Supplemental Digital Content is Available in the Text.
Keywords: complex interventions, fidelity, Huntington disease, logic models, physical activity intervention development
Abstract
Background and Purpose:
We studied the development and delivery of a 14-week complex physical activity intervention for people with Huntington disease, where detailed information about the intervention was fully embedded in the trial design process.
Methods:
Intervention Development: The intervention was developed through a series of focus groups. The findings from the focus groups informed the development of a logic model for the physical activity intervention that was broadly consistent with the framework of self-determination theory. Intervention Delivery: Key components underpinning the delivery of the intervention were implemented including a defined coach training program and intervention fidelity assessment methods. Training of coaches (physical therapists, occupational therapists, research nurses, and exercise trainers) was delivered via group and 1:1 training sessions using a detailed coach's manual, and with ongoing support via video calls, and e-mail communication as needed. Detailed documentation was provided to determine costs of intervention development and coach training.
Results:
Intervention delivery coaches at 8 sites across the United Kingdom participated in the face-to-face training. Self-report checklists completed by each of the coaches indicated that all components of the intervention were delivered in accordance with the protocol. Mean (standard deviation) intervention fidelity scores (n = 15), as measured using a purpose-developed rating scale, was 11 (2.4) (out of 16 possible points). Coaches' perceptions of intervention fidelity were similarly high. The total cost of developing the intervention and providing training was £30,773 ($47,042 USD).
Discussion and Conclusions:
An important consideration in promoting translation of clinical research into practice is the ability to convey the detailed components of how the intervention was delivered to facilitate replication if the results are favorable. This report presents an illustrative example of a physical activity intervention, including the development and the training required to deliver it. This approach has the potential to facilitate reproducibility, evidence synthesis, and implementation in clinical practice.
Video Abstract available for more insights from the authors (see Supplemental Digital Content 1, http://links.lww.com/JNPT/A122).
INTRODUCTION
Huntington disease (HD) is a dominantly inherited neurodegenerative condition that affects the brain, causing dysfunction and death of medium spiny striatal projection neurons and thus disruption of corticostriatal pathways, with resultant gradual impairment of cognition and motor function, along with behavioral problems including apathy, anxiety, and irritability.1 Currently there is no disease-modifying treatment available for this condition, and very little known regarding effective symptomatic treatment. Encouraging regular physical activity throughout the developing disease may offer a means to enrich the lives of people with HD and their carers by helping to maintain independence, improve health, and subsequently reduce health and care costs.
The benefits of physical activity in maintaining cardiovascular health and reducing mortality in the general population are widely recognised,2 and there is an ever-increasing public health focus on physical activity for maintenance of health. Exercise interventions also seem to have similar, if not potentially better, mortality outcomes among a range of chronic diseases compared with drug interventions.3 There is also a growing interest in the potential of regular physical activity in people with neurodegenerative conditions, such as multiple sclerosis, Parkinson disease, and Huntington disease. Exercise and physical activity are secondary prevention strategies that have the potential to significantly impact the progression and management of neurodegenerative diseases, including maintaining function and improving postural control, gait, and health-related quality of life.4–6 However, many healthy individuals and those with neurodegenerative diseases have difficulty maintaining adherence to exercise programs. Developing interventions that are aimed at specifically promoting adherence and facilitating exercise uptake have thus been the focus of emerging research.
One of the challenging aspects of this developing research is achieving effective translation from research to practice. Even if studies demonstrate positive effects, implementation may not readily occur. One of the possible contributing factors to this may be the lack of detailed description of the various components of physical therapy interventions, which are often complex in nature. Guidelines for reporting interventions stress the importance of having well-defined, detailed descriptions of intervention components, including duration, dose or intensity, mode of delivery, essential processes, and a means of monitoring fidelity.7 Furthermore, elements of the intervention should have explicit descriptions of theoretical foundations. It is encouraging that there has been a gradual increase in research focusing on understanding the components of physical activity interventions in neurologic diseases. For example, a series of theory-based interventions underpinned by established associations between social cognitive theory (SCT) constructs and physical activity have been developed for people with multiple sclerosis.8 These interventions aimed to support behavior change through focusing on participants' self-efficacy, goal-setting, and outcome expectations.9–11 The Blue Prescription intervention has been implemented for people with multiple sclerosis in New Zealand, with a focus on combining professional help with self-help to increase physical activity.12 This study was underpinned by concepts related to motivational interviewing and promoting self-efficacy. In the Netherlands, Van Nimwegen and colleagues13 developed a physical therapy intervention for patients with Parkinson disease called ParkFit, which was also explicitly based on behavior change theories, such as SCT and the transtheoretical model of health behavior change.
Although theoretical frameworks do provide some support for the interventions mentioned previously, there is a lack of consistent linkage of these frameworks within the evaluation of such interventions to inform implementation into clinical practice. For example, logic models, which graphically depict the proposed relationship between activities and expected outcomes,14 are not routinely described and many studies in neurologic physical therapy, even if the intervention is described in detail, do not extend the approach to explicitly measure whether the intervention was delivered as it was intended (ie, fidelity). An additional challenge in designing physical activity interventions for patients with neurodegenerative diseases is the need to ensure that any theoretical framework is grounded in and relevant to the particular experiences and needs of the specific population. Given that these complex diseases require a high degree of care over the disease trajectory, it is particularly important to understand and account for the views of patients, families, and carers so as to make the intervention acceptable to the intended population.15
The purpose of this report is to describe the development and delivery of ENGAGE-HD, a single-blind, exploratory phase II multisite randomized, controlled trial of a 14-week physical activity intervention compared with a social contact control intervention (ISRCTN65378754).16 Multicenter research ethical approval was granted by South East Wales Research Ethics Committee B (approval number: 14/WA/0034).
Forty-six participants with genetically confirmed HD were recruited to the study; twenty-two participants were allocated to the physical activity intervention; 6 participants in this arm were withdrawn, and a total of 16 completed the intervention. The physical activity intervention involved 6 home visits from activity coaches, delivered over 14 weeks, with interim supporting phone calls. Although the protocol for this study has recently been published,16 this report did not provide aspects that might facilitate successful clinical implementation of the intervention. Here, we present details of the study that are essential to promote effective knowledge translation, with consideration of user perspectives, incorporation of a theoretically grounded logic model, coach training program, fidelity methods, and costs of intervention development and delivery.
METHODS
Development of the ENGAGE-HD Intervention
The ENGAGE-HD intervention is grounded in an established behavioral change theory, chosen because it was judged (by analyses of focus group results described below) to be the most appropriate for the complex needs of this population. A structured logic model then guided intervention delivery, and there was a system in place for promoting and evaluating therapist fidelity. Each of these unique features, which we argue should be more widely utilized in design of clinical trials, particularly in patients with complex health conditions such as neurodegenerative diseases, will ultimately help to facilitate translation of the results from this randomized trial into clinical practice.
Focus Groups
The underlying theoretical framework for the ENGAGE-HD intervention was developed through a series of focus groups. A purposive maximum variation sampling approach17 was used to capture varied perspectives from people with HD, their family members, carers, and professionals. People with HD and their caregivers (both formal carers and informal carers, ie, family members) were invited by post via regional care advisors of the Huntington's Disease Association of England and Wales (HDA). The HDA maintains a confidential mailing list of members who have agreed to be contacted in this way. All correspondence was initiated by the HDA, and no personally identifiable details were provided to the research team without the consent of the involved individuals. Eight focus groups including a total of 56 people were conducted. Of these, 26 were people with HD (46.4%; 18 male), 24 were carers or family members (42.9%; 18 female), and 6 were professionals (10.7%; 2 physical therapists, a physical therapy assistant, a health care assistant, an occupational therapist, and a nurse). The number of participants in each group ranged between 3 and 12. Several participants were at an early stage of disease progression and still able to live relatively independently; one participant was gene positive but asymptomatic. Others were at a much later stage of the disease and more severely disabled by their symptoms. Across all participants, there was involvement in a variety of activities ranging from relatively low intensity such as walking or gardening to more vigorous exercise such as running.
Focus group facilitators were all registered physical therapists with experience working with people with HD and their families. A single facilitator moderated each focus group using a semistructured topic guide covering 4 key areas about the physical activity experiences of people with HD: (1) descriptions of these experiences; (2) impact of the disease; (3) carer's experience; and (4) clarifying enablers for regular physical activity. When moderating the group, special attention was given to the needs of people with HD and the role of the family members and carers. Reframing, repetition, and expansion of the questions as required were used to encourage full participation of all present. A second facilitator was also present in each group to capture field notes. HDA care advisors were also in attendance at all meetings. In 3 of the 5 locations (Cardiff, Southampton, and Liverpool), 2 focus groups were conducted in parallel. Focus groups were digitally audio-recorded and transcribed verbatim; the accuracy of the transcripts was confirmed by both the focus group leader and the field notes.
Focus group audio-recordings and transcripts were analyzed thematically.18 These themes were identified as patterns in the discourse of focus group participants that corresponded with the research questions. The coding frame was developed inductively through an iterative process of data analysis. A second researcher double coded 25% of the data (2 of the 8 focus groups). Where 95% agreement was reached between the 2 coders, no action was taken. Alternately the coders reviewed areas of discrepancy and resolved these. There were no coding discrepancies that could not be resolved. QSR NVivo10 software was used (QSR International Pty Ltd 2014).
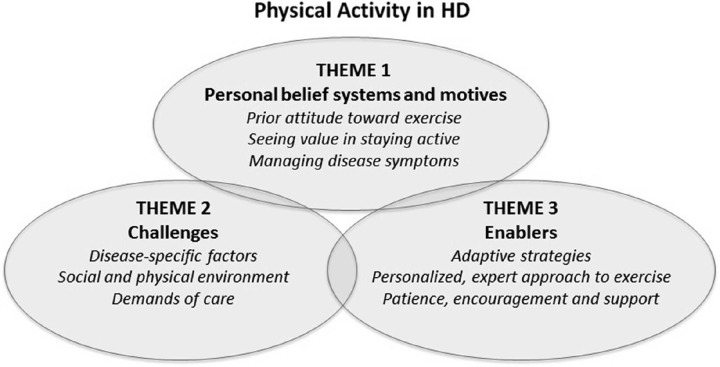
Three themes emerged from the focus groups: personal beliefs and motives, enablers, and challenges (Figure 1). Although many of the enablers and challenges to physical activity were not unique to this population—the challenge of integrating physical activity into existing schedules, for instance—other disease-specific factors were highlighted. In particular, it was clear that people with HD wanted to be provided with a range of options and be able to choose what sort of activities they might take part in, rather than being prescribed a rigid exercise plan. Although some patients enjoyed the social aspect of group activities such as golf, for instance, most were reluctant to exercise in public because of what they perceived to be a social stigma attached to their disease. Likewise, carers spoke of the need to tailor activity plans to the ability of the person with HD, rather than seeing each person as “just an HD sufferer.” For many patients whose activity levels were very limited, family members and carers suggested that starting with simple activities such as getting out of a chair without any assistance would allow people with HD to gradually build confidence. Patients themselves spoke of wanting specialist support and advice, to help them find activities that might be suitable for their condition. Finally, carers highlighted the need for patience, encouragement, and empathy when working with individuals with HD, in order to slowly build trust and help patients overcome the fear of falls or experiencing pain when exercising.
Figure 1.
The 3 themes that emerged from the focus groups: personal beliefs and motives, enablers, and challenges.
The findings from the focus group were interpreted as being broadly consistent with the framework of self-determination theory (SDT).19 Self-determination theory is a theory of human motivation that has been applied across a range of health behaviors, including physical activity. Self-determination theory suggests that motivation in general, and indeed with respect to physical activity, can be placed along a continuum from extrinsically motivated and regulated (for rewards or to satisfy an external demand) to the more autonomous, intrinsically integrated, and self-determined behavior as the motives become internalized. Self-determination is said to arise from feelings of autonomy (being in control of behavior and having choices), competence (experiencing a sense of mastery or skill), and relatedness (feeling connected to and understood by others).
Our participants described a range of regulatory styles along a continuum from intrinsic to extrinsic that could potentially have an impact on sustained physical activity behaviors. Some participants talked about physical activity as enjoyable and essential to their quality of life, and others participated only with sufficient encouragement from carers. In their talk of wanting to maximize independence, to challenge themselves and to improve their health, people with HD described intrinsic goals, which are associated with enhanced participation in exercise.19,20 However, these participants also experienced considerable challenges through their HD symptoms, such as loss of insight, balance, and motor function, which could negatively impact their ability to safely perform physical activity. Much like interventions that have incorporated a SDT framework with a psychiatric population, where motivational mechanisms were not different from those in the normal population even in the presence of disease-specific barriers to physical activity,21 we suggest the motivational processes underlying physical activity behavior in people with HD may be at least partially explained using this theory.
Development of Logic Model
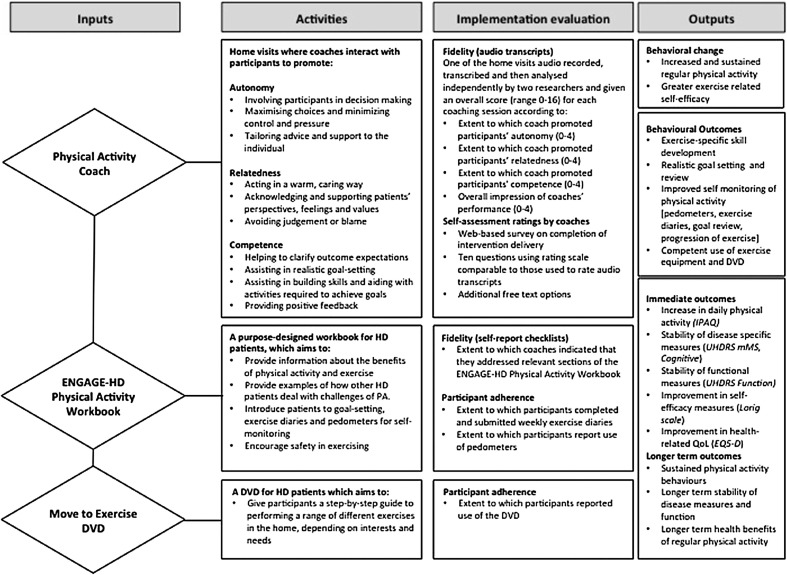
This complex intervention consisted of 3 main elements, namely the participant/coach interaction (underpinned by SDT), a purpose developed ENGAGE-HD Workbook, and an exercise DVD (Move to Exercise).6,22 Each of these elements is described in detail below. Figure 2 presents this in the form of a logic model, describing the key elements (inputs) and activities of the physical activity intervention (outputs).
Figure 2.
The logic model describing inputs, activities, and outputs. This has been adapted from the ENGAGE-HD trial protocol paper (open access article distributed under the terms of the Creative Commons Attribution License, http://creativecommons.org/licenses/by/4.016, to provide additional detail with respect to methods for implementation evaluation).
Participant/Coach Interaction
The coaching visits and the participant/coach interaction in the ENGAGE-HD intervention specifically aimed to develop self-determined physical activity behaviors through intentionally promoting feelings of autonomy, competence, and relatedness. Specifically, coaches were encouraged to promote autonomy by involving participants in any decisions, minimizing control or pressure, and tailoring advice and support to the individual. Competence was promoted through helping patients to clarify potential outcomes of physical activity, working with participants to set realistic and measurable goals, and providing positive feedback. Finally, relatedness was promoted through acting in a warm and caring way, expressing empathy and avoiding judgment and blame.
ENGAGE-HD Workbook
The ENGAGE-HD workbook was used as a guide for the interactions. Workbook-based approaches have been used to promote self-management approaches in other diseases and disorders, including the Bridges program used in patients post-stroke.23 During the first home visit, the coach introduced the participant to the program and the workbook. The workbook is structured into 5 distinct sections: (1) Exercise-Who Me?; (2) HD Experiences in Increasing Physical Activity; (3) Goals; (4) My Physical Activity Plan; and (5) Recording Progress. The initial interactions consider benefits of physical activity and each participant's individual exercise history. Participants are encouraged to identify specific areas in their lives (both formal and informal) that could be altered to promote physical activity for general well-being, and also to set specific physical activity goals. Instructions were provided for assuring safety of exercise, including use of perceived exercise scales, and also for use of pedometers (provided to participants) to measure physical activity. Further discussion topics on physical activity include implementing a daily activity plan, monitoring exercise intensity, and dealing with safety, weather, equipment, and typical barriers (eg, time, boredom, lack of equipment, lack of specific knowledge, and support).
Move to Exercise DVD
The final component of the ENGAGE-HD intervention is the Move to Exercise DVD. The Move to Exercise DVD was developed after consultation with people with HD, their family members, and physical therapists specializing in neurodegenerative diseases,24 and has been found to be acceptable and of benefit to people with HD.6,22 The individual DVD sections can be used differentially based on an individual's specific needs and targets, and the coaches work with the participants to identify relevant sections that may be appropriate for them. Although the exercise DVD is optional, it provides a specific activity, in addition to walking programs, that the coaches can focus on to facilitate increased physical activity.
Delivery of the ENGAGE-HD Intervention
An intervention in a clinical trial must be delivered in a systematic manner to facilitate translation of the intervention into clinical practice, if the results of the trial indicate it is safe and has potential for benefit. Key components of effective delivery are training of the coaches delivering the intervention, providing ongoing support, assessing costs for training and support, and fidelity monitoring.
Training of Coaches
The coaches delivering the ENGAGE-HD interventions were either (a) health care professionals (eg, physical therapists, occupational therapists, or nurses) with experience of delivering exercise-related activities or with specific experience with HD; or (b) exercise professionals. All staff had to meet specific health competencies, namely Skills for Life Competencies, developed by the National Health System (NHS) in the UK. (Competencies can be found at Skills for Life, accessed May 29, 2015: https://tools.skillsforhealth.org.uk/competence/show/html/id/2603/). Nevertheless, across the sites, the coaches would likely have a wide range of backgrounds and experiences, hence the need for centralized and standardized training and support.
The training model was for a team, including the intervention coordinator, trial chief investigator, and trial manager to travel to the site location and conduct a 6-hour training session in a small group setting (Table 1 for overview of the training program). Training for the coaches included a 1.5-hour, one-to-one session with either the chief investigator or the intervention coordinator. Both the chief investigator and the intervention coordinator were research physical therapists with extensive experience working with the HD community in both clinical practice and research, who oversaw development of the training materials and ongoing support of the coaching staff. A coach's manual was provided to each coach, and was used as a guide for each of the training sessions. The coaching manual gave an explicit, session-by-session guide, familiarized the coaches with the specific challenges of working with patients with HD, and offered a background to the intervention's SDT framework.
Table 1. Summary of Training, Support, and Monitoring for Physical Activity Coaches.
| Time | Description | |
|---|---|---|
| Initial training | 6-h training session for all site staff, including coaches with individualized 1.5-h training | • Delivered by either trial PI or the intervention coordinator |
| • Review of coach's manual, with explicit, session-by-session guide | ||
| • Familiarized the coaches with the specific challenges of working with patients with HD | ||
| • Offered a background to the intervention's SDT framework | ||
| Ongoing support | Minimum 2 discussions, others as needed | • Before first visit, coaches had video discussion with the intervention coordinator to assist them in interpreting a participant's baseline assessment scores |
| • After the first or second home visit, coaches had a further discussion with the intervention coordinator to discuss goal setting and address any concerns or issues | ||
| • Coaches were further encouraged to contact the intervention coordinator if they had any questions about the home visits as the intervention progressed, either by e-mail or videoc-onferencing |
Abbreviations: HD, Huntington disease; PI, principal investigator; SDT, self-determination theory.
Ongoing Support and Monitoring
In addition to the initial training sessions and coaching manuals, coaches received ongoing support from the intervention coordinator. This support is particularly important in helping to guide coaches who have had little or no experience of working with patients with this relatively rare disease. Before each coach visited a participant for the first time, they were able to have a discussion with the intervention coordinator to assist them to interpret a participant's baseline assessment scores (including measures of walking ability, cognitive function, a motor score, and a breakdown of scores on the Physical Performance Test). This allowed them to appropriately anticipate the ability level and potential needs of each participant. After the initial home visits, coaches had a further discussion with the intervention coordinator to develop realistic goals for the participants, based on each participant's particular interests and their current ability levels. Coaches were further encouraged to contact the intervention coordinator if they had any questions about the home visits as the intervention progresses, either by e-mail or video-conferencing.
Fidelity Monitoring
Fidelity of an intervention measures the extent to which the intervention was delivered in the way it was intended. In this study, fidelity was measured for each of the 3 elements of the intervention: the coach interactions, the Physical Activity Workbook, and the Move to Exercise DVD. Fidelity was measured by a combination of self-report checklists, independent analysis of audio-recordings, and a self-assessment completed by the intervention coaches.
After each of the 6 home visits, coaches were required to complete a short self-report checklist, indicating whether the content of each of the sessions was consistent with what was specified in the protocol and training manual. For visit 1, for instance, the checklist asks whether the coach introduced the ENGAGE-HD program, talked to the participant about the exercise workbook and DVD, and whether he or she discussed the idea of setting a series of activity-based goals. The checklists also recorded the number of minutes that coaches spent delivering each session.
Recognizing the limitation of self-report measures of intervention fidelity, we also included an independent assessment of the quality of the coaching sessions, based on audio-recordings of one of the coach home visits. The fidelity of the coach interactions was measured by assessing the extent to which each coach demonstrated efforts to promote a patient's autonomy, relatedness, and competence. Coaches were asked to audio-record one of their later home visits (typically the third of 6 visits). The audio files were transcribed and then independently rated by a member of the study team, using a rating scale that represented the core features of the intervention as described in the logic model (Table 2). For rating on the scale, coaches were given a 0 to 4 rating for the 3 SDT areas (autonomy, competence, and relatedness) and a final 0 to 4 score to reflect an overall impression of the coach's performance. Each recorded coaching session was accordingly scored from 0 to 16. Scoring of the sessions using this rating scale had 2 purposes: (1) the lead intervention coordinator was able to use the transcripts to provide coaches with constructive feedback on their interactions in-between visits, promoting ongoing fidelity; and (2) individual fidelity scores could be used as a potential mediating factor when exploring measures of benefit (blinded outcome measures). To ensure that the fidelity rating tool could be readily implemented in a clinical setting utilizing relatively novice raters, the study team member (who was a researcher and not involved in delivery of the intervention) and the intervention coordinator independently rated 3 audio files and compared ratings for agreement. For each of the 5 possible levels within each of the 4 items (autonomy, competence, relatedness, and overall impression), the ratings for the 2 raters were within one point of each other, and for 2 of the 3 total scores, there was 100% agreement.
Table 2. Rating Tool Used to Assess Fidelity of Delivering the ENGAGE-HD Intervention.
| Item | Description | Score | ||||
|---|---|---|---|---|---|---|
| 1. Autonomy | • Involves participants in decision making | Not at all | A great extent | |||
| • Minimizes control and pressure | 0 | 1 | 2 | 3 | 4 | |
| • Maximizes participants’ choices | ||||||
| • Provides a rationale for suggestions | ||||||
| • Allows the participant to overtly express the pros and cons of changing behavior | ||||||
| • Tailors advice and support | ||||||
| 2. Relatedness | • Acts in a warm and caring way | Not at all | A great extent | |||
| • Expresses empathy | 0 | 1 | 2 | 3 | 4 | |
| • Acknowledges and supports patients’ perspectives, feelings, and values | ||||||
| • Avoids judgment or blame | ||||||
| 3. Competence | • Helps to clarify outcome expectations (what a person might expect as result of the changes that they have made) | Not at all | A great extent | |||
| 0 | 1 | 2 | 3 | 4 | ||
| • Assists in realistic goal-setting and developing a tailored activity plan | ||||||
| • Assists in building skills and developing coping strategies required to achieve specific goals | ||||||
| • Provides positive feedback | ||||||
| 4. General impression | • Overall perception of participant/coach interaction is positive | Not at all | A great extent | |||
| • Coach is in command of the session and demonstrates ability to direct conversation and maintain focus | 0 | 1 | 2 | 3 | 4 | |
Fidelity of the intervention was further evaluated by asking coaches to complete a self-assessment of their perceived ability to deliver the intervention as it was intended to be delivered. We surveyed those coaches that had delivered the intervention for their opinions on the content and structure of the intervention and the issues surrounding its delivery. A set of 10 questions with a mix of rating scales (directly comparable to those scores used to rate fidelity) and free text answers were developed and delivered to the coaches via a web-based survey. The questions covered each coach's views on the training provided (including the audio-recording of one visit to assess fidelity), adherence of the intervention to SDT, accompanying materials used in the delivery of the intervention, and the intervention in general. Respondents were asked to identify themselves so that their answers could be linked to individual fidelity scores.
Costs of Intervention Development and Training
The costs of developing the intervention included costs of conducting the focus groups, encompassing recruitment material, venue hire and refreshments, travel reimbursement for staff and participants, staff time attending the focus groups and interpreting output, and transcription costs. These costs also included the costs of developing the workbook and the exercise DVD. This included staff time to develop the content, design fees for the workbook and the DVD, and licensing fees for the DVD.
A spreadsheet was used to record the travel and subsistence costs for the training team, the number of hours spent travelling to training, the number of hours spent in training (for both the training team and staff being trained), venue hire, and refreshment costs. The midpoint of the pay grade for each staff member attending training was used to calculate the hourly cost of their time, including UK National Insurance and pension on-costs. The cost of training varied by site and was largely influenced by travel and subsistence costs, reflecting the distance of the intervention site from the training team's base in Cardiff, UK.
RESULTS
Training the Coaches
Intervention delivery coaches were trained at a total of 8 sites. Coaches were a mixture of research nurses, physical therapists, occupational therapists, clinical researchers, and exercises trainers/scientists (Table 3). Almost all of the coaches had some experience with working with patients with neurodegenerative diseases, and many had direct experience in working with patients with HD.
Table 3. Qualifications and Backgrounds of Physical Activity Coaches.
| Coach # | Qualifications/Background | Number of Participants in Physical Activity Intervention | Experience of Working on Physical Therapy Interventions | Experience of Working With Patients With Neurologic Conditions | Experience of Working With Patients With HD |
|---|---|---|---|---|---|
| 1 | Research nurse, health visitor | 5 | No | Yes | Yes |
| 2 | Research nurse | 3 | No | Yes | No |
| 3 | Physical therapist | 3 | Yes | Yes | Yes |
| 4 | Occupational therapist | 2 | Yes | Yes | Yes |
| 5 | Research nurse | 2 | No | Yes | No |
| 6 | Exercise instructor | 1 | Yes | Yes | Yes |
| 7 | Exercise scientist, neurovascular researcher | 1 | Yes | No | No |
Abbreviation: HD, Huntington disease.
Over the course of the recruitment period, it became necessary to train additional staff for some sites because of staffing issues. These additional staff received telephone, web-based, or face-to-face training either individually or in pairs.
In addition to the site training, the intervention coordinator provided ongoing coaching and training for the physical activity coaches. The intervention coordinator had a minimum of 2 additional contacts with the coach per participant, which were carried out over web-based video conferencing or phone. In addition to these set contact times, there was frequent e-mail communication and occasional additional video coaching as needed (a range of 1-4 additional contacts, including e-mail and video conferencing). During these sessions, the intervention coordinator was able to provide detailed advice and guidance to assure the coaches provided the intervention as intended, and further to provide advice and support for any HD-specific issues. The coordinator documented all contact.
Challenges to Delivery of Intervention
The most notable challenge of delivering the intervention was training and support of the coaches, who had a wide range of health professional backgrounds and experiences. Although all staff met the defined competencies, there were some staff with fairly limited experience in delivering physical activity interventions, thus requiring greater initial contact and support. Disease-specific issues also needed consideration in planning the intervention delivery for all of the coaches. Coaches needed to be considerate of patient's individual schedules and preferences for appointment times, and often needed to work closely with family members and carers. In all cases, family members were integral to the intervention delivery, both to schedule and to facilitate uptake of the physical activity program. Some participants struggled with formulating physical activity goals, HD-specific concerns, such as apathy and behavioral concerns, also resulted in an increased need for support and advice from the intervention coordinator.
Fidelity of the Intervention
Seventeen participants completed physical activity interventions, which were delivered by 7 coaches (Table 3). The self-report checklists completed by each of the coaches at the first home visit 1 indicate that in 100% of sessions (16/16), coaches introduced the participants to the Physical Activity Workbook, gave the participants the exercise DVD and discussed the concept of goal-setting with the participant in 100% of the sessions (16/16). Sessions lasted on average 72.3 minutes.
Fidelity scores for coach interactions, based on audio transcripts of the third intervention session, were assessed for 15 of the 16 participants. Overall scores ranged from 7 to 14 out of a possible 16 points, with a mean (standard deviation) score across the coaches of 11.0 (2.4). Coach interactions scored an average of 2.5/4 for autonomy, 3.0/4 for relatedness, 2.7/4 for competence, and 2.8/4 for the overall impression.
All 7 of the coaches completed self-assessment surveys pertaining to intervention fidelity. Self-assessment scores were on average higher than those assigned by the independent rater, namely 3.1/4 for autonomy, 3.3/4 for relatedness, and 3.0/4 for competence. In relation to the process of audio-recording a session, one coach reported that they found it “distracting” and another reported that the process may have influenced their behavior as they were acutely more aware of asking open questions during the session. Only one coach reported that the recording of the session may have affected participants adversely, making the discussion less free than it might otherwise have been. For the remainder of the coaches, they reported no difficulties or undue influences from recording the session.
Three coaches reported perceived barriers to delivering the intervention. Generally, these were logistical issues; the difficulty of scheduling home visits as per protocol in conjunction with other commitments (both for the coaches and participants) or when there had been a change in the participant's home life or disease state. One coach (a research nurse) responded that lack of confidence may have prevented the coach from being as assertive as was perhaps needed.
Costs of Intervention Development and Training
The total cost of developing the intervention was £30,773 ($47,042 USD). This included the cost of developing the workbook, developing and producing the DVD, and conducting the 5 focus groups. The total cost for delivering training at all of the sites, and for the additional staff training throughout the trial to date, was £18,821 ($28,771). Costs for delivering the intervention are not reported here, and will be reported with the main study findings.
DISCUSSION
This report describes the approach used for the delivery of a trial of a complex intervention in people with a neurodegenerative disease; the intent of the approach was to seamlessly ensure the implementation of research to clinical practice. Therapist-led interventions aimed at increasing patients' physical activity require an interaction between therapist and patient, and can typically be considered complex interventions; that is, interventions involving many interactive components.25 Importantly, the theoretical basis for the complex intervention should be explicitly defined,26 a recommendation that has been echoed by researchers evaluating physical therapy interventions for patients with neurologic disorders.27
An important consideration in promoting translation of clinical research, such as the intervention presented here, is the ability to convey the detailed components of how the intervention was delivered to facilitate replication if the results are favorable. In this report, we present a detailed description of a physical activity intervention as an illustrative example, including the development and the training required to deliver it. This approach has the potential to facilitate reproducibility, evidence synthesis, and implementation in clinical practice. Additional details pertaining to the design of the study, including assessments, the control group, and additional details of intervention delivery have been previously reported.16
The ENGAGE-HD intervention included a theoretically grounded logic model, where components of the intervention were defined to inform evaluation. Crucially, the concepts related to the theoretical framework have been integrated throughout all aspects of this randomized controlled trial design: during the development of the intervention, its delivery, and its evaluation. We argue that this approach is essential to ensure knowledge translation to clinical practice. The intervention reporting is consistent with TIDieR guidelines,7 and was developed in line with the Medical Research Council of the United Kingdom's Framework for Development and Evaluation of Complex interventions (MRC framework).26 The MRC framework advocates the use of a cyclical development process, whereby all the components are fully developed and evaluated in an iterative process so as to ultimately ensure widespread and sustainable implementation of a specific intervention.
It is useful to inform the understanding of the components and mechanisms of the intervention to make inferences about whether the intervention worked, how it may have worked, and which factors contributed to its success or failure. One approach for making explicit the relationship between various interacting elements of an intervention is through the development of a logic model.28 Logic models are typically a graphical representation of how an intervention is supposed to work, illustrating the various inputs, activities, outputs, and expected outcomes. Such an approach provides a clear framework for monitoring and evaluating different aspects of study implementation.29 In this study, we present the development of a logic model for an intervention that was explicitly developed on the basis of particular experiences and needs of the population with HD.
In therapist-led interventions, another aspect to consider is that of fidelity of intervention delivery (ie, the extent to which the intervention is delivered as intended).30 Reporting of treatment fidelity is fairly commonplace in psychotherapy and counseling interventions, and specific tools have been developed for its measurement.31,32 Yet, researchers have identified a failure to monitor, evaluate, and promote treatment fidelity within physical therapy trials. Hildebrand and colleagues, for instance, argue that “in occupational therapy (OT) and physical therapy (PT) outcomes research, treatment fidelity methods have not been utilized, which in our view is a serious gap that impedes novel treatment development and testing in these rehabilitation fields.”33 In those studies where fidelity has been measured, results have often indicated variable delivery of intervention techniques. In this study, we developed a fidelity monitoring system that included review of self-report checklists, as well as review and rating of transcribed audiotapes from actual sessions. This rating scale enabled independent raters to determine the extent to which the intervention was being delivered as intended. The results from the independent fidelity ratings suggest that the intervention was being delivered as intended; however, coaches tended to rate themselves higher on average than the independent rater. As the coach's ratings were completed at the end of the overall study, this may have been a reflection of their increase in confidence and competence as the study progressed. Review of these audiotaped sessions also enabled the intervention coordinator to provide feedback to the coaches to make modifications to ongoing sessions.
A final important aspect that is included in this report is related to understanding the costs involved in the development and delivery of the interventions. Clearly, a full-scale health economics evaluation is imperative for phase III trials; however, we argue that preliminary costs need to be documented at an early stage in intervention development. Indeed, feasibility of an intervention should extend not only to adherence and acceptability but also to costs and training and support requirements. In our intervention, we have purposely allowed coaching staff with differing levels of skills and expertise, and some staff therefore required greater remote support in terms of training and delivery of the intervention. To further inform future implementation, we will conduct sensitivity analyses regarding staff costs, including testing the effect of using staff at a higher/lower grade to conduct the training and delivery of the intervention. The outcome of this work will be reported with the main study results. It is only by recording and considering these factors at an early stage that we can make suggestions as to the best configuration for implementation in the future.
CONCLUSIONS
In order for a physical activity intervention to have the potential for effective translation and implementation into clinical practice, detailed information about the theoretical underpinnings, fidelity monitoring, and cost of development must be provided. This approach is novel and not yet routinely utilized in physical therapy trials. We argue here that in order for physical therapy research to take the critical steps forward in translating to the clinic, these principles must be embedded in future clinical trial designs. In this report, we have demonstrated how this can be achieved within a physical activity trial for individuals with a neurodegenerative disease. However, it is only once full-scale evaluation of the trial is complete can we then consider the potential effects of the components of the intervention, training support, and fidelity on the effectiveness of the intervention.
Supplementary Material
ACKNOWLEDGMENTS
We extend our special thanks to Cath Stanley, Karen Crowder, Jacqueline Peacock, Charles Whaley, Carol Dutton, Mike Cummings, Ann Pathmanaban, Anita Daly, Eve Payler, Heather Thomas, Veena Agarwal, Astrid Burrell, and all of the Huntington disease families in Oxford, Cardiff, Liverpool, Plymouth, Dorset, and Southampton who attended focus groups where they shared their experiences of physical activity and how they could be best supported to be active with Huntington disease. We also thank Dr Fiona Jones of Bridges (http://www.bridges-stroke.org.uk/management_group.php) who kindly worked with us to develop ideas “based on Bridges” that could be relevant to the HD population. We are also extremely grateful to our colleagues, from the EHDN Physiotherapy working group, Jessie van der Bent and Karin Bunnig at the Huntingtoncentre TOPAZ Overduin, for sharing their ideas and successes of conducting regular walking programs and other activities for people with HD.
In the implementation of this trial, we acknowledge all the research staff at each of the participating sites (NHS Grampian, Birmingham and Solihull Mental Health NHS Foundation Trust, North Staffordshire Combined NHS Healthcare Trust, Sheffield Children's NHS Foundation Trust, North Bristol NHS Trust, University Hospital Southampton NHS Foundation Trust, Central Manchester University Hospitals NHS Foundation Trust, and Cardiff University).
Footnotes
The study was funded by Health and Social Care Wales, United Kingdom. The South East Wales Trials Unit is funded by the Wales Assembly Government through Health and Care Research Wales.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal's Web site (www.jnpt.org).
The authors declare no conflict of interest.
REFERENCES
- 1.Walker FO. Huntington's disease. Semin Neurol. 2007;27(2):143–150. [DOI] [PubMed] [Google Scholar]
- 2.Blair SN, Morris JN. Healthy hearts—and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19(4):253–256. [DOI] [PubMed] [Google Scholar]
- 3.Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23(5):631–640. [DOI] [PubMed] [Google Scholar]
- 5.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalil H, Quinn L, van Deursen R, Martin R, Rosser A, Busse M. Adherence to use of a home-based exercise DVD in people with Huntington disease: participants' perspectives. Phys Ther. 2012;92(1):69–82. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 8.Motl RW. Lifestyle physical activity in persons with multiple sclerosis: the new kid on the MS block. Mult Scler. 2014;20(8):1025–1029. [DOI] [PubMed] [Google Scholar]
- 9.Coote S, Gallagher S, Msetfi R, et al. A randomised controlled trial of an exercise plus behaviour change intervention in people with multiple sclerosis: the step it up study protocol. BMC Neurol. 2014;14(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motl RW, Dlugonski D. Increasing physical activity in multiple sclerosis using a behavioral intervention. Behav Med. 2011;37(4):125–131. [DOI] [PubMed] [Google Scholar]
- 11.Dlugonski D, Motl RW, Mohr DC, Sandroff BM. Internet-delivered behavioral intervention to increase physical activity in persons with multiple sclerosis: sustainability and secondary outcomes. Psychol Health Med. 2012;17(6):636–651. [DOI] [PubMed] [Google Scholar]
- 12.Mulligan H, Treharne GJ, Hale LA, Smith C. Combining self-help and professional help to minimize barriers to physical activity in persons with multiple sclerosis: a trial of the “Blue Prescription” approach in New Zealand. J Neurol Phys Ther. 2013;37(2):51–57. [DOI] [PubMed] [Google Scholar]
- 13.Van Nimwegen M, Speelman AD, Smulders K, et al. Design and baseline characteristics of the ParkFit study, a randomized controlled trial evaluating the effectiveness of a multifaceted behavioral program to increase physical activity in Parkinson patients. BMC Neurol. 2010;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin SL, Heath GW. A six-step model for evaluation of community-based physical activity programs. Prev Chronic Dis. 2006;3(1):A24. [PMC free article] [PubMed] [Google Scholar]
- 15.Ho AK, Hocaoglu MB. Impact of Huntington's across the entire disease spectrum: the phases and stages of disease from the patient perspective. Clin Genet. 2011;80(3):235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busse M, Quinn L, Dawes H, et al. Supporting physical activity engagement in people with Huntington's disease (ENGAGE-HD): study protocol for a randomized controlled feasibility trial. Trials. 2014;15:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polite DF, Hungluer BP. Nursing Research: Principles and Methods. Philadelphia: Lippincott; 1999. [Google Scholar]
- 18.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. [Google Scholar]
- 19.Teixeira PJ, Carraça EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self-determination theory: a systematic review. Int J Behav Nutr Phys Act. 2012;9:78. 10.1186/1479-5868-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortier MS, Duda JL, Guerin E, Teixeira PJ. Promoting physical activity: development and testing of self-determination theory-based interventions. Int J Behav Nutr Phys Act. 2012;9:20. 10.1186/1479-5868-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorensen M. Motivation for physical activity of psychiatric patients when physical activity was offered as part of treatment. Scand J Med Sci Sport. 2006;16:391–398. [DOI] [PubMed] [Google Scholar]
- 22.Khalil H, Quinn L, van Deursen R, et al. What effect does a structured home-based exercise programme have on people with Huntington's disease? A randomized, controlled pilot study. Clin Rehabil. 2013;27(7):646–658. [DOI] [PubMed] [Google Scholar]
- 23.McKenna S, Jones F, Glenfield P, Lennon S. Bridges self-management program for people with stroke in the community: a feasibility randomized controlled trial. Int J Stroke. 2015;10(5):697–704. [DOI] [PubMed] [Google Scholar]
- 24.Quinn L, Busse M, Khalil H, Richardson S, Rosser A, Morris H. Client and therapist views on exercise programmes for early-mid stage Parkinson's disease and Huntington's disease. Disabil Rehabil. 2010;32(11):917–928. [DOI] [PubMed] [Google Scholar]
- 25.Michie S, Abraham C, Eccles MP, Francis JJ, Hardeman W, Johnston M. Strengthening evaluation and implementation by specifying components of behaviour change interventions: a study protocol. Implement Sci. 2011;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2769032&tool=pmcentrez&rendertype=abstract. Accessed September 18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis T, Motl R. Physical activity behavior change in persons with neurologic disorders: overview and examples from Parkinson disease and multiple sclerosis. J Neurol Phys Ther. 2013;37(2):85–90. [DOI] [PubMed] [Google Scholar]
- 28.Kellogg WK. Logic Model Development Guide. Michigan: WK Kellogg Foundation; 2004. [Google Scholar]
- 29.Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015;350:h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dusenbury L, Brannigan R, Falco M, Hansen WB. A review of research on fidelity of implementation: implications for drug abuse prevention in school settings. Heal Educ Res. 2003;18(2):237–256. [DOI] [PubMed] [Google Scholar]
- 31.Nuro KF, Maccarelli L, Martino S, et al. Yale Adherence and Competence Scale (YACSII) Guidelines. Yale University Psychotherapy Development Center; 2005. [Google Scholar]
- 32.Lane C, Huws-Thomas M, Hood K, Rollnick S, Edwards K, Robling M. Measuring adaptations of motivational interviewing: The development and validation of the behavior change counseling index (BECCI). Patient Educ Couns. 2005;56(2):166–173. [DOI] [PubMed] [Google Scholar]
- 33.Hildebrand MW, Host HH, Binder EF, et al. Measuring treatment fidelity in a rehabilitation intervention study. Am J Phys Med Rehabil. 2012;91(8):715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.