Abstract
Analysis of 786 NF1 mutation-positive subjects with clinical diagnosis of neurofibromatosis type 1 (NF1) allowed to identify the heterozygous c.5425C>T missense variant (p.Arg1809Cys) in six (0.7%) unrelated probands (three familial and three sporadic cases), all exhibiting a mild form of disease. Detailed clinical characterization of these subjects and other eight affected relatives showed that all individuals had multiple cafè-au-lait spots, frequently associated with skinfold freckling, but absence of discrete cutaneous or plexiform neurofibromas, Lisch nodules, typical NF1 osseous lesions or symptomatic optic gliomas. Facial features in half of the individuals were suggestive of Noonan syndrome. Our finding and revision of the literature consistently indicate that the c.5425C>T change is associated with a distinctive, mild form of NF1, providing new data with direct impact on genetic counseling and patient management.
Introduction
Neurofibromatosis type 1 (NF1) (MIM #162200) is an autosomal dominant condition with a birth incidence of 1/3500 live births,1 manifesting a considerable inter- and intra-familial clinical variability. Major diagnostic characteristics include café-au-lait spots (CaLS), skinfold freckling (SF), Lisch nodules (LN) and neurofibromas (NF), which occur in the majority of patients and represent key features of this disorder. In addition, a number of other features, including macrocephaly, learning problems, short stature, hypertelorism and thorax abnormalities, are found in NF1 patients, but are not pathognomonic for the trait. About a quarter of these patients develop one or more complications leading to significant morbidity and increased mortality. They include various malignant tumors, scoliosis, long-bone dysplasia and cardiovascular manifestations, such as vasculopathies, hypertension or congenital heart disease. Diagnosis is made based on the diagnostic criteria defined at the NIH 1987 NF consensus conference.2
NF1 is caused by inactivating intragenic mutations or deletions of the NF1 gene (MIM #613113), which spans 350 kb and contains 61 translated exons.3 NF1 encodes neurofibromin, a multifunctional protein acting as a RAS-specific GTPase activating protein down-regulating RAS function. RasGTPase activating protein activity is mediated by the GTPase activating protein related domain (GRD) (residues 1198–1530). Located adjacent to the C-terminus of the GRD, two additional functionally relevant regions, the Sec-14p homologous (Sec) (residues 1560–1698) and plekstrin homology (PH)-like (residues 1715–1816) domains, form a bipartite lipid-binding module.4
To date, only two clinically relevant genotype–phenotype correlations have been reported in NF1. The first applies to patients heterozygous for germline large genomic deletions encompassing the NF1 gene.5 These deletions occur in about 4% of all NF1 cases.6 Differently from subjects with intragenic mutations, these patients display a severe phenotype with facial dysmorphism, cognitive deficits, cardiovascular anomalies and early onset multiple cutaneous neurofibromas (CNFs), together with a higher lifetime risk for developing malignant peripheral nerve sheath tumors. The second correlation relates to the association between a 3-nt deletion in exon 17 of the NF1 gene (c.2970_2972delAAT, p.Met992del) and absence of CNFs, subcutaneous neurofibromas (SCNFs) and external plexiform neurofibromas (PNFs).7 The disease in these patients is milder, with an increased rate of pulmonary valve stenosis (PVS). Of note a phenotype resembling NF1, Legius syndrome (LS, MIM #611431), is caused by heterozygous inactivating mutations in SPRED1 (Sprouty-related, EVH1 domain containing 1, MIM #609291). This disorder is less severe than NF1, and characterized by CaLS and SF, without CNFs and PNFs, LN, osseous lesions and optic pathway gliomas (OPGs).8
Here, we report on a recurrent missense NF1 variant, predicting the p.Arg1809Cys amino-acid change, identified in six unrelated families sharing a distinctive disorder within the NF1 phenotypic spectrum, but characterized by high prevalence of CaLS and SF, without NFs and other NF1-associated malignancies.
Materials and methods
Patients were referred for diagnostic testing to the Molecular Genetics Laboratory of CSS-Mendel Institute (Rome, Italy) and the Medical Genetics Unit of Padua University Hospital (Padua, Italy), because of a clinical suspect of NF1. Participating practitioners filled out a clinical questionnaire about each patient. Clinical and genetic analyses were conducted with the approval of the institutional review boards of the participating institutions. Informed consent was obtained from all patients or their legal guardians. Permission for publication of photographs was obtained. NF1, PTPN11 and SPRED1 mutation analysis was performed by direct sequencing on all their coding exons and flanking intronic portions (NF1, NM_000267.3; PTPN11, NM_002834.3; SPRED1, NM_152594.2). NF1 exon numbering followed that used in the NCBI Reference Sequence (NG_009018.1), which places c.5425C>T variant within exon 38. This exon corresponds to exon 29 according to the ‘historical NF Consortium NF1 exon numbering'.9 Bidirectional Sanger sequencing was performed using the ABI BigDye Terminator Sequencing Kit v.3.1 (Applied Biosystems, Foster City, CA, USA) and ABI 3130, and ABI3100 Genetic Analyzers (Applied Biosystems). Primer sequences and PCR conditions are available upon request. The multiplex ligation-dependent probe amplification kits no. P081/P082 and no. P295-B1 (MRC-Holland, Amsterdam,the Netherlands) were used to screen for the presence of intragenic deletions or duplications in the NF1 and SPRED1 genes, respectively, according to the manufacturer's instructions. High-resolution melting (HRM) scanning was used to search for the occurrence of the c.5425C>T change in population-matched unaffected controls. HRM optimization strategies for genotyping are available on request. In familial cases, paternity was confirmed by STR analysis. The six probands plus three additional family members carrying c.5425C>T have been entered in the LOVD NF1 database, including a description of their phenotype (www.LOVD.nl/NF1, patient IDs 00019904, 00019941–00019948).
Results
Mutation screening allowed to identify 786 NF1 mutation-positive subjects. Among them, six unrelated patients were found to be heterozygous for c.5425C>T transition in exon 38 (Supplementary Figure S1). The predicted amino-acid change is non-conservative, resulting in the replacement of a positively charged residue with a cysteine residue (p.Arg1809Cys). Different evidences supported the pathogenic role of this missense change. First, Arg1809 is invariantly conserved among orthologs (Supplementary Figure S2). Consistently, this amino-acid change was considered as deleterious by different in silico prediction programs (SIFT, score =0.00; PolyPhen-2, score=1.00; Mutation Taster, P=0.99). Second, p.Arg1809Cys was the only variant within the entire coding region (CDS) and exon–intron junctions of NF1 gene identified in all affected individuals. Copy number changes involving NF1 and SPRED1, and functionally relevant variants in the entire CDS of SPRED1 and PTPN11 were excluded in all subjects. To exclude that c.5425C>T was a common variant in the Italian population, 460 healthy unrelated subjects were screened by means of HRM scanning (Supplementary Figure S3), and none of them was positive for this missense change. In agreement with our finding, this change had not been reported in public databases (dbSNP138 and 1000 Genomes). Finally, genotyping of parental DNAs demonstrated the de novo origin of this missense variant in the three families with sporadic occurrence of disease, as well as cosegregation in the three families transmitting the trait (Figure 1a).
Figure 1.
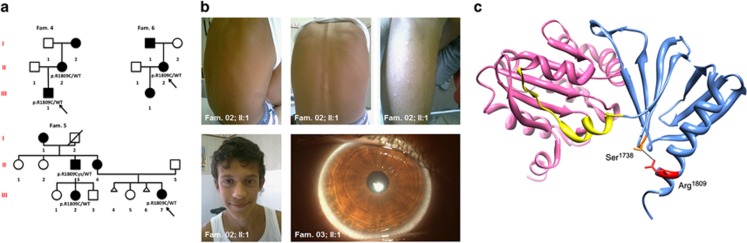
The c.5425C>T change in NF1 is associated with a distinctive NF1 form. (a) Pedigrees of the three families cosegregting NF1 and the NF1 mutation. Squares and circles indicate males and females, respectively; open symbols indicate unaffected individuals, filled symbols indicate affected individuals; arrows indicate index cases, and symbols with a slash indicate deceased family members. WT, wild-type allele. (b) Representative clinical features of subjects heterozygous for the c.5425C>T missense change in NF1. This mutation is associated with a mild phenotype consisting mainly of pigmentary signs (CaLS and SF) and lack of NFs. Other typical findings, including LN, OPGs and osseous dysplasia are uncommon or absent, whereas a high incidence of facial features resembling NS is observed. (c) Location of Arg1809 in the tridimensional structure of the neurofibromin Sec/PH-like bipartite module. The cartoon shows the ribbon representation of the module together with location of the residue (lateral chain reported as stick representation) mutated in the studied NF1 subjects. The Sec domain (residues 1560–1698) (pink), which is implicated in lipid binding, is connected to a regulatory PH-like domain (residues 1715–1816) (light blue) by a partly helical linker (residues 1699–1714) (yellow). Arg1809 (red) is in close contact with the backbone of Ser1738 (dark blue). The H-bond between these residues is also shown (black line).
Clinical features of the subjects heterozygous for the transition are shown in Figure 1b, summarized in Table 1, and compared with those reported in available large NF1 cohorts in Supplementary Table S1. All examined individuals with the c.5425C>T change displayed a distinctive and mild form of NF1. Specifically, they exhibited six or more CaLS, which were frequently associated with SF. Remarkably, none of the patients had discrete CNFs, SCNFs, evident PNFs, OPG or other malignancies. Other major NF1 features, including LN and typical osseous lesions were not found in any of these subjects. Macrocephaly, thoracic anomalies, reduced growth and learning problems were observed to occur in these subjects with similar prevalence compared with the NF1 general population. None of them had PVS or other congenital heart disease. Notably, half of the examined cases displayed facial features suggestive of Noonan syndrome (NS, MIM #163950), including ptosis, low-set posteriorly rotated ears, hypertelorism, triangular face, as well as short neck.10
Table 1. Clinical features of 14 subjects from six families with c.5425C>T change in NF1.
| Cases | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Features | Family 1 | Family 2 | Family 3 | Family 4 | Family 5 | Family 6 | |||||||||
| I:2 | II:2 | III:1 | I:1 | II:3 | II:4 | III:2 | III:7 | I:1 | II:2 | III:1 | Total | ||||
| Age (years) at observation | 17 | 16 | 10 | 54 | 35 | 6 | 87 | 65 | 62 | 29 | 21 | 60 | 39 | 3.3 | |
| Sex | M | M | F | F | F | M | F | M | F | F | F | M | F | F | 5M/9F |
| CaLS | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 14/14 (100%) |
| Freckling | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 9/10 (90%) | ||||
| Lish nodules | No | No | No | No | No | No | No | No | No | No | 0/10 (0%) | ||||
| Cutaneous neurofibromas | No | No | No | No | No | No | No | No | No | No | No | No | No | 0/13 (0%) | |
| Subcutaneous neurofibromas | No | No | No | No | No | No | No | No | No | No | No | No | No | 0/13 (0%) | |
| External plexiform neurofibromas | No | No | No | No | No | No | No | No | No | No | No | No | No | 0/13 (0%) | |
| Spinal neurofibromas | No | 0/1 (0%) | |||||||||||||
| Optic pathway glioma | Noa | No | No | No | No | Noa | No | Noa | No | Noa | No | 0/11 (0%) | |||
| Short stature | No | No | No | Yes | No | No | No | No | No | No | 1/10 (10%) | ||||
| Macrocephaly | Yes | No | Yes | No | No | No | No | No | No | No | 2/10 (20%) | ||||
| Other neoplasias | No | No | No | No | No | No | No | No | No | No | No | 0/11 (0%) | |||
| Thoracic anomalies | PE | PE, SW | PE | No | No | No | No | No | No | No | No | 3/11 (27%) | |||
| Learning problems | No | Yes | No | No | No | No | No | No | No | No | No | No | No | 1/13 (8%) | |
| Scoliosis | No | Yes | No | No | No | No | No | No | Yes | No | 2/10 (20%) | ||||
| Pulmonary valve stenosis | No | No | No | No | No | No | No | No | No | No | 0/10 (0%) | ||||
| NS features | P, TF, CP LSPRE | H, LSPRE, SN | LSE, P | LSE, H | No | No | No | No | No | 4/9 (44%) | |||||
| Others | No | MJL, SH, EEoE, FF, CD, NoPVF | No | No | No | No | No | No | No | ||||||
Abbreviations: CD, celiac disease; CP, close palate; EEoE, easy extensibility of the ears; F, female; FF, flat feet; H, Hypertelorism; LSE, low set ears; LSPRE, low set posteriorly rotated ears; M, male; MJL, mild joint laxity; NoPVF, narrowing of the peripheral nasal visual field; NS, Noonan syndrome; P, ptosis; PE, pectus excavatum; SH, skin hyperelasticity; SN, short neck; SW, scapular winging; TF, triangular face.
Absent on MRI scanning of the brain.
Discussion
The c.5425C>T missense change had previously been reported as either a rare variant11 or a NF1-causing mutation.12, 13 In the present study, we provided evidence for its de novo occurrence in three sporadic individuals, and cosegregation with the trait in other three pedigrees, including families with three generations. This per se provides unequivocal proof that c.5425C>T has functional relevance and underlies a trait within the NF1 phenotypic spectrum. Based on the examined NF1 cohort, we estimate that this variant might account for approximately 1% of NF1 mutation-positive cases. This frequency, however, may represent an underestimate of its prevalence in the general population as the mild phenotype, which is expected to result in an overlooked diagnosis.
Arg1809 is located within the PH-like domain of the GTPase. This domain is adjacent to the C-terminus of the GRD, and forms a bipartite module with the Sec domain.4 Specifically, Arg1809 is located at the end of the C-terminal α-helix, close to the end of PH-like domain. A number of crystal structures of PH domains have been determined. Although residue conservation is not high, their overall structure is well preserved, and characterized by two orthogonal β-sheets made of seven β-strands, followed by an α-helix. The loops of the β-sheets are very different in length, and their structures are difficult to be determined due to their high flexibility,14 which likely underlies the functional specificity of individual domains. Based on the available Sec/PH-like bipartite module of neurofibromin (RCSB-PDB accession number 2D4Q) Arg1809 is solvent exposed, facing away from the protein core (Figure 1c), with its lateral chain hydrogen bonded with the backbone of residue Ser1738, contributing to the intradomain interaction between the C-terminal α-helix and the loop connecting the β2- and β3-strands. These considerations suggest that substitution of Arg1806 to cysteine might affect such intradomain interaction, and possibly lead to a rearrangement of the entire secondary structure of the domain with possible impact on lipid-binding properties of the adjacent Sec domain.4
The clinical phenotype associated with the c.5425C>T substitution was observed to be mild in all cases, with no patient developing CNFs, SCNFs or evident PNFs. This is remarkable considering that CNFs are an hallmark of NF1, and virtually all NF1 adult patients develop CNFs. These benign tumors are a major cause of morbidity in NF1 and present observation has immediate clinical impact. The absence of subcutaneous and plexiform lesions in present cohort should be taken with more caution. Whereas CNFs are mostly visible and palpable, SCNFs and internal PNFs are more difficult to recognize and quantify. MRI scanning studies should be performed to investigate the presence of internal plexiform tumors among subjects carrying the c.5425C>T change. We also observed a significant lower incidence of LN among these subjects, compared with the general NF1 population (Supplementary Table S1). LN represent one of the most common features of the disease. Based on published data, they were found in two of three members of a family with c.5425C>T substitution and mild features of NF1 and NS without congenital heart disease and CNFs.13 In another family, this change was associated with CaLS and a severe NS phenotype with several additional defects, such as Arnold–Chiari I malformation, hypoplastic corpus callosum, syringomyelia, hydronephrosis, malrotation of the bowel and premature menopause, coexisting, however, with a de novo PTPN11 mutation (p.Phe285Leu) in the index patient.12 It is likely that the athypical symptoms observed in this patient resulted from the additive effect of two mutations affecting different genes, as reported in some subjects with neurofibromatosis-Noonan syndrome (MIM #601321).15, 16 These observations are in line with the present findings pointing to an association between c.5425C>T change and facial features of NS, including ptosis, low-set posteriorly rotated ears, hypertelorism, short neck and triangular face. Although none of the patients exhibited PVS, accurate cardiac screening on larger cohorts might be considered to assess whether the c.5425C>T substitution results in a higher risk of developing PVS or other congenital heart diseases.
Multigenerational families with multiple CaLS were initially recognized by Riccardi,17 who suggested the possibility of a disorder distinct from NF1. Consistent with this hypothesis, prior NF1 mutation testing, linkage studies argued for genetic heterogeneity of this phenotype.18, 19 Successively, studies allowed to identify a 3-bp deletion in exon 22 of the gene (c.2970_2972delAAT, p.Met992del) as responsible for a mild form of NF1 with multiple CaLS and SF as cardinal signs, in the absence of CNFs.7 Genetic heterogeneity for familial CaLS in association with SF without LN, NFs or other features of NF1, was confirmed by the identification of inactivating mutations in SPRED18 as the molecular event underlying the NF1 clinically related LS. The present data add a novel genotype–phenotype correlation to the clinical spectrum of NF1. Similarly to the previously reported c.2970_2972delAAT, c.5425C>T causes a mild phenotype with major features consisting in pigmentary signs (CaLS and SF), without neurofibromas or other NF1-related malignancies. The present and published data recommend to consider NF1 mutation screening in adults with multiple CaLS, with or without SF and no other NF1 diagnostic features, starting from exons 22 and 38 (exons 17 and 29, following the previously used NF1 exon numbering), before to eventually extend the analysis to the remaining coding exons.
Acknowledgments
We would like to express our gratitude to the patients and their family, who made this study possible. We thank Angela Alberico and Stefania Cavone for skilfull technical assistance. This work was supported, in part, by funding from AIRC (IG 13360) and Telethon-Italy (GGP13107) to MT, and Italian Ministry of Health (RF-2010-2310935 and RC-2014) to ADL.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Huson SM, Hughes R: The Neurofibromatoses: A Clinical and Pathogenetic Overview. Chapman and Hall: London, 1994. [Google Scholar]
- Stumpf DA, Alksne JF, Annegers JF et al: Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol 1988; 45: 575–578. [PubMed] [Google Scholar]
- Cawthon RM, Weiss R, Xu GF et al: A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell 1990; 62: 193–201. [DOI] [PubMed] [Google Scholar]
- D'Angelo I, Welti S, Bonneau F, Scheffzek K: A novel bipartite phospholipid-binding module in the neurofibromatosis type 1 protein. EMBO Rep 2006; 7: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayes LM, Burke W, Riccardi VM et al: Deletions spanning the neurofibromatosis 1 gene: identification and phenotype of five patients. Am J Hum Genet 1994; 54: 424–436. [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Bottillo I, Dasdia MC et al: Deletions of NF1 gene and exons detected by multiplex ligation-dependent probe amplification. J Med Genet. 2007; 44: 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya M, Huson SM, Davies M et al: An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970-2972 delAAT): evidence of a clinically significant NF1 genotype-phenotype correlation. Am J Hum Genet 2007; 80: 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brems H, Chmara M, Sahbatou M et al: Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nat Genet 2007; 39: 1120–1126. [DOI] [PubMed] [Google Scholar]
- De Luca A, Buccino A, Gianni D et al: NF1 gene analysis based on DHPLC. Hum Mutat 2003; 21: 171–172. [DOI] [PubMed] [Google Scholar]
- Roberts AE, Allanson JE, Tartaglia M, Gelb BD: Noonan syndrome. Lancet 2013; 381: 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ars E, Kruyer H, Morell M et al: Recurrent mutations in the NF1 gene are common among neurofibromatosis type 1 patients. J Med Genet 2003; 40: e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström AM, Ekvall S, Strömberg B et al: A severe form of Noonan syndrome and autosomal dominant café-au-lait spots - evidence for different genetic origins. Acta Paediatr 2009; 98: 693–698. [DOI] [PubMed] [Google Scholar]
- Ekvall S, Sjörs K, Jonzon A, Vihinen M, Annerén G, Bondeson ML: Novel association of neurofibromatosis type 1-causing mutations in families with neurofibromatosis-noonan syndrome. Am J Med Genet A 2014; 164: 579–587. [DOI] [PubMed] [Google Scholar]
- Scheffzek K, Welti S: Pleckstrin homology (PH) like domains - versatile modules in protein-protein interaction platforms. FEBS Lett 2012; 586: 2662–2673. [DOI] [PubMed] [Google Scholar]
- Bertola DR, Pereira AC, Passetti F et al: Neurofibromatosis-Noonan syndrome: molecular evidence of the concurrence of both disorders in a patient. Am J Med Genet A 2005; 136: 242–245. [DOI] [PubMed] [Google Scholar]
- Prada CE, Zarate YA, Hagenbuch S, Lovell A, Schorry EK, Hopkin RJ: Lethal presentation of neurofibromatosis and Noonan syndrome. Am J Med Genet A 2011; 155A: 1360–1366. [DOI] [PubMed] [Google Scholar]
- Riccardi VM: Neurofibromatosis: clinical heterogeneity. Curr Probl Cancer 1982; 7: 1–34. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Hulsebos T, Steijlen PM, der Kinderen DJ, vd Steen A, Hamel BC: Exclusion of the neurofibromatosis 1 locus in a family with inherited café-au-lait spots. Am J Med Genet 1993; 46: 472–474. [DOI] [PubMed] [Google Scholar]
- Abeliovich D, Gelman-Kohan Z, Silverstein S et al: Familial café au lait spots: a variant of neurofibromatosis type 1. J Med Genet 1995; 32: 985–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.