality: I; recommendation strength: strong)
Action Statement Profile
Aggregate evidence quality: Level I. Based on 3 level I and 1 level II randomized controlled trials.
Benefits: Improved outcomes in patients receiving vestibular rehabilitation when compared with controls given either no exercise or sham exercises.
-
Risk, harm, and cost:
Increased cost and time spent traveling associated with supervised vestibular rehabilitation.
-
Benefit-harm assessment:
Preponderance of benefit.
-
Value judgments:
Importance of optimizing and accelerating recovery of balance function and decreasing distress, improving functional recovery to activities of daily living, and reducing fall risk.
-
Role of patient preferences:
Cost and availability of patient time and transportation may play a role.
-
Exclusions:
Individuals who have already compensated sufficiently to their vestibular loss and no longer experience symptoms or gait and balance impairments do not need formal vestibular rehabilitation.
Possible additional exclusions include active Meniere disease or those with impairment of cognitive or general mobility function that precludes adequate learning and carryover or otherwise impedes meaningful application of therapy.
Supporting Evidence and Clinical Interpretation
Strong evidence indicates that vestibular rehabilitation provides clear and substantial benefit to patients with chronic unilateral vestibular hypofunction. Therefore, with the exception of extenuating circumstances, vestibular rehabilitation should be offered to patients who are still experiencing symptoms (eg, dizziness, dysequilibrium, motion sensitivity, and oscillopsia) or imbalance because of unilateral vestibular hypofunction.
A level I randomized controlled trial studied 21 patients with chronic unilateral vestibular hypofunction (based on caloric testing) of 2 weeks to 3 years of duration who also had impairment of Dynamic Visual Acuity as well as a measure of severity of oscillopsia (Visual Analog Scale).19 Patients were randomized to vestibular (n = 13) versus placebo exercises (n = 8). The vestibular exercises included adaptation and substitution exercises to improve gaze stability, whereas the placebo exercises involved saccadic eye movements against a Ganzfeld (a large featureless surface) with head stationary. Vestibular and placebo exercises were performed 4 to 5 times per day for 20 to 30 minutes plus 20 minutes of balance and gait exercises daily with individual programs adjusted as needed. Patients were seen once a week in the clinic for 4 weeks and adherence was monitored. The vestibular exercise group showed improvement in Dynamic Visual Acuity (P < 0.001) with 12 of the 13 returned to normal, whereas no change in Dynamic Visual Acuity was seen in the control group and no control subject returned to normal. Thus, vestibular exercises facilitate recovery of gaze stability as measured by Dynamic Visual Acuity. There was no indication of failure to improve on the basis of age, and improvement was seen even if exercises were administered 12 months after symptom onset. Improvement in Dynamic Visual Acuity did not correlate with improvement in oscillopsia measured by the Visual Analog Scale.
In a level I randomized controlled trial, Loader et al49 studied 24 patients with chronic unilateral vestibular hypofunction who were randomly assigned to either a treatment group (n = 12, exposure to optokinetic stimuli while standing) or a control group (n = 12, no treatment). The outcome consisted of a measure of postural stability in stance (Sensory Organization Test). The treatment group was required to read randomly presented texts while standing. Patients attended 10 treatment sessions over a 3-week period, with each session lasting approximately 30 minutes. The control group only had their balance tested before and after a 3-week period. Neither group performed a home exercise program. There were no differences between groups before the initiation of treatment, but after the 3-week intervention period, the treatment group had significantly better postural stability. Two limitations of the study are that there is a difference in how the 2 groups were treated (the control group having limited contact with the therapists) and that the treatment group practiced standing balance, which is closely related to the outcome measure, whereas the control group did not.
In another level I randomized controlled trial study, Giray et al50 examined 41 patients with chronic vestibular dysfunction treated with vestibular rehabilitation for 4 weeks (n = 20) versus a no-treatment control group (n = 21). Interestingly, the ratio of male to female was 11:2. They specifically excluded patients with benign paroxysmal positional vertigo and Meniere disease or any orthopedic or neurological comorbid condition that would confound recovery. All participants had chronic uncompensated unilateral vestibular hypofunction based on caloric testing. No mention was made of allocation concealment in the randomization process. Patients were seen in the clinic twice per week for 4 weeks for 30 to 45 minutes and monitored for adherence. Between supervised sessions, patients did a twice-daily home exercise program for a total of 30 to 40 minutes per day. The home exercise program included a combination of adaptation (without and with target moving in pitch and yaw planes for 1 minute each for 3 times per day), substitution, habituation, and balance exercises. The vestibular rehabilitation group made improvements from pre and posttreatment in all measures, including disequilibrium on the basis of the Visual Analog Scale (P < 0.003), Dizziness Handicap Inventory (P < 0.001), Berg Balance Scale (P < 0.013), and Modified Clinical Test for Sensory Interaction on Balance (P < 0.004), whereas the control group did not change in any of the measures. Furthermore, there were significant differences (P < 0.05) in change scores of all measures for the vestibular rehabilitation group compared with the control group.
Enticott et al16 reported, in their level II study, that on average, all subjects significantly improved pre- to posttherapy for the Dizziness Handicap Inventory and Activities-Specific Confidence Scale (P < 0.05). However, the experimental group (vestibular exercises) improved to a greater extent than the control group (strength and endurance exercises) on the Dizziness Handicap Inventory and Activities-Specific Balance Confidence (P < 0.05). On average, all subjects significantly improved pre- to posttherapy for tandem walk, step test, tandem stance, and single-leg stance test (P < 0.05). The experimental group improved to a greater extent than the control group on the tandem walk, step tests, and posturography on foam and eyes closed conditions (P < 0.05). Limitations of the study include no blinding and that some patients had other vestibular disorders in addition to unilateral vestibular hypofunction. Nine subjects had vestibular migraine. Three subjects had benign paroxysmal positional vertigo, which initially had not resolved, but had resolved by the end of the study.
Finally, although not a traditional randomized controlled trial, Shepard and Telian51 provide support specifically for the use of habituation exercises. In this level III study of patients with chronic vestibular deficits, Shepard and Telian compared the efficacy of customized vestibular exercise programs with a more generic exercise program using a delayed treatment paradigm. Subjects first were assessed to establish a baseline and identify specific deficits-related motion-provoked symptoms or balance and gait impairments and then re-assessed at 1 month before initiating any exercises. This delayed treatment model served as a control for spontaneous recovery. Subjects who had not shown spontaneous recovery were then stratified by age and by pretreatment disability to receive a customized or generic exercise program. The customized program included habituation exercises for motion-provoked or positional sensitivity and balance and gait retraining. The generic exercise program consisted of 1 active head movement, a Dix-Hallpike movement with head in neutral position, 1 balance exercise and graded walking. After 3 months of therapy, only the vestibular rehabilitation group showed a significant reduction in dizziness during routine daily activities. The vestibular rehabilitation group also showed a significant improvement on both static and dynamic posturography, a reduction in motion sensitivity, and a decrease in asymmetry of vestibular function. The generic exercise group improved only in their performance of static balance tests.
Several other treatment modalities have been explored as possible interventions for patients with unilateral vestibular hypofunction. In a level III study, Verdecchia et al52 present the results from a cohort of 69 patients with chronic unilateral vestibular hypofunction. All patients performed a vestibular rehabilitation program of gaze stability, balance, and gait exercises to which the complementary use of video game equipment (Wii) was added. Outcome measures included the perception of handicap, fall risk, and gaze stability (clinical Dynamic Visual Acuity). As a group, patients improved significantly in all measures (P < 0.0001). Aquatic physiotherapy may also be beneficial for people with chronic unilateral vestibular hypofunction.53 In one study, patients performed 10 sessions of aquatic physiotherapy consisting of eye, head, and body movements that stimulate the vestibular system and other systems involved in body balance, which frequently generate dizziness in patients with unilateral vestibular hypofunction. As a group, patients had lower Brazilian Dizziness Handicap Inventory total scores, lower intensity of dizziness, and better postural stability after aquatic physiotherapy. They found no association between age, time since symptom onset, and use of antivertigo medication with rehabilitation outcomes.
A. Action Statement 3: EFFECTIVENESS OF VESTIBULAR REHABILITATION IN PERSONS WITH BILATERAL VESTIBULAR HYPOFUNCTION. Clinicians should offer vestibular rehabilitation to patients with bilateral vestibular hypofunction. (Evidence quality: I; recommendation strength: strong)
Action Statement Profile
Aggregate evidence quality: Level I. Based on 4 level I randomized controlled trials.
Benefits: Improved function and decreased symptoms in patients receiving vestibular rehabilitation when compared with controls given sham exercises.
-
Risk, harm, and cost:
Increased symptom intensity and imbalance when performing the exercises.
Increased cost and time spent traveling associated with supervised vestibular rehabilitation.
-
Benefit-harm assessment:
Preponderance of benefit.
-
Value judgments:
Benefit of gaze stability and balance exercises in patients with bilateral vestibular hypofunction has been demonstrated in level I studies. However, the number of subjects in these studies was small (with the exception of one study) and the outcome measures utilized were variable.
-
Role of patient preferences:
Cost and availability of patient time and transportation may play a role.
-
Exclusions:
Possible exclusions include impairment of cognitive or general mobility function that precludes adequate learning and carryover or otherwise impedes meaningful application of therapy.
Supporting Evidence and Clinical Interpretation
Strong evidence indicates that vestibular rehabilitation provides clear and substantial benefit to patients with bilateral vestibular hypofunction, so with the exception of extenuating circumstances vestibular rehabilitation should be offered to patients who are still experiencing symptoms (eg, dizziness, dysequilibrium, and oscillopsia) or imbalance because of bilateral vestibular hypofunction. Four level I, randomized controlled trials assessed the effectiveness of vestibular exercises in individuals with bilateral vestibular hypofunction. Herdman et al20 examined the influence of gaze stability exercises (a combination of adaptation and substitution exercises) as compared with a vestibular-neutral placebo treatment (saccadic eye movements without head movement against a Ganzfeld) on Dynamic Visual Acuity in 13 patients with bilateral vestibular hypofunction. All participants were seen weekly in the clinic by a physical therapist and were instructed to perform the home exercise program of eye exercises (either gaze stability or saccadic eye movements) 4 to 5 times per day for a total of 20 to 40 minutes. All participants performed balance and gait exercises as part of a home exercise program for 20 minutes per day. As a group, the individuals performing the gaze stability exercises demonstrated an improvement in their Dynamic Visual Acuity as compared with the placebo group.
A level I study by Krebs et al22 examined 8 individuals with bilateral vestibular hypofunction who performed either an exercise program consisting of gaze stability exercises and balance and gait activities or a placebo exercise program. The vestibular exercises involved a staged progression of gaze stability, balance, and gait exercises (eg, phase I—gaze stability with fixed target and slow head movement; phase II—gaze stability with fixed target and fast head movement; phase III—gaze stability with moving target and fast head movement). Participants were seen for weekly outpatient physical therapy visits and were instructed to perform the home exercise program 1 to 2 times per day for 8 weeks. The group performing the vestibular exercises demonstrated increased gait speed and postural stability, as compared with those who performed a placebo exercise program of progressive isometric exercises. Both groups demonstrated improvements in Dizziness Handicap Inventory scores; however, there were no differences between the experimental and control groups in improvement in perceived disability.
There is one additional level I randomized controlled trial that included a significant proportion of individuals with bilateral vestibular hypofunction (53 of the 86) who completed 12 weeks of vestibular rehabilitation.54 On the basis of improved gait biomechanics (preferred gait speed, decreased double support time, and decreased vertical center of mass excursion), Krebs and colleagues determined that patients with vestibular hypofunction benefitted from vestibular rehabilitation as compared with a placebo control group. As described previously, vestibular rehabilitation included a staged progression of gaze stability, balance, and gait retraining exercises.22 Participants were seen for 6 weeks of supervised visits and were instructed to perform a home exercise program at least once per day and 5 times per week for an additional 6 weeks. Patients with unilateral and bilateral vestibular hypofunction benefitted equally from vestibular rehabilitation. Although the unilateral vestibular hypofunction group had more stable and faster gait characteristics at baseline than the bilateral vestibular hypofunction group, both groups' gait characteristics improved significantly with rehabilitation.54
Rine et al55 used a similar intervention approach as that described by Krebs and colleagues22 but modified it for children's motor abilities, attention span, and motivational factors. The investigators reported a significant improvement in motor development scores and a trend toward improvement in sensory organization test scores in the treatment group as compared with the placebo group. This study by Rine and colleagues is the only experimental study in children in which vestibular dysfunction was confirmed by laboratory tests. The results suggest that children with bilateral peripheral vestibular dysfunction respond similarly to adults to vestibular rehabilitation, although more research is needed. The difference in vestibular rehabilitation provided to the children was that it was delivered in the form of games to engage the children. Together, these level I studies provide strong support for the use of vestibular rehabilitation in patients with bilateral vestibular hypofunction to improve gaze and postural stability.
There are 5 level III and IV studies that have examined change with vestibular rehabilitation using a variety of outcomes.56–60 Patten et al56 (level III) found that individuals with bilateral vestibular hypofunction improved in coordinated head-trunk control after vestibular rehabilitation although no change in preferred gait speed was noted. Gillespie and Minor57 (level III), using retrospective chart review, identified 35 patients with confirmed bilateral vestibular hypofunction on the basis of clinical, caloric, and rotary chair testing. The majority of patients (32 of the 35) underwent vestibular rehabilitation that included gaze stability exercises (adaptation and substitution) as well as gait and balance exercises. Patients were instructed to perform gaze stability exercises at least 3 times per day. Outcome measures included Dynamic Visual Acuity, static balance in Romberg, and gait speed as well as subjective measures of symptoms. Half of the patients improved with vestibular rehabilitation. Improvement was defined as normalization of at least 2 of the 3 measures. The group that did not improve had more comorbidities (2.5) than the group that did improve (1.7), and having 4 or more comorbidities was associated with poorer outcomes. Taken together, these studies demonstrate improvements in measures of gaze stability, static postural stability, gait, and symptoms. However, it is apparent from these studies that not all individuals improved, individuals did not improve on all measures, and there was a great deal of variability in outcome measures.
R. Research Recommendation 2: With the advent of new diagnostic tools, it is possible to assess the functioning of each component of the vestibular apparatus. Researchers should examine rehabilitation outcomes in persons with damage to semicircular canal versus otolith components of the vestibular apparatus. Furthermore, researchers should examine the impact of the magnitude and range of hypofunction relative to functional recovery.
R. Research Recommendation 3: There is a paucity of research on the effectiveness of vestibular rehabilitation in children. Researchers should examine rehabilitation outcomes in children with confirmed vestibular dysfunction based on vestibular laboratory tests. In addition, researchers should examine the concept of a critical period of balance development in children in the context of providing vestibular rehabilitation. This is especially important in light of the number of children who are receiving cochlear implants at a very young age and the surgical procedure may affect vestibular function.
A. Action Statement 4: EFFECTIVENESS OF SACCADIC OR SMOOTH-PURSUIT EXERCISES IN PERSONS WITH PERIPHERAL VESTIBULAR HYPOFUNCTION (UNILATERAL OR BILATERAL). Clinicians should not offer saccadic or smooth-pursuit eye exercises in isolation (ie, without head movement) as specific exercises for gaze stability to patients with unilateral or bilateral vestibular hypofunction. (Evidence quality: I; recommendation strength: strong)
Action Statement Profile
Aggregate evidence quality: Level I. Based on 3 level I randomized controlled trials.
-
Benefits:
Poorer outcomes in patients performing only saccadic or smooth-pursuit eye movements without head movement when compared with vestibular rehabilitation.
-
Risk, harm, and cost:
Smooth-pursuit and saccadic eye movement exercises do not appear to harm patients with unilateral or bilateral vestibular hypofunction.
Delay in patients receiving an effective exercise program.
Increased cost and time spent traveling associated with ineffective supervised exercises.
-
Benefit-harm assessment:
Preponderance of harm.
-
Value judgments:
Importance of prescribing an effective exercise program rather than exercises that will not improve gaze stability, symptom complaint, or balance while walking.
-
Role of patient preferences:
It is doubtful that patients would choose to perform an ineffective exercise.
-
Exclusions:
None.
Supporting Evidence and Clinical Interpretation
Three level I studies have used either saccadic or smooth-pursuit eye movements in isolation (ie, without head movement) as control (placebo) exercises.18–20 Note: The saccadic and smooth-pursuit eye movements used in all 3 of these studies are voluntary saccades and smooth-pursuit eye movements without head movement, of the type used when reading or following a moving object. The voluntary saccade and smooth-pursuit eye movements should not be confused with compensatory eye movements (saccadic or high-velocity, slow-phase eye movements) seen after a head impulse (high acceleration of the head in yaw through a small amplitude) in some patients with vestibular hypofunction that potentially are facilitated by gaze stability exercises. In one study, patients scheduled for resection of vestibular schwannoma were randomly assigned to either an exercise group (vestibular rehabilitation; n = 11) or a control group (n = 8).18 Exercises were started 3 days after resection of the vestibular schwannomas and continued until the patients were discharged from the hospital (average = postoperative day 6). The control group performed vertical and horizontal smooth-pursuit eye movements. Patients in both groups walked at least once each day. The vestibular rehabilitation group was older (mean age 59 years vs 48 years in controls, (P < 0.04), but both groups were similar in other respects. Both groups reported significantly more dizziness after surgery than before (P < 0.05) and more postural sway on postoperative day 3 than preoperatively (P < 0.05). By postoperative days 5 to 6, patients in the control group reported significantly greater subjective disequilibrium than the vestibular group who performed gaze stabilization exercises. In addition, none of the control groups were able to walk and turn their head without a loss of balance, whereas 50% of the exercise groups were able to walk and turn their head without losing their balance.
Herdman et al,19 in a level I study in patients with chronic unilateral vestibular hypofunction, used saccadic eye movements as the exercise for the control group. Patients were randomized to vestibular rehabilitation (n = 13) versus placebo exercises (n = 8). The vestibular group was taken through supervised adaptation and substitution exercises to improve gaze stability, whereas the control group performed saccadic eye movements against a Ganzfeld (a large featureless background) with their head stationary. Exercises were done 4 to 5 times daily for 20 to 30 minutes plus 20 minutes of gait and balance exercises for 4 weeks, with adherence monitored and progressed as indicated. On average, there was no change in Dynamic Visual Acuity in the control group and no control subject achieved normal Dynamic Visual Acuity for their age. In contrast, the vestibular treatment group showed improvement in Dynamic Visual Acuity (P < 0.001), and 12 of the 13 individuals improved their Dynamic Visual Acuity to normal. The same experimental design was used to examine the effect of exercises in patients with bilateral vestibular hypofunction.20 As a group, the individuals performing the control saccadic eye movement exercises showed no improvement in Dynamic Visual Acuity whereas those performing gaze stability exercises improved significantly. Thus, saccadic eye movement exercises did not facilitate recovery of gaze stability as measured by Dynamic Visual Acuity.
B. Action Statement 5: EFFECTIVENESS OF DIFFERENT TYPES OF EXERCISES IN PERSONS WITH ACUTE OR CHRONIC UNILATERAL VESTIBULAR HYPOFUNCTION. Clinicians may provide targeted exercise techniques to accomplish specific goals appropriate to address identified impairments and functional limitations. (Evidence quality: II; recommendation strength: moderate)
Action Statement Profile
Aggregate evidence quality: Level II. Based on 1 level I and 2 level II randomized controlled trials examining whether one type of vestibular exercise is more beneficial than another. In addition, 2 level II studies compared a traditional vestibular exercise with a novel exercise.
-
Benefits:
Unknown.
-
Risk, harm, and cost:
Increased cost and time spent traveling associated with supervised vestibular rehabilitation.
-
Benefit-harm assessment:
Unknown; there is a potential for patients to perform an exercise that will not address their primary problems.
-
Value judgments:
Importance of identifying the most appropriate exercise approach to optimize and accelerate recovery of balance function and decreasing distress, improving functional recovery to activities of daily living, and reducing fall risk.
-
Role of patient preferences:
Cost and availability of patient time and transportation may play a role.
-
Exclusions:
Possible exclusions include active Meniere disease or those with impairment of cognitive or general mobility function that precludes adequate learning and carryover or otherwise impedes meaningful application of therapy.
Supporting Evidence and Clinical Interpretation
On the basis of the few randomized trials, clinicians may offer targeted exercise techniques to accomplish specific goals for improvement in exercise programs (eg, exercises related to gaze stability and visual motion sensitivity for improved stability of the visual world and decreased sensitivity to visual motion; head movements in a habituation format to decrease sensitivity to head movement provoked symptoms; and activities related to body sway control for improved general stance and gait).
Few studies have examined whether any one vestibular exercise is more beneficial than another. A few studies have compared a standard vestibular exercise (eg, Cawthorne-Cooksey exercises) with a novel exercise (eg, moving platform practice). Of the 14 randomized clinical trials initially thought to compare the standard vestibular exercise approaches (gaze stabilization, adaptation, habituation, substitution, Cawthorne-Cooksey), only 3 actually compared different exercise approaches with vestibular rehabilitation for peripheral vestibular hypofunction. Two other randomized controlled trials examined the concept that particular exercises should be used to accomplish specific goals.
In a level I randomized trial, Pavlou et al61 compared patients performing a customized exercise program (n = 20; balance, gait, Cawthorne-Cooksey, gaze stability) with patients performing exercises in an optokinetic environment (n = 20). Outcome measures included the Sensory Organization Test, the Berg Balance Scale, and several symptom complaint measures including the Vertigo Symptom Scale, Situational Characteristics Questionnaire, and Hospital Anxiety and Depression Scale. Both groups improved significantly in the Sensory Organization Test and symptom scores; however, the optokinetic stimulus group improved more in the symptom measures. Although the optokinetic stimulus group seems to have improved more in the Sensory Organization Test score, the customized exercise group had higher (better) scores to begin with and therefore there may have been a ceiling effect for that group.
In a level II study, Clendaniel62 studied 7 patients with chronic uncompensated unilateral vestibular hypofunction on the basis of caloric testing or clinical examination. Patients were randomized (no mention of allocation concealment) to habituation exercises (n = 4) designed to reduce patient sensitivity to head movement or gaze stabilization exercises (n = 3) designed to improve visual acuity during head movement. Both patient groups also performed balance and gait exercises and were provided a home exercise program. Both groups were to perform the exercises 3 times daily over a 6-week period. Exercise adherence averaged 69.7% (range 34%-90%). In this preliminary study, both exercise interventions resulted in improved self-reported ability to perform daily activities, decreased sensitivity to movement, and better visual acuity during head movements. However, because of the small number of subjects in the study and the fact that some patients had normal values on the outcome measures at baseline, further research is strongly recommended.
In another level II study, Szturm et al63 examined postural stability (Sensory Organization Test) and vestibular asymmetry (rotary chair and optokinetic testing) in patients with chronic uncompensated unilateral vestibular hypofunction. Patients were randomly assigned to perform either vestibular rehabilitation (gaze stability and balance exercises performed in the clinic and as a home program) or control exercises (Cawthorne-Cooksey exercises performed only as an unsupervised home program). The vestibular rehabilitation group showed improvement in both postural stability and vestibular symmetry, whereas those performing the Cawthorne-Cooksey exercises did not. The study, however, has several limitations. First, not all patients seem to have unilateral vestibular hypofunction on the basis of the investigators' criteria (approximately 25% in each group). Second, the investigators examined vestibulo-ocular reflex gain asymmetry by rotational testing, which is insensitive to unilateral vestibular hypofunction. Finally, because one group was supervised and the other group was not, the differences in outcome may be attributed to a supervision effect rather than to the type of exercise.
Two studies provide support for using particular exercises for specific problems. One, a level I study by McGibbon et al64 randomly assigned 53 patients with vestibular hypofunction and documented gait and balance impairments to either a group-based vestibular exercise intervention or a group-based Tai Chi exercise intervention. Fifteen subjects dropped out of the study and another 12 were unable to perform the step-up/step-down test; thus, the final sample size was 26, and 8 subjects had unilateral and 5 subjects had bilateral vestibular hypofunction in each treatment group. Subjects met once a week for 10 weeks in small groups for 70 minutes of exercise. The study demonstrated that balance exercises (Tai Chi) selectively improved whole body stability during a step-up and step-down test, whereas vestibular exercises (adaptation and eye-head exercises) selectively improved gaze stability. The role of severity of vestibular hypofunction (unilateral vs bilateral) is unclear.
In a level II study, Jauregui-Renaud et al65 compared the effectiveness of Cawthorne-Cooksey exercises, Cawthorne-Cooksey exercises plus training in breathing rhythm, and Cawthorne-Cooksey exercises plus proprioceptive exercises. The outcome measures included disability (Dizziness Handicap Inventory) and static balance in patients with chronic vestibular hypofunction. Although all 3 groups showed improvement in Dizziness Handicap Inventory scores and in static balance, the group performing Cawthorne-Cooksey exercises plus breathing training was more likely to have a meaningful clinical improvement in Dizziness Handicap Inventory scores and the patients performing Cawthorne-Cooksey plus proprioceptive exercises had decreased sway during static balance tests. Although not conclusive, the results from these 2 studies support the concept of exercise specificity in the treatment of patients with vestibular hypofunction.
Pavlou et al66 examined the effect of different virtual reality experiences on outcome in patients with unilateral peripheral vestibular hypofunction. Patients were randomly allocated to a virtual reality regime incorporating exposure to a static (group S) or dynamic (group D) virtual reality environment. Participants practiced vestibular exercises, twice weekly for 4 weeks, inside a virtual crowded square environment. Both groups also received a vestibular exercise home program to practice on days not attending clinic. A third group (D1) completed both the static and dynamic virtual reality training. Outcome measures included the Dynamic Gait Index and questionnaires concerning symptom triggers and psychological state. Those groups who performed exercises within the dynamic virtual reality environment (D and D1) had significantly better Visual Vertigo Scores than those who performed exercises inside the static virtual reality environment (S). In contrast, depression scores increased only in group S. The Dynamic Gait Index did not differ across groups; however, many subjects were already within the normal range before the initiation of the intervention. The investigators concluded that use of dynamic virtual reality environments should be considered as a useful adjunct to vestibular exercises for patients with chronic vestibular disorders and visual vertigo symptoms.
R. Research Recommendation 4: There is sufficient evidence that vestibular exercises compared with no or placebo exercises is effective; thus, future research efforts should be directed to comparative effectiveness research. Researchers should directly compare different types of vestibular exercise in large clinical trials to determine optimal exercise approaches.
B. Action Statement 6: EFFECTIVENESS OF SUPERVISED VESTIBULAR REHABILITATION. Clinicians may offer supervised vestibular physical therapy in patients with unilateral or bilateral peripheral vestibular hypofunction. (Evidence quality: I-III; recommendation strength: moderate)
Action Statement Profile
Aggregate evidence quality: Level II. Based on numerous level I, II, and III studies.
Benefits: Possibly better adherence with a supervised exercise program.
-
Risk, harm, and cost:
There is an increased cost and time spent traveling associated with supervised vestibular rehabilitation.
Without feedback from the supervising physical therapist, the patient may under- or overcomply with the exercise prescription resulting in either lack of progress/improvement or increased symptoms potentially leading to stopping therapy.
-
Benefit-harm assessment:
Preponderance of benefit for supervision.
Evidence suggests that patients drop out at higher rates when unsupervised.
-
Value judgments:
Supervised vestibular rehabilitation appears to promote adherence and continued performance of vestibular exercises, which may lead to improved outcomes.
Persons with impairment of cognition or moderate-severe mobility dysfunction may need supervision to benefit from vestibular rehabilitation.
People who are fearful of falling may not do well in an unsupervised exercise program.
-
Role of patient preferences:
Cost and availability of patient time and transportation may play a role.
-
Exclusions:
Patients who live at a distance may not be able to participate in supervised vestibular rehabilitation.
Supporting Evidence and Clinical Interpretation
Several studies (Levels I63 and II21,45,67–69) demonstrate that patients may respond better to customized, supervised rehabilitation than to generic exercises or solely a home program. The reason for these differences may be that supervised vestibular rehabilitation promotes adherence and continued performance of vestibular exercises, which may lead to improved outcomes.
Two studies examined the effect of supervision during the acute stages of vestibular dysfunction with different outcomes. Kammerlind et al70 in a level I study compared a supervised versus a home training group of vestibular exercises that included gaze stability, balance, and gait exercises. All patients received oral and written instructions for the vestibular exercises in the hospital and were instructed to exercise 15 minutes per day. The supervised group received 3 additional supervised physical therapy sessions in the hospital. Once discharged home, the supervised group received 10 additional supervised visits. At 1 week, 10 weeks, and 6 months postdischarge, both groups improved in measures of balance and symptoms of vertigo, but were not different from each other. A level I study in postsurgical acute patients compared patients who started exercises in the hospital with a control group who did no exercise.40 In patients younger than 50 years, outcomes were equally good whether or not exercises were performed. The average age of Kammerlind et al's participants was 52 years, so the study outcomes may reflect the age of patients versus the role of supervision.70
Teggi et al,45 in a level II study, compared a supervised exercise program with usual activity for patients hospitalized for an acute episode of vertigo. Participants were randomly assigned to attend 10 therapy sessions (n = 20) within 10 days of baseline assessment or were instructed to perform daily activities (n = 20). Twenty-five days later, the group that underwent a supervised exercise program had better outcomes on all measures (Dynamic Gait Index, computerized Clinical Test of Sensory Interaction on Balance, Dizziness Handicap Inventory, and a Visual Analog Scale for anxiety), with the greatest change noted in the Dynamic Gait Index. The results of this study are confounded by differences in exercises (vestibular exercises vs daily activities) and may explain the difference in outcomes compared with Kammerlind et al.45,70
Kao et al,67 in a level II study, compared supervised and home-based (unsupervised) vestibular rehabilitation. Both groups performed seated and standing eye movements and adaptation exercises, as well as walking with head turns. The supervised group received an initial evaluation and individualized treatment plan followed by three 30-minute sessions per week with a physical therapist. The home group participants received an individualized treatment plan on the basis of an initial evaluation and were not seen again by the physical therapist until outcomes were assessed at 2 months. The subjects self-selected their treatment group, with 28 choosing supervised rehabilitation and 13 choosing home-based or unsupervised rehabilitation. Both groups improved, but there were greater improvements in the supervised group compared with the home group for the Dynamic Gait Index (86% vs 14%) and Dizziness Handicap Inventory (74% vs 26%). There are several limitations of this study that limit generalizability including small sample size, no randomization, and assessors that were not blinded to group.
Optokinetic training for visual vertigo was utilized in a level I study.71 Sixty patients were randomized into 3 groups: a supervised training group that utilized a full field environmental rotator, a supervised training group provided with a DVD, and an unsupervised training group using a DVD. All subjects also received a customized program of gaze and postural stability exercises to perform at home. The outcome measures were Visual Vertigo Symptoms, Sensory Organization Test, and Functional Gait Assessment. The Sensory Organization Test and Functional Gait Assessment improved significantly for the supervised groups (full field and DVD groups), and anxiety scores improved for the supervised DVD group. The study has a major limitation related to the high dropout rate of 55% in the unsupervised group compared with 10% in the supervised groups. Pavlou et al71 concluded that supervision promotes greater adherence and improvements in postural stability and psychological state. Yardley et al72, in a level I study, reported “fair” self-reported adherence to an exercise booklet for persons with vestibular disorders. In a subsequent study, she reported that additional advice or encouragement might improve adherence in a home-based program.
Monitoring of the exercise program may have value, as demonstrated by Shepard et al73 in a level III study. The investigators reported that nausea, emesis, and vertigo provoked by exercises could be managed by stopping the exercise session and resuming the exercises at the next session. In most cases, they found this approach to successfully allow continued participation. In those cases where this was not successful, they suggested that antiemetic or vestibular suppressant medication may be required. Recommendations for use of antiemetic drugs should be carefully considered because of concerns about slowing central compensation. For example, Strupp et al42 limited antiemetic use to a maximum of 3 days because of concerns for slowed vestibular compensation.
Failure to return to the clinic,66,71,74 failure to comply with the exercise program,67,74 and illness have been noted as reasons for why people do not complete a program of vestibular exercises. In Pavlou's work, those with an unsupervised exercise program had higher dropout rates.66,71 It is not known why the dropout rate was higher in the unsupervised group.
R. Research Recommendation 5. Researchers should include measures of adherence to understand the impact of supervision. Researchers need to incorporate intent-to-treat research designs to understand dropout rates related to supervision.
D. Action Statement 7: OPTIMAL EXERCISE DOSE OF TREATMENT IN PEOPLE WITH PERIPHERAL VESTIBULAR HYPOFUNCTION (UNILATERAL AND BILATERAL). Clinicians may prescribe a home exercise program of gaze stability exercises consisting of a minimum of 3 times per day for a total of at least 12 minutes per day for patients with acute/subacute vestibular hypofunction and at least 20 minutes per day for patients with chronic vestibular hypofunction. (Evidence quality: V; recommendation strength: expert opinion)
Action Statement Profile
Aggregate evidence quality: Level V. Based on lack of direct evidence on exercise dose. Best practice based on the clinical experience of the guideline development team and guided by the evidence.
-
Benefit:
Improved outcomes with appropriate exercise dose.
-
Risk, harm, and cost:
Risk of provoking temporary dizziness during and after performance of exercises.
Risk of increased nausea and possible emesis when exercises are performed during the most acute stage.
Some physicians may want to delay exercises during the early postoperative stage in some patients because of risk of bleeding or cerebrospinal fluid leak.
Increased cost and time spent traveling associated with supervised vestibular rehabilitation.
-
Benefit-harm assessment:
Preponderance of benefit over harm.
-
Value judgments:
Benefit of gaze stability exercises in patients with unilateral vestibular hypofunction has been demonstrated in numerous level l and level II studies; however, the frequency and intensity of the exercises is based on extrapolation from research studies rather than based on direct evidence.
-
Role of patient preferences:
Minimal.
-
Exclusions:
Patients at risk for bleeding or cerebrospinal fluid leak.
Supporting Evidence and Clinical Interpretation
There are few studies to date that have examined in what ways (if any) exercise dose (frequency and intensity) affects outcomes in patients with unilateral or bilateral vestibular hypofunction. Two studies examined the influence of exercise intensity on outcomes.75,76 Cohen et al compared 2 groups of patients who performed the same exercise but at different levels of intensity. One group performed exercises with rapid head movements (ie, approximately 1-2 Hz) and the other group performed exercises with slow head movements (approximately 0.04 Hz), 5 times per day for a total of 4 weeks. They reported both groups improved equally in vertigo intensity, vertigo frequency, and on a functional repetitive head movement task, suggesting that the dose intensity (frequency of head movement) was not a factor in recovery. There are some limitations to the study that confound the interpretation of the data however. First, it is not clear that the groups were equivalent at baseline on the timed repetitive head movement task and second, the data suggest that the time to perform the repetitive head movement task did not improve until 4 months after initiation of exercises.
Although far from ideal, some information on exercise dose can be found by comparing the findings from multiple studies.
Acute and subacute postoperative patients: Two level I and 1 level II studies have examined the effect of gaze stabilization exercises on the recovery of patients during the early postoperative period after vestibular schwannoma resection.16,18,40 Patients performed gaze stabilization exercises 3 to 5 times daily for a total of 12 to 20 minutes a day and reported improvement in subjective complaints of imbalance,18,40 Dizziness Handicap Inventory,16 and stability while walking with voluntary head movements.18 These results suggest that as little as 12 minutes of gaze stabilization exercises a day over 3 exercise periods may be sufficient to induce recovery in patients during the acute and subacute stages after vestibular schwannoma resection.
Chronic unilateral vestibular hypofunction: Four studies (2 level I and 2 level II), each examining the effect of vestibular rehabilitation on outcomes in patients with chronic unilateral vestibular hypofunction, included sufficient details on the type, frequency, and duration of exercise to provide some guideline as to exercise dose in these patients. In these studies, patients performed the gaze stability exercises 3 to 5 times per day for a total of 20 to 40 minutes each day.19,20,67,77 Patients performing these exercises improved compared with a control group. The data suggest that a minimum performance of the exercises 3 times per day for a total of 20 minutes daily may be sufficient to induce recovery.
R. Research Recommendation 6. Researchers should examine the impact of frequency, intensity, time, and type of exercises on rehabilitation outcomes. Researchers should determine the difficulty of exercises and how to progress patients in a systematic manner.
D. Action Statement 8: DECISION RULES FOR STOPPING VESTIBULAR REHABILITATION IN PERSONS WITH PERIPHERAL VESTIBULAR HYPOFUNCTION (UNILATERAL AND BILATERAL). Clinicians may use achievement of primary goals, resolution of symptoms, or plateau in progress as reasons for stopping therapy. (Evidence quality: V; recommendation strength: expert opinion)
Action Statement Profile
-
Aggregate evidence quality: Level V. Based on extrapolation from methodology and results in 69 studies, it may be advisable to consider the following in the decision to stop treatment:
Goals are met, a plateau has been reached, or the patient is no longer symptomatic.
Nonadherence/patient choice.
Deterioration of clinical status or a prolonged increase in symptoms.
Fluctuating/unstable vestibular conditions (eg, Meniere) and comorbid musculoskeletal, neurologic, cardiac, visual, cognitive, psychological, or disability-related conditions affecting ability to participate.
Overall length of treatment.
-
Benefits:
More efficient management of treatment duration, avoiding cessation of treatment before optimal recovery is achieved, or continuing treatment for unreasonably protracted periods.
-
Risk, harm, and cost:
Prematurely stopping treatment before maximum gains are achieved.
Protracted treatment is costly to the payer, the patient, and the clinician who are not seeing documented improvement, and to other patients who are waiting to receive treatment.
-
Benefit-harm assessment:
Preponderance of benefit over harm.
-
Value judgments:
No concrete stopping rules have been explored in the research; however, numerous level I through IV studies provide comments and findings that can assist in the decision-making process.
-
Role of patient preferences:
It is the patient's decision whether or not to participate in vestibular rehabilitation and when to stop vestibular rehabilitation.
-
Patient exclusions:
Patients with impaired cognition or moderate to severe mobility dysfunction may need a greater number of treatment sessions, so using the treatment duration based on research (which typically excludes these patients) may not be appropriate.
Patients with moderate to severe motion sensitivity may also benefit from a greater number of treatment sessions.
In a level II study, patients taking vestibular-suppressant medication required additional treatment sessions (11 weeks vs 9 weeks before plateau).68
Supporting Evidence and Clinical Interpretation
There are no studies that have specifically examined decision rules for stopping vestibular rehabilitation in those with unilateral or bilateral peripheral vestibular hypofunction. An investigator's a priori decision relative to the research design determines the length of the intervention; thus, the duration of treatment is protocol-driven and not based on patient outcomes. Furthermore, the length of the study intervention may affect a patient's willingness to participate in the study. Thus, we cannot extrapolate from research studies to create clinical stopping rules on the basis of current research design.
Implicit reasons for stopping therapy in a clinic setting ideally include the patient no longer being symptomatic, goals being met, or a plateau being reached.2,78 For example, Hall et al's level III study reported discharge from treatment when 75% of goals were met.17 Multiple studies cited nonadherence as a reason to discontinue treatment. Only a few studies provided specific criteria, such as missing at least 3 treatment sessions or 30% of therapy sessions.42,69,79 Some reasons that patients report nonadherence with vestibular rehabilitation include the following: unrelated health issues, finding the exercises too provoking, family or work conflicts, litigation, travel or time inconvenience, loss of interest or motivation, and feeling better.
Deterioration of clinical status was cited as a reason for 9 of the 37 patients showing an increased Dizziness Handicap Inventory score in a level II study by Perez et al80 and seems an obvious reason to pause or stop treatment; however, if worsening of subjective complaints is a factor in the consideration to stop treatment, the following studies may provide some guidance. A level IV study found that nausea, body shift, dizziness, and stress were increased during first 2 weeks of intervention, but subsided by week 2.81 Szturm's level I randomized controlled trial study found that the adverse effects of moderate to strong dizziness, nausea, and disorientation during exercises subsided within 2 to 5 weeks.63 Thus, worsening symptoms during the 1 or 2 weeks of the vestibular rehabilitation program should not necessarily be considered a reason for stopping therapy. However, more persistent worsening symptoms should be carefully considered a reason to discontinue therapy.
Numerous factors were identified by researchers to exclude patients from studies or to drop subjects from study participation. These factors may also provide guidance for stopping or deferring therapy if a patient is not showing progress. Factors include (1) progressive, fluctuating, or unstable vestibular conditions (ie, vestibular schwannoma, episodes of spontaneous vertigo, unrepaired perilymphatic fistula, and active Meniere disease); (2) musculoskeletal conditions affecting the ability to stand or perform exercises; (3) central nervous system or other neurologic diseases or conditions (eg, head injury) affecting balance, motor control, muscle strength, or somatosensation; (4) significant cardiac problems; (5) severe visual disorders or blindness; (6) cognitive impairment affecting comprehension; (7) severe migraine; and (8) psychological conditions. In Shepard et al's level II study in 1993, those with head injury showed a substantially less reduction in symptoms than the rest of the subjects and comprised a significantly higher percentage of those showing no change or worsening.68
Pretreatment disability could also be considered when deciding whether or not to discontinue therapy in a patient, as patients with high disability scores may be more resistant to change and may be less likely to improve on the basis of 2 level II studies68,82 and 2 level III studies.60,73
On the basis of expert opinion extrapolated from the evidence, clinicians may consider providing adequate supervised vestibular rehabilitation sessions for the patient to understand the goals of the program and how to manage and progress themselves independently. Sixty-one of the prospective studies reported that treatment duration for vestibular rehabilitation ranged from 5 days to 16 weeks (average = 6.7 weeks). However, the researchers did not provide justification for the length of treatment time chosen for their studies. In 20 retrospective studies that reflect clinical practice (based on chart review), treatment duration for vestibular rehabilitation ranged from 2 to 38 weeks (average = 10.0 weeks); however, some patients with bilateral vestibular hypofunction may need a longer course of treatment than individuals with unilateral vestibular hypofunction. As a general guide, persons without significant comorbidities that affect mobility and with acute or subacute unilateral vestibular hypofunction may only need 1 time per week supervised sessions for 2 to 3 sessions; persons with chronic unilateral vestibular hypofunction may need 1 time per week supervised sessions for 4 to 6 weeks; and persons with bilateral vestibular hypofunction may need a longer course of treatment (1 time per week supervised sessions for 8-12 weeks) than persons with unilateral vestibular hypofunction.
Finally, on the basis of expert opinion, the advisory panel recommends that before stopping therapy for patients who remain symptomatic or have not met their goals, consultation with another vestibular physical therapist colleague would be advisable.
R. Research Recommendation 7: Researchers should determine optimal duration of vestibular rehabilitation for favorable outcomes and the factors that impact functional recovery.
C. Action Statement 9: FACTORS THAT MODIFY REHABILITATION OUTCOMES. Clinicians may evaluate factors that could modify rehabilitation outcomes. (Evidence quality: I-III; recommendation strength: weak to strong)
Action Statement Profile
Aggregate evidence quality: Age: Level I. Based on 4 level I randomized controlled trials and 2 level II quasiexperimental studies. Sex: Level III. Based on 1 level II and 2 level III studies. Time from onset: Level III. Based on 1 level I randomized controlled trial and 3 level III studies, 1 with contradictory results to the others. Comorbidities: Level III. Based on 1 level I randomized controlled trial, 2 level II and 1 level III studies. Use of vestibular-suppressant medications: Level III. Based on 1 level II and 1 level III studies.
-
Benefits:
Older patients obtain similar benefits from vestibular rehabilitation.
-
Risk, harm, and cost:
Peripheral neuropathy may increase risk of falling and negatively impact rehabilitation outcomes.
-
Benefit-harm assessment:
Vestibular rehabilitation has been shown to improve outcomes regardless of the time from onset; however, the potential harm (decreased quality of life, falls) to initiating rehabilitation later warrants initiating rehabilitation as soon as possible.
-
Value judgments:
Little evidence is available to make decisions about how to consider factors that may affect outcomes.
-
Role of patient preferences:
Cost and availability of patient time and transportation may play a role, especially with older patients who may have transportation issues.
-
Exclusions:
None.
Supporting Evidence and Clinical Interpretation
Several non-disease-related modifying factors—including age, sex, time from onset of symptoms to start of rehabilitation, comorbidities, and use of vestibular-suppressant medications—have been evaluated for their impact on vestibular rehabilitation outcomes.
Age: Increased age does not affect potential for improvement with vestibular rehabilitation. Clinicians should offer vestibular rehabilitation to older adults with the expectation of good outcomes. (Evidence quality: I; recommendation strength: strong)
Sex: Sex may not impact rehabilitation outcomes and clinicians may offer vestibular rehabilitation to males and females with expectation of similar outcomes. (Evidence quality: III; recommendation strength: weak)
Time from onset (acute): Earlier intervention improves rehabilitation outcomes; thus, vestibular rehabilitation may be started as soon as possible after acute onset of vertigo. (Evidence quality: II; recommendation strength: moderate)
Time from onset (chronic): Vestibular exercises have been shown to improve outcomes regardless of the time from onset; however, the potential for harm related to decreased quality of life or falls suggests that clinicians may initiate rehabilitation as soon as possible. (Evidence quality: I-III; recommendation strength: moderate)
Comorbidities: Anxiety, migraine, and peripheral neuropathy may negatively impact rehabilitation outcomes. (Evidence quality: III; recommendation strength: weak)
Vestibular-suppressant medications: Long-term use of valium or meclizine may negatively impact patient recovery. (Evidence quality: II-III; recommendation strength: moderate)
Supporting Evidence and Clinical Interpretation
Several non-disease-related modifying factors have been evaluated in various studies. These factors include age, sex, time from onset of symptoms until starting vestibular rehabilitation, comorbidities, and use of vestibular-suppressant medications. The level of evidence for these studies ranged from level I to level III.
Eleven studies evaluated the effect of age and none demonstrated a significant effect of age on the efficacy of vestibular rehabilitation. Six studies evaluated the influence of age on vestibular rehabilitation in patients with unilateral vestibular hypofunction; of these, 3 studies had an evidence level of I,19,40,83 1 study had an evidence level of II,69 and 2 studies had an evidence level of III.2,17 Four studies evaluated the influence of age on vestibular rehabilitation in patients with various diagnoses including both peripheral and central vestibular deficits; of these, 1 study had an evidence level of II,67 and 3 studies had an evidence level of III.82,84,85 One level I study evaluated the influence of age on vestibular rehabilitation in patients with bilateral peripheral vestibular deficits.20
Three studies evaluated the effect of sex, and none demonstrated a significant effect of sex on the efficacy of vestibular rehabilitation. Two of these—1 level II69 and 1 level III2—evaluated the influence of sex on vestibular rehabilitation in patients with unilateral vestibular hypofunction. One level II study evaluated the influence of sex on vestibular rehabilitation in patients with various diagnoses including both peripheral and central vestibular deficits.67
Two level I studies examined the effects of vestibular exercises solely in the acute stage after resection of vestibular schwannoma.16,18 Both studies provide evidence that early intervention is beneficial. Herdman et al18 started vestibular exercises 3 days postsurgery and continued until discharge from the hospital. Participants randomized to receive gaze stability exercises were less symptomatic and had better postural stability at discharge than the placebo group. Enticott et al16 compared a cohort of patients who were randomized to vestibular exercises (gaze stability exercises) versus a control group starting on postoperative day 3. The vestibular group had lower perceived disability (based on the Dizziness Handicap Inventory) over the course of 12 weeks.
Six studies of patients with chronic vestibular hypofunction evaluated the effect of time from onset of symptoms until starting vestibular rehabilitation. Four studies evaluated patients with unilateral vestibular hypofunction with conflicting results. One level III study indicated that earlier intervention produced better results.86 The other 3 studies, one of which had level I evidence19 and 2 with level III evidence,2,17 showed no effect of duration of symptoms before initiation of vestibular rehabilitation therapy. A level II study of patients with various diagnosis including both peripheral and central vestibular deficits also found no effect of time from onset of symptoms until starting vestibular rehabilitation.82 One level I study determined that time from onset of symptoms did not affect the outcomes of the vestibular rehabilitation in individuals with bilateral vestibular hypofunction.20 In each of these studies, participants improved with vestibular rehabilitation; thus, these studies demonstrate that vestibular rehabilitation improves outcomes regardless of the time from onset.
Five studies evaluated the effect of comorbidities on response to vestibular rehabilitation. Two studies evaluated the influence of anxiety. In a study of patients with unilateral peripheral vestibular deficits, anxiety was found to result in decreased balance confidence on the basis of level III evidence.2 In a study of patients with various diagnoses, higher anxiety was associated with poorer scores on the Dynamic Gait Index on the basis of level II evidence.45 In persons with psychological conditions (anxiety/depression), addressing psychological needs as an adjunct to physical therapy may increase the success of the intervention on the basis of evidence from level I, II, and III studies.45,72,87,88
A single study reported a negative effect of peripheral neuropathy on vestibular rehabilitation in patients with peripheral vestibular disorders on the basis of level II evidence. Aranda et al89 examined a mixed population of individuals with unilateral or bilateral vestibular hypofunction and diabetes with or without peripheral neuropathy. They found that individuals with peripheral neuropathy had no improvement on measures of standing balance with eyes open and closed on a firm surface, and eyes open on a compliant surface; individuals without peripheral neuropathy demonstrated significant improvements in these test conditions. These findings suggest that peripheral neuropathy may have a negative impact on recovery of function.
Two studies (1 level I90 and 1 level III91) investigated the impact of migraine on rehabilitation outcomes and found that individuals with vestibular dysfunction and migraine had poorer outcomes in terms of quality of life as measured by the Dizziness Handicap Inventory. Another level I study reported that patients with migraine improved in symptoms of visual vertigo more than patients without migraine.71 These study findings are in contrast to Vitkovic et al90 and Wrisley et al91 and may reflect the use of an optokinetic stimulus.
Two studies have examined the impact of medications on outcomes. A level II study found that patients with vestibular hypofunction who were treated with valium or meclizine daily had no improvement in postural sway over a 6-week treatment period.21 These patients did report a decrease in dizziness and in symptomatic complaints over time with these medications. Another study, on the basis of level III evidence, reported that patients with various disorders who were using centrally active medications, such as vestibular suppressants, antidepressants, tranquilizers, and anticonvulsants, required a longer duration of therapy to achieve the same benefit as compared with patients who were not using medications.73
R. Research Recommendation 8. Researchers should perform longitudinal studies. Researchers should examine time from onset and see whether it affects short- and long-term outcomes.
A. Action Statement 10: THE HARM/BENEFIT RATIO FOR VESTIBULAR REHABILITATION IN TERMS OF QUALITY OF LIFE/PSYCHOLOGICAL STRESS. Clinicians should offer vestibular rehabilitation to persons with peripheral vestibular hypofunction. (Evidence quality: I-III; recommendation strength: strong)
Action Statement Profile
Aggregate evidence quality: Level I-III. Based on randomized trials and descriptive studies. No targeted randomized trials are available to directly answer the question to the harm/benefit ratio of vestibular rehabilitation for persons with vestibular hypofunction; however, quality of life measures have been used as primary outcome measures in a number of studies.
-
Benefits:
There are improved quality of life and psychological outcomes in persons undergoing vestibular rehabilitation when compared with controls who receive sham or no exercise interventions.
-
Risk, harm, and cost:
Neck pain, motion sickness, and nausea have been reported as side effects of rehabilitation and these can affect quality of life.
Dizziness as a side effect of the exercises could increase psychological distress in some patients.
-
Benefit-harm assessment:
Preponderance of benefit, although not all patients improve with vestibular rehabilitation.
-
Value judgments:
There is sufficient evidence of improved quality of life and reduced psychological distress with vestibular rehabilitation.
-
Role of patient preferences:
Cost and availability of patient time, location of the vestibular rehabilitation clinic, and transportation may play a role.
-
Exclusions:
None.
Supporting Evidence and Clinical Interpretation
Loss of vestibular function can result in postural instability, visual blurring with head movement, and subjective complaints of dizziness and/or imbalance. Although vestibular rehabilitation was not provided, Sun et al92 recently reported via a quality of life survey that persons with bilateral vestibular loss had impaired quality of life plus loss of work days as a result of their dizziness.
Quality of life has been reported to improve postvestibular rehabilitation for persons with unilateral vestibular dysfunction (level I: Johansson et al,93 Rossi-Izquierdo et al,94 Winkler and Esses95; level II: Clendaniel,62 Badaracco et al,96 Enticott et al,16 Gottshall et al,97 Mantello et al,98 Meli et al,99 Morozetti et al,100 Murray et al,87 Perez et al,80 Schubert et al,77 Tee et al,101 Teggi et al,45 Topuz et al69; level III: Cowand et al,102 Patatas et al84; level IV: Bittar et al103) and bilateral loss (level I: Krebs et al22; level III: Brown et al,58 Gillespie and Minor57) on the basis of improvements in the Dizziness Handicap Inventory. Although the Dizziness Handicap Inventory was designed to measure the handicapping effects of dizziness, it has also been used as a measure of quality of life to record improvements over time. Others have utilized the Activities-Specific Balance Confidence scale to note beneficial changes over time in patients' balance (level I: Enticott et al16; level II: Gottshall et al,97 Badaracco et al,96 Meli et al99; level III: Brown et al58). The improvements in the Dizziness Handicap Inventory and the Activities-Specific Balance Confidence scale suggest that persons are less dizzy and have improved perception of balance after a course of vestibular rehabilitation.
Harm/benefit ratios were not specifically noted in any of the literature reviewed related to quality of life and psychological distress. Occasional mentions were made about side effects of the vestibular rehabilitation program and that not all patients improve. Herdman et al2 recently reported in a level III study that anxiety and depression were associated with lower balance confidence scores, a quality of life measure in persons with unilateral hypofunction. This suggests that coexisting anxiety and depression might potentially diminish potential beneficial effects of an exercise program. Cohen and Kimball,75 in a level II study, reported nausea as a side effect of the exercise program, which could affect quality of life. Although nausea is a common side effect of exercise, it has not been routinely reported in the literature as being “harmful” or resulting in dropouts from a vestibular exercise program.
Telian et al,82 in a level II study, reported that a majority of patients (82% of the patients, n = 65) indicated that they had improved, whereas 12% reported feeling worse. Almost half of their subjects had central vestibular disorders. Of the 12% who were worse after vestibular rehabilitation therapy, it is not reported whether these people had central or peripheral vestibular diagnoses. Bittar et al,104 in a level IV study, also reported that 14% of their subjects were not any better after rehabilitation, which is similar to the Telian et al. report.82 Therefore, there is the possibility that people will undergo the exercise program and not change their quality of life.
Meli et al99 (level III) studied 42 people prospectively and followed up at 6 months to determine whether they had improved after a course of vestibular rehabilitation. The Medical Outcomes Study 36 item-short form improved in their subjects, except bodily pain and vitality. Younger subjects reported worse Medical Outcomes Study 36 item-short form scores, suggesting that dizziness may have more effect on their lives with work and possibly a busier schedule than the older adults studied.
Return to work is an important measure of the benefit of any exercise program; however, virtually no researchers have incorporated a measure of return to work. Chen et al,81 in a level IV trial, reported that in 3 of 3 of their subjects they were able to return to work and drive. All had chronic symptoms before starting the Wiimote gaze stabilization exercise program. Improvements in driving have been noted in others with chronic unilateral hypofunction after an exercise program.105 In 2 level II studies and 3 level III studies, patients' perceived disability has been reported to positively change after rehabilitation. This disability scale includes ability to work as a portion of the instrument, yet no studies specifically report how frequently people are able to return to work effectively after vestibular rehabilitation (level II: Giray et al,50 Shepard et al,68 Telian et al82; level III: Shepard et al,73 Telian et al60).
In 2 randomized trials (level I), Pavlou et al66,71 reported that the autonomic/somatic anxiety scores decreased (improved anxiety) with vestibular rehabilitation. Pavlou et al also reported positive changes on the Hospital Anxiety and Depression–A and B Scale plus the Spielberger State Trait Anxiety Inventory, suggesting that after rehabilitation their subjects were less anxious. Teggi et al45 reported that a Visual Analog Scale for anxiety improved when compared with control subjects at 25 days posthospitalization for acute vertigo (level II). The exercise group participated in 10 sessions that included dynamic posturography training and gaze stabilization exercises. There is emerging evidence that psychological distress and anxiety are decreased with exercise in persons with vestibular hypofunction.
R. Research Recommendation 9. Researchers should examine the concept of return to work. Areas for study include job requirements that may be difficult for patients with vestibular hypofunction, job modification, or assistive technology to allow return to work, criteria for return to work or disability assignment, indicators for return to safe driving.
GUIDELINE IMPLEMENTATION RECOMMENDATIONS
The following strategies are provided as suggestions for clinicians to implement the action statements of this CPG, but are not an exhaustive list. Many variables affect the successful translation of evidence into practice, and clinicians need to assess their own practice environment and clinical skills to determine the best approach to implement the action statements as individuals.
Strategies for implementation:
Keep a copy of the vestibular rehabilitation CPG in a convenient clinic location.
Seek training in the use of the recommended intervention approaches.
Build relationships with referral sources to encourage early referral of persons with peripheral vestibular hypofunction.
Measure outcomes of care using recommended outcome measures across the ICF domains.
Share the JNPT Perspectives for Patients that accompanies this article with patients and others who are interested in learning about the management of dizziness related to vestibular disorders.
SUMMARY OF RESEARCH RECOMMENDATIONS
R. Research Recommendation 1: Researchers should examine the concept of a critical period for optimal vestibular compensation through studies that examine early versus delayed intervention. Researchers should identify factors that predict which patients will recover without the benefit of vestibular rehabilitation and which patients will need vestibular rehabilitation to optimize outcomes.
R. Research Recommendation 2: With the advent of new diagnostic tools, it is possible to assess the functioning of each component of the vestibular apparatus. Researchers should examine rehabilitation outcomes in persons with damage to semicircular canal versus otolith components of the vestibular apparatus. Furthermore, researchers should examine the impact of the magnitude and range of hypofunction relative to functional recovery.
R. Research Recommendation 3: There is a paucity of research on the effectiveness of vestibular rehabilitation in children. Researchers should examine rehabilitation outcomes in children with confirmed vestibular dysfunction on the basis of vestibular laboratory tests. In addition, researchers should examine the concept of a critical period of balance development in children in the context of providing vestibular rehabilitation. This is especially important in light of the number of children who are receiving cochlear implants at a very young age and the surgical procedure may affect vestibular function.
R. Research Recommendation 4: There is sufficient evidence that vestibular exercises compared with no or placebo exercises are effective; thus, future research efforts should be directed to comparative effectiveness research. Researchers should directly compare different types of vestibular exercise in large clinical trials to determine optimal exercise approaches.
R. Research Recommendation 5: Researchers should include measures of adherence to understand the impact of supervision. Researchers need to incorporate intent-to-treat research designs to understand dropout rates related to supervision.
R. Research Recommendation 6: Researchers should examine the impact of frequency, intensity, time, and type of exercises on rehabilitation outcomes. Researchers should determine the difficulty of exercises and how to progress patients in a systematic manner.
R. Research Recommendation 7: Researchers should determine optimal duration of vestibular rehabilitation for favorable outcomes and the factors that impact functional recovery.
R. Research Recommendation 8: Researchers should perform longitudinal studies. Researchers should examine time from onset and see whether it affects short- and long-term outcomes.
R. Research Recommendation 9: Researchers should examine the concept of return to work. Areas for study include job requirements that may be difficult for patients with vestibular hypofunction, job modification, or assistive technology to allow return to work, criteria for return to work or disability assignment, indicators for return to safe driving.
ACKNOWLEDGMENTS
We are grateful to Jacob O'Dell, MS, SPT, for countless hours of research and assistance with data management throughout this project.
The academic librarians from East Tennessee State University (Richard Wallace, MSLS, EdD, AHIP; Nakia Woodward, MSIS, AHIP), Emory University (Amy Allison, MLS, AHIP), and the University of Pittsburgh (Linda Hartman, MLS, AHIP) performed the systematic literature searches.
We gratefully acknowledge Thomas Getchius, Director, Clinical Practice at the American Academy of Neurology for his generosity in sharing his expertise and the American Academy of Neurology Clinical Practice Guideline Process Manual.
We also thank John Engberg, PhD, who, as our patient representative, provided valuable feedback to the process and content of the guideline.
We are grateful to members of the Neurology Section and Vestibular Special Interest Group who volunteered their time and efforts to perform critical appraisals of the literature. The physical therapist critical appraisal team included Carmen Abbott, Eric Anson, Kathryn Brown, Lisa Brown, Janet Callahan, Diron Cassidy, Jennifer Braswell Christy, Pam Cornwell, Renee Crumley, Elizabeth Dannenbaum, Pamela Dunlap, Lisa Farrell, Julie Grove, John Heick, Janet Helminski, Lisa Heusel-Gillig, Janene Holmberg, Jennifer Kelly, Brooke Klatt, Jodi Krause, Karen Lambert, Rob Landel, Lara Martin, Joann Moriarty-Baron, Laura Morris, Charles Plishka, Nora Riley, Britta Smith, Debbie Struiksma, Derek Steele, Brady Whetten, and Wendy Wood.
We acknowledge the support of Matthew Elrod, PT, DPT, MEd, NCS, and Anita Bemis-Dougherty, PT, DPT, MAS, of the APTA. The Neurology Section provided support and guidance throughout the process, specifically through the efforts of Beth Crowner, PT, DPT, NCS, MPPA, Director of Practice, Neurology Section.
Footnotes
REVIEWERS: Kathryn E. Brown, PT, MS, NCS; Beth Crowner, PT, DPT, NCS, MPPA; Kathleen Gill-Body, DPT, MS, NCS, FAPTA; Joseph Godges, DPT, MA; Tim Hanke PT, PhD; Tina Stoeckman, PT, DSc, MA; Michael Schubert, PT, PhD; Rose Marie Rine, PT, PhD; Irene Ward PT, DPT, NCS.
The APTA provided grant funding to support the development and preparation of this document.
All members of the workgroup and advisory board submitted written conflict-of-interest forms and CVs which were evaluated by a member of the Neurology Section Clinical Practice Director (Beth Crowner, PT, DPT, NCS, MPPA) and found to be free of financial and intellectual conflict of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal's Web site (www.jnpt.org).
The American Physical Therapy Association Neurology Section welcomes comments on this guideline. Comments may be sent to Neuro@apta.org.
REFERENCES
- 1.Fetters L, Tilson J. Evidence Based Physical Therapy. FA Davis; 2012. [Google Scholar]
- 2.Herdman SJ, Hall CD, Delaune W. Variables associated with outcome in patients with unilateral vestibular hypofunction. Neurorehabil Neural Repair. 2012;26(2):151–162. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal Y, Carey J, Della Santina C, Schubert M, Minor L. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch Intern Med. 2009;169(10):938–944. [DOI] [PubMed] [Google Scholar]
- 4.Herdman SJ, Blatt P, Schubert MC, Tusa RJ. Falls in patients with vestibular deficits. Am J Otol. 2000;21(6):847–851. [PubMed] [Google Scholar]
- 5.Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006;12(5):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Englander F, Hodson TJ, Terregrossa RA. Economic dimensions of slip and fall injuries. J Forensic Sci. 1996;41(5):733–746. [PubMed] [Google Scholar]
- 7.Adamec I, Skorić MK, Handžić J, Habek M. Incidence, seasonality and comorbidity in vestibular neuritis. Neurol Sci. 2015;36(1):91–95. [DOI] [PubMed] [Google Scholar]
- 8.Neuhauser H, Von Brevern M, Radtke A, et al. Epidemiology of vestibular vertigo: a neurotologic survey of the general population. Neurology. 2005;65(6):898–904. [DOI] [PubMed] [Google Scholar]
- 9.Kroenke K, Hoffman RM, Einstadter D. How common are various causes of dizziness? A critical review. South Med J. 2000;93(2):160–167. [PubMed] [Google Scholar]
- 10.Ward BK, Agrawal Y, Hoffman HJ, Carey JP, Della Santina CC. Prevalence and impact of bilateral vestibular hypofunction: results from the 2008 US National Health Interview Survey. JAMA Otolaryngol Head Neck Surg. 2013;139(8):803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Reilly RC, Morlet T, Nicholas BD, Josephson G, Horlbeck D, Lundy L, Mercado A. Prevalence of vestibular and balance disorders in children. Otol Neurotol. 2010;31(9):1441–1444. [DOI] [PubMed] [Google Scholar]
- 12.Tribukait A, Brantberg K, Bergenius J. Function of semicircular canals, utricles and saccules in deaf children. Acta Otolaryngol. 2004;124(1):41–48. [DOI] [PubMed] [Google Scholar]
- 13.Jacot E, Van Den Abbeele T, Debre HR, Wiener-Vacher SR. Vestibular impairments pre-and post-cochlear implant in children. Int J Pediatr Otorhinolaryngol. 2009;73(2):209–217. [DOI] [PubMed] [Google Scholar]
- 14.Dillon CF, Gu Q, Hoffman HJ, Ko C-W. Vision, hearing, balance, and sensory impairment in Americans aged 70 years and over: United States, 1999-2006. NCHS Data Brief. 2010(31):1–8. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Home and Recreational Safety. http://www.cdc.gov/HomeandRecreationalSafety/Falls/index.html. Accessed April 3, 2015.
- 16.Enticott JC, O'Leary S J, Briggs RJ. Effects of vestibulo-ocular reflex exercises on vestibular compensation after vestibular schwannoma surgery. Otol Neurotol. 2005;26(2):265–269. [DOI] [PubMed] [Google Scholar]
- 17.Hall CD, Schubert MC, Herdman SJ. Prediction of fall risk reduction as measured by dynamic gait index in individuals with unilateral vestibular hypofunction. Otol Neurotol. 2004;25(5):746–751. [DOI] [PubMed] [Google Scholar]
- 18.Herdman SJ, Clendaniel RA, Mattox DE, Holliday MJ, Niparko JK. Vestibular adaptation exercises and recovery: acute stage after acoustic neuroma resection. Otolaryngol Head Neck Surg. 1995;113(1):77–87. [DOI] [PubMed] [Google Scholar]
- 19.Herdman SJ, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2003;129(8):819–824. [DOI] [PubMed] [Google Scholar]
- 20.Herdman SJ, Hall CD, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2007;133(4):383–389. [DOI] [PubMed] [Google Scholar]
- 21.Horak FB, Jones-Rycewicz C, Black FO, Shumway-Cook A. Effects of vestibular rehabilitation on dizziness and imbalance. Otolaryngol Head Neck Surg. 1992;106(2):175–180. [PubMed] [Google Scholar]
- 22.Krebs DE, Gill-Body KM, Riley PO, Parker SW. Double-blind, placebo-controlled trial of rehabilitation for bilateral vestibular hypofunction: preliminary report. Otolaryngol Head Neck Surg. 1993;109(4):735–741. [DOI] [PubMed] [Google Scholar]
- 23.Yardley L, Beech S, Zander L, Evans T, Weinman J. A randomized controlled trial of exercise therapy for dizziness and vertigo in primary care. Br J Gen Pract. 1998;48(429):1136–1140. [PMC free article] [PubMed] [Google Scholar]
- 24.McDonnell MN, Hillier SL. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2015;1:CD005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porciuncula F, Johnson CC, Glickman LB. The effect of vestibular rehabilitation on adults with bilateral vestibular hypofunction: a systematic review. J Vestib Res. 2012;22(5–6):283–298. [DOI] [PubMed] [Google Scholar]
- 26.Fife TD, Iverson DJ, Lempert T, et al. Practice parameter: therapies for benign paroxysmal positional vertigo (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70(22):2067–2074. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharyya N, Baugh RF, Orvidas L, et al. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139(5 Suppl 4):S47–81. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan SL, Coulter C, Fetters L. Physical therapy management of congenital muscular torticollis: an evidence-based clinical practice guideline: from the Section on Pediatrics of the American Physical Therapy Association. Pediatr Phys Ther. 2013;25(4):348–394. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Menière's disease. J Vestib Res. 2015;25(1):1–7. [DOI] [PubMed] [Google Scholar]
- 30.Gandolfi MM, Reilly EK, Galatioto J, Judson RB, Kim AH. Cost-effective analysis of unilateral vestibular weakness investigation. Otol Neurotol. 2015;36(2):277–281. [DOI] [PubMed] [Google Scholar]
- 31.Voelker CC, Lucisano A, Kallogjeri D, Sinks BC, Goebel JA. Comparison of the gaze stabilization test and the dynamic visual acuity test in unilateral vestibular loss patients and controls. Otol Neurotol. 2015;36(4):746–753. [DOI] [PubMed] [Google Scholar]
- 32.Yeh SC, Huang MC, Wang PC, et al. Machine learning-based assessment tool for imbalance and vestibular dysfunction with virtual reality rehabilitation system. Comput Methods Programs Biomed. 2014;116(3):311–318. [DOI] [PubMed] [Google Scholar]
- 33.Jongkees L. Thermic test and electronystagmography. Acta Otorhinolaryngol Belg. 1965;19(2):455–464. [PubMed] [Google Scholar]
- 34.Berryhill W, Graham M. Chemical and physical labyrinthectomy for Meniere's disease. Otolaryngol Clin North Am. 2002;35(3):675–682. [DOI] [PubMed] [Google Scholar]
- 35.Cawthorne T. Vestibular injuries. Proc R Soc Med. 1946;39(5):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grill E, Bronstein A, Furman J, Zee DS, Müller M. International Classification of Functioning, Disability and Health (ICF) Core Set for patients with vertigo, dizziness and balance disorders. J Vestib Res. 2012;22(5–6):261–271. [DOI] [PubMed] [Google Scholar]
- 37.Strupp M, Arbusow V, Brandt T. Exercise and drug therapy alter recovery from labyrinth lesion in humans. Ann N Y Acad Sci. 2001;942(1):79–94. [DOI] [PubMed] [Google Scholar]
- 38.Jeong S-H, Kim H-J, Kim J-S. Vestibular neuritis. Semin Neurol. 2013;33(3):185–194. [DOI] [PubMed] [Google Scholar]
- 39.Mruzek M, Barin K, Nichols DS, Burnett CN, Welling DB. Effects of vestibular rehabilitation and social reinforcement on recovery following ablative vestibular surgery. Laryngoscope. 1995;105(7 Pt 1):686–692. [DOI] [PubMed] [Google Scholar]
- 40.Vereeck L, Wuyts FL, Truijen S, De Valck C, Van de Heyning PH. The effect of early customized vestibular rehabilitation on balance after acoustic neuroma resection. Clin Rehabil. 2008;22(8):698–713. [DOI] [PubMed] [Google Scholar]
- 41.Sparrer I, Duong Dinh TA, Ilgner J, Westhofen M. Vestibular rehabilitation using the Nintendo® Wii Balance Board-a user-friendly alternative for central nervous compensation. Acta Otolaryngol. 2013;133(3):239–245. [DOI] [PubMed] [Google Scholar]
- 42.Strupp M, Arbusow V, Maag KP, Gall C, Brandt T. Vestibular exercises improve central vestibulospinal compensation after vestibular neuritis. Neurology. 1998;51(3):838–844. [DOI] [PubMed] [Google Scholar]
- 43.Venosa AR, Bittar RS. Vestibular rehabilitation exercises in acute vertigo. Laryngoscope. 2007;117(8):1482–1487. [DOI] [PubMed] [Google Scholar]
- 44.Marioni G, Fermo S, Zanon D, Broi N, Staffieri A. Early rehabilitation for unilateral peripheral vestibular disorders: a prospective, randomized investigation using computerized posturography. Eur Arch Otorhinolaryngol. 2013;270(2):425–435. [DOI] [PubMed] [Google Scholar]
- 45.Teggi R, Caldirola D, Fabiano B, Recanati P, Bussi M. Rehabilitation after acute vestibular disorders. J Laryngol Otol. 2009;123(4):397–402. [DOI] [PubMed] [Google Scholar]
- 46.Tjernström F, Fransson P-A, Kahlon B, et al. Vestibular PREHAB and gentamicin before schwannoma surgery may improve long-term postural function. J Neurol Neurosurg Psychiatry. 2009;80(11):1254–1260. [DOI] [PubMed] [Google Scholar]
- 47.Magnusson M, Kahlon B, Karlberg M, Lindberg S, Siesjö P. Preoperative vestibular ablation with gentamicin and vestibular'prehab'enhance postoperative recovery after surgery for pontine angle tumours-first report. Acta Otolaryngol. 2007;127(12):1236–1240. [DOI] [PubMed] [Google Scholar]
- 48.Magnusson M, Karlberg M, Tjernström F. “PREHAB”: vestibular prehabilitation to ameliorate the effect of a sudden vestibular loss. Neurorehabilitation. 2011;29(2):153–156. [DOI] [PubMed] [Google Scholar]
- 49.Loader B, Gruther W, Mueller CA, et al. Improved postural control after computerized optokinetic therapy based on stochastic visual stimulation in patients with vestibular dysfunction. J Vestib Res. 2007;17(2–3):131–136. [PubMed] [Google Scholar]
- 50.Giray M, Kirazli Y, Karapolat H, Celebisoy N, Bilgen C, Kirazli T. Short-term effects of vestibular rehabilitation in patients with chronic unilateral vestibular dysfunction: a randomized controlled study. Arch Phys Med Rehabil. 2009;90(8):1325–1331. [DOI] [PubMed] [Google Scholar]
- 51.Shepard NT, Telian SA. Programmatic vestibular rehabilitation. Otolaryngol Head Neck Surg. 1995;112(1):173–182. [DOI] [PubMed] [Google Scholar]
- 52.Verdecchia DH, Mendoza M, Sanguineti F, Binetti AC. Outcomes after vestibular rehabilitation and Wii(R) therapy in patients with chronic unilateral vestibular hypofunction. Acta Otorrinolaringol Esp. 2014;65(6):339–345. [DOI] [PubMed] [Google Scholar]
- 53.Gabilan YPL, Perracini MR, Munhoz MSL, Gananc FF. Aquatic physiotherapy for vestibular rehabilitation in patients with unilateral vestibular hypofunction: exploratory prospective study. J Vestib Res. 2008;18(2–3):139–146. [PubMed] [Google Scholar]
- 54.Krebs DE, Gill-Body KM, Parker SW, Ramirez JV, Wernick-Robinson M. Vestibular rehabilitation: useful but not universally so. Otolaryngol Head Neck Surg. 2003;128(2):240–250. [DOI] [PubMed] [Google Scholar]
- 55.Rine RM, Braswell J, Fisher D, Joyce K, Kalar K, Shaffer M. Improvement of motor development and postural control following intervention in children with sensorineural hearing loss and vestibular impairment. Int J Pediatr Otorhinolaryngol. 2004;68(9):1141–1148. [DOI] [PubMed] [Google Scholar]
- 56.Patten C, Horak FB, Krebs DE. Head and body center of gravity control strategies: adaptations following vestibular rehabilitation. Acta Otolaryngol. 2003;123(1):32–40. [DOI] [PubMed] [Google Scholar]
- 57.Gillespie MB, Minor LB. Prognosis in bilateral vestibular hypofunction. Laryngoscope. 1999;109(1):35–41. [DOI] [PubMed] [Google Scholar]
- 58.Brown KE, Whitney SL, Wrisley DM, Furman JM. Physical therapy outcomes for persons with bilateral vestibular loss. Laryngoscope. 2001;111(10):1812–1817. [DOI] [PubMed] [Google Scholar]
- 59.Calder JH, Jacobson GP. Acquired bilateral peripheral vestibular system impairment: rehabilitative options and potential outcomes. J Am Acad Audiol. 2000;11(9):514–521. [PubMed] [Google Scholar]
- 60.Telian SA, Shepard NT, Smith-Wheelock M, Hoberg M. Bilateral vestibular paresis: diagnosis and treatment. Otolaryngol Head Neck Surg. 1991;104(1):67–71. [DOI] [PubMed] [Google Scholar]
- 61.Pavlou M, Lingeswaran A, Davies RA, Gresty MA, Bronstein AM. Simulator based rehabilitation in refractory dizziness. J Neurol. 2004;251(8):983–995. [DOI] [PubMed] [Google Scholar]
- 62.Clendaniel RA. The effects of habituation and gaze stability exercises in the treatment of unilateral vestibular hypofunction: a preliminary results. J Neurol Phys Ther. 2010;34(2):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szturm T, Ireland DJ, Lessing-Turner M. Comparison of different exercise programs in the rehabilitation of patients with chronic peripheral vestibular dysfunction. J Vestib Res. 1994;4(6):461–479. [PubMed] [Google Scholar]
- 64.McGibbon CA, Krebs DE, Wolf SL, Wayne PM, Scarborough DM, Parker SW. Tai Chi and vestibular rehabilitation effects on gaze and whole-body stability. J Vestib Res. 2004;14(6):467–478. [PubMed] [Google Scholar]
- 65.Jauregui-Renaud K, Villanueva Padron LA, Cruz Gomez NS. The effect of vestibular rehabilitation supplemented by training of the breathing rhythm or proprioception exercises, in patients with chronic peripheral vestibular disease. J Vestib Res. 2007;17(1):63–72. [PubMed] [Google Scholar]
- 66.Pavlou M, Kanegaonkar RG, Swapp D, Bamiou DE, Slater M, Luxon LM. The effect of virtual reality on visual vertigo symptoms in patients with peripheral vestibular dysfunction: a pilot study. J Vestib Res. 2012;22(5–6):273–281. [DOI] [PubMed] [Google Scholar]
- 67.Kao CL, Chen LK, Chern CM, Hsu LC, Chen CC, Hwang SJ. Rehabilitation outcome in home-based versus supervised exercise programs for chronically dizzy patients. Arch Gerontol Geriatr. 2010;51(3):264–267. [DOI] [PubMed] [Google Scholar]
- 68.Shepard NT, Smith-Wheelock M, Telian SA, Raj A. Vestibular and balance rehabilitation therapy. Ann Otol Rhinol Laryngol. 1993;102(3):198–205. [DOI] [PubMed] [Google Scholar]
- 69.Topuz O, Topuz B, Ardiç FN, Sarhus M, Ögmen G, Ardiç F. Efficacy of vestibular rehabilitation on chronic unilateral vestibular dysfunction. Clin Rehabil. 2004;18(1):76–83. [DOI] [PubMed] [Google Scholar]
- 70.Kammerlind ASC, Ledin TRE, Odkvist LM, Skargren EIB. Effects of home training and additional physical therapy on recovery after acute unilateral vestibular loss—a randomized study. Clin Rehabil. 2005;19(1):54–62. [DOI] [PubMed] [Google Scholar]
- 71.Pavlou M, Bronstein AM, Davies RA. Randomized trial of supervised versus unsupervised optokinetic exercise in persons with peripheral vestibular disorders. Neurorehabil Neural Repair. 2013;27(3):208–218. [DOI] [PubMed] [Google Scholar]
- 72.Yardley L, Donovan-Hall M, Smith HE, Walsh BM, Mullee M, Bronstein AM. Effectiveness of primary care-based vestibular rehabilitation for chronic dizziness. Ann Int Med. 2004;141(8):598–605. [DOI] [PubMed] [Google Scholar]
- 73.Shepard NT, Telian SA, Smith-Wheelock M. Habituation and balance retraining therapy: a retrospective review. Neurol Clin. 1990;8(2):459–475. [PubMed] [Google Scholar]
- 74.Cohen HS, Kimball KT. Decreased ataxia and improved balance after vestibular rehabilitation. Otolaryngol Head Neck Surg. 2004;130(4):418–425. [DOI] [PubMed] [Google Scholar]
- 75.Cohen HS, Kimball KT. Increased independence and decreased vertigo after vestibular rehabilitation. Otolaryngol Head Neck Surg. 2003;128(1):60–70. [DOI] [PubMed] [Google Scholar]
- 76.Cohen HS, Kimball KT. Changes in a repetitive head movement task after vestibular rehabilitation. Clin Rehabil. 2004;18(2):125–131. [DOI] [PubMed] [Google Scholar]
- 77.Schubert MC, Migliaccio AA, Clendaniel RA, Allak A, Carey JP. Mechanism of dynamic visual acuity recovery with vestibular rehabilitation. Arch Phys Med Rehabil. 2008;89(3):500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keim RJ, Cook M, Martini D. Balance rehabilitation therapy. Laryngoscope. 1992;102(11):1302–1307. [DOI] [PubMed] [Google Scholar]
- 79.Aquaroni Ricci N, Aratani MC, Caovilla HH, Freitas Gananca F. Effects of conventional versus multimodal vestibular rehabilitation on functional capacity and balance control in older people with chronic dizziness from vestibular disorders: design of a randomized clinical trial. Trials. 2012;13:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perez N, Santandreu E, Benitez J, Rey-Martinez J. Improvement of postural control in patients with peripheral vestibulopathy. Eur Arch Otorhinolaryngol. 2006;263(5):414–420. [DOI] [PubMed] [Google Scholar]
- 81.Chen PY, Hsieh WL, Wei SH, Kao CL. Interactive wiimote gaze stabilization exercise training system for patients with vestibular hypofunction. J Neuroeng Rehabil. 2012;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Telian S, Shepard N, Smith-Wheelock M, Kemink J. Habituation therapy for chronic vestibular dysfunction: preliminary results. Otolaryngol Head Neck Surg. 1990;103(1):89–95. [DOI] [PubMed] [Google Scholar]
- 83.Cohen HS, Kimball KT, Jenkin HA. Factors affecting recovery after acoustic neuroma resection. Acta Oto-Laryngologica. 2002;122(8):841–850. [PubMed] [Google Scholar]
- 84.Patatas OHG, Gananca CF, Gananca FF. Quality of life of individuals submitted to vestibular rehabilitation. Braz J Otorhinolaryngol. 2009;75(3):387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitney SL, Wrisley DM, Marchetti GF, Furman JM. The effect of age on vestibular rehabilitation outcomes. Laryngoscope. 2002;112(10):1785–1790. [DOI] [PubMed] [Google Scholar]
- 86.Bamiou DE, Davies RA, McKee M, Luxon LM. Symptoms, disability and handicap in unilateral peripheral vestibular disorders—effects of early presentation and initiation of balance exercises. Scand Audiol. 2000;29(4):238–244. [DOI] [PubMed] [Google Scholar]
- 87.Murray K, Carroll S, Hill K. Relationship between change in balance and self-reported handicap after vestibular rehabilitation therapy. Physiother Res Int. 2001;6(4):251–263. [DOI] [PubMed] [Google Scholar]
- 88.Nishino LK, Gananca CDF, Manso A, Herrerias De Campos CA, Korn GP. Personalized vestibular rehabilitation: medical chart survey with patients seen at the ambulatory of otoneurology of I.S.C.M.S.P. Rev Bras Otorrinolaringol. 2005;71(4):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aranda C, Meza A, Rodriguez R, Mantilla MT, Jauregui-Renaud K. Diabetic polyneuropathy may increase the handicap related to vestibular disease. Arch Med Res. 2009;40(3):180–185. [DOI] [PubMed] [Google Scholar]
- 90.Vitkovic J, Winoto A, Rance G, Dowell R, Paine M. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol. 2013;260(12):3039–3048. [DOI] [PubMed] [Google Scholar]
- 91.Wrisley DM, Whitney SL, Furman JM. Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol. 2002;23(4):483–487. [DOI] [PubMed] [Google Scholar]
- 92.Sun DQ, Ward BK, Semenov YR, Carey JP, Della Santina CC. Bilateral vestibular deficiency: quality of life and economic implications. JAMA Otolaryngol Head Neck Surg. 2014;140(6):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johansson M, Akerlund D, Larsen HC, Andersson G. Randomized controlled trial of vestibular rehabilitation combined with cognitive-behavioral therapy for dizziness in older people. Otolaryngol Head Neck Surg. 2001;125(3):151–156. [DOI] [PubMed] [Google Scholar]
- 94.Rossi-Izquierdo M, Santos-Pérez S, Soto-Varela A. What is the most effective vestibular rehabilitation technique in patients with unilateral peripheral vestibular disorders? Eur Arch Otorhinolaryngol. 2011;268(11):1569–1574. [DOI] [PubMed] [Google Scholar]
- 95.Winkler PA, Esses B. Platform tilt perturbation as an intervention for people with chronic vestibular dysfunction. J Neurol Phys Ther. 2011;35(3):105–115. [DOI] [PubMed] [Google Scholar]
- 96.Badaracco C, Labini FS, Meli A, De Angelis E, Tufarelli D. Vestibular rehabilitation outcomes in chronic vertiginous patients through computerized dynamic visual acuity and Gaze stabilization test. Otol Neurotol. 2007;28(6):809–813. [DOI] [PubMed] [Google Scholar]
- 97.Gottshall KR, Hoffer ME, Moore RJ, Balough BJ. The role of vestibular rehabilitation in the treatment of Meniere's disease. Otolaryngol Head Neck Surg. 2005;133(3):326–328. [DOI] [PubMed] [Google Scholar]
- 98.Mantello EB, Moriguti JC, Rodrigues-Junior AL, Ferrioli E. Vestibular rehabilitation's effect over the quality of life of geriatric patients with labyrinth disease. Braz J Otorhinolaryngol. 2008;74(2):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meli A, Zimatore G, Badaracco C, De Angelis E, Tufarelli D. Vestibular rehabilitation and 6-month follow-up using objective and subjective measures. Acta Otolaryngol. 2006;126(3):259–266. [DOI] [PubMed] [Google Scholar]
- 100.Morozetti PG, Ganança CF, Chiari BM. Comparison of different protocols for vestibular rehabilitation in patients with peripheral vestibular disorders. J Soc Bras Fonoaudiol. 2011;23(1):44–50. [DOI] [PubMed] [Google Scholar]
- 101.Tee LH, Chee NWC, Zhu M, Jin J. Functional outcomes after customized vestibular rehabilitation in subjects with chronic vestibular dysfunction. Physiother Sing. 2010;13(2):4–7. [Google Scholar]
- 102.Cowand JL, Wrisley DM, Walker M, Strasnick B, Jacobson JT. Efficacy of vestibular rehabilitation. Otolaryngol Head Neck Surg. 1998;118(1):49–54. [DOI] [PubMed] [Google Scholar]
- 103.Bittar RSM, Pedalini MEB, Lorenzi MC, Formigoni LG. Treating vertigo with vestibular rehabilitation: results in 155 patients. Rev Laryngol Otol Rhinol. 2002;123(1):61–65. [PubMed] [Google Scholar]
- 104.Bittar RS, Pedalini ME, Ramalho JO, Yoshimura R. Critical analysis of vestibular rehabilitation outcome according to dizziness etiology. Braz J Otorhinolaryngol. 2007;73(6):760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murray KJ, Hill K, Phillips B, Waterston J. Does otolith organ dysfunction influence outcomes after a customized program of vestibular rehabilitation? J Neurol Phys Ther. 2010;34(2):70–75. [DOI] [PubMed] [Google Scholar]