Abstract
Aims:
The use of platelet-rich plasma (PRP) alone in periodontal defects has been controversial and inconclusive. Hence, the present study was designed with the aim to assess the clinical and radiographic effectiveness of PRP alone in infrabony defects.
Materials and Methods:
Thirty infrabony defects were treated with either autologous PRP with open flap debridement (OFD) or autologous PRP + demineralized freeze dried bone graft (DFDBA) with OFD or OFD alone. Clinical parameters recorded were gingival index, plaque index, probing depth (PD), clinical attachment level (CAL), and gingival recession (REC). Radiographic parameters included defect depth reduction, defect resolution, and crestal bone level. All the parameters were recorded at baseline and 12 months postoperatively.
Results:
Mean PD reduction and CAL gain were greater in PRP + DFDBA (4.88 ± 1.12 mm and 4.26 ± 1.85 mm) and PRP (4.86 ± 2.12 mm and 4.10 ± 1.47 mm) groups than the control group (2.69 ± 1.37 mm and 1.27 ± 0.89 mm).
Conclusions:
Within the limits of the study, all the three groups showed significant improvement in clinical parameters from baseline to postoperative 12 months. The amount of defect depth reduction and defect resolution treated with PRP alone group were significantly < PRP + DFDBA. The results pertaining to these parameters were significantly better than the control group.
Keywords: Demineralized freeze-dried bone graft, infrabony defects, platelet-rich plasma, regeneration
INTRODUCTION
Regeneration is defined as the reproduction or reconstitution of a lost or injured a part of the body in such a way that the architecture and function of the lost or injured tissues are completely restored.[1] The complex series of events associated with periodontal regeneration involves recruitment of locally derived progenitor cells to the site. The progenitor cells can subsequently differentiate into periodontal ligament-forming cells, cementoblasts, or bone-forming osteoblasts.[2] Therefore, the key to periodontal regeneration is to stimulate the progenitor cells to re-occupy the defects.[3] Growth factors are vital modulators during this process which can induce the migration, attachment, proliferation, and differentiation of periodontal progenitor cells.[4]
This led periodontal researchers and clinicians to focus on the use of polypeptide growth factors for periodontal regeneration.[5,6,7] It was shown that osteoblasts proliferate in response to platelet-derived growth factor (PDGF) alone or with the addition of a progression factor to induce mitosis.[8,9] Transforming growth factor-β (TGF-β), a multifunctional growth factor that is chemotactic for bone cells, increases the differentiated function of osteoblasts, osteoblast precursors, and extracellular matrix formation, such as type I collagen,[10] and stimulates the proliferation of gingival fibroblastic cells, the formation of blood vessels, the re-modeling of extracellular matrix, and the formation of granulation tissue during the healing of periodontal tissue.[11] Platelet-rich plasma (PRP) is an autologous source of PDGF and TGF-β that is obtained by isolating and concentrating human platelets to 338% by gradient density centrifugation.[12]
Various researchers have investigated if a combination of regenerative therapies would promote maximum resolution of the defects. Since then, bone replacement grafting has been combined with a synthetic cell-binding peptide (P-15), guided tissue regeneration (GTR), enamel matrix derivative, and PRP.[13,14,15,16,17] However, other studies[18,19,20,21] suggested that the use of PRP failed to improve the results obtained with GTR and bone substitutes. de Obarrio et al.[22] presented case reports in which platelet gel was used successfully in combination with demineralized freeze-dried bone graft (DFDBA) for the treatment of periodontal osseous defects. The combination of PRP and DFDBA was compared to the combination of DFDBA and saline in a study by Piemontese et al.[23] and PRP alone in a study by Ilgenli et al.[24]
Therefore, this study was conducted with the aim to compare the clinical and radiographic effectiveness of PRP when used alone and when added to DFDBA in the treatment of periodontal endosseous defects. The study also evaluates the effects of the two treatment modalities independently when compared to control group, that is, receiving open flap debridement (OFD) alone.
MATERIALS AND METHODS
Patient and defect selection
Ten patients (7 males and 3 females) were selected from the outpatient department of the Institute of Dental Sciences, Bareilly. Signed consent was obtained from patients, and the study was approved by the Ethics Committee of MJP Rohilkhand University, Bareilly. Thirty infrabony defects were included in the study.
The inclusion criteria were adult patients in good general health and diagnosed with chronic advanced periodontitis based on the 1999 consensus classification of periodontal diseases,[25] presence of three deep intrabony defects (three-walled) with a probing depth (PD) >5 mm located in the interproximal area in maxillary or mandibular posterior teeth in three different quadrants. Radiographic evidence of the defects should exist. Base line radiographs should reveal a defect depth (distance from cemento-enamel junction [CEJ] to the base of the defect) of minimum 4 mm.
Exclusion criteria included smoking, antibiotic, or anti-inflammatory treatment or the known use of any medication with the potential to affect periodontal tissues within the preceding 6 months, and pregnancy.
The defects were assigned randomly to three groups. The control group (C) consisted of sites treated with OFD alone. Whereas, test group A consisted of sites treated with PRP alone and test group B received PRP in combination with DFDBA. The patient and defect characteristics of the three groups at baseline yielded no significant differences among any of the patient-associated variables or baseline levels of defect depth.
Presurgical therapy
Prior to the surgery, each patient was given careful instructions on proper oral hygiene measures. Full mouth supra- and sub-gingival scaling and root planning procedures were performed under local anesthesia. In addition, occlusal stents were fabricated at this stage with cold-cured acrylic resin on a cast model obtained from an alginate impression. To assess the tissue changes reproducibly, grooves were placed on the occlusal stents to compare the pre- and post-surgical measurements. Six to Eight weeks following phase I therapy, periodontal evaluation was performed to confirm the suitability of the sites for this study.
Clinical parameters recorded at baseline and 12 months after surgery were plaque index (PI),[26] gingival index (GI),[26] PD, and clinical attachment level (CAL). Measurements were performed with a manual periodontal probeδ and recorded. Intra-examiner calibration was achieved by examination of 10 patients twice, 24 h apart before beginning the study. Calibration was accepted if measurements at baseline and 24 h were similar to ± 1 mm at the 90% level. The method was previously described by Pradeep et al.[27]
In addition to, clinical measurements and radiographs were taken to measure the radiographic defect depth reduction and defect resolution. The standardized radiographs were taken using the conventional long-cone technique. Defect fill was assessed by measuring distance between CEJ and base of the defect (BD). The distance between alveolar crest (AC) and BD depicted defect resolution. Change in AC level was also seen as a measurement of distance between CEJ and AC.
Preparation of platelet-rich plasma
Approximately, 20 ml blood was drawn from each patient by venipuncture of the antecubital vein, on the day of surgery. Blood was collected in sterile plastic test tubes that contained anticoagulant. The blood containing test tubes were shaken gently to enhance complete mixing of the blood with anticoagulant. They were kept at room temperature for a minimum of 45 min to minimize the complement activity. Later, blood containing test tubes were centrifuged using centrifugal machine at 3000, revolutions/min for 10 min, which resulted in the separation of three basic fractions because of differential densities: The bottom red blood cells (RBCs), middle PRP, and the top layer of platelet poor plasma (PPP). A total of 2–3 ml of the top layer, corresponding to the PPP, was aspirated with a pipette and collected in a separate sterile plastic tube. The PRP was collected along with the top 1–2 mm of RBC fraction because it is also rich in newly synthesized platelets.[28]
PRP was activated to a gel form using 10% CaCl2 and autologous thrombin, the latter being prepared from PPP as described by Su et al.[29]
Surgical procedure
Following administration of local anesthesia, buccal, and lingual sulcular incisions were made, and mucoperiosteal flaps were elevated. Care was taken to preserve as much interproximal soft tissue as possible.
Meticulous defect debridement and root planning were carried out with the use of ultrasonic instruments and curettes. No osseous re-contouring was done. DFDBA graft particles were mixed with coagulated autologous PRP preparation (1:1) in a sterile dappen dish.[28] This mixture was properly condensed in the test site to the level of the surrounding bony walls in test group B. Autologous PRP, in the gel form was packed into the defect in the test group A.
Postoperative care
All patients were prescribed 500 mg amoxicillin three times daily for 1 week, and instructed to rinse with 0.12% chlorhexidine for at least 3 weeks, twice a day. Dressing and sutures were removed 1 week postoperatively. Patients were examined weekly for 1 month after surgery and then at 3, 6, 9, and 12 months. Postoperative care included reinforcement of oral hygiene and mechanical plaque control whenever necessary. Subgingival instrumentation was avoided before 12 months to prevent the disruption of developing attachment apparatus.
Posttreatment assessments
All the clinical parameters, PI, GI, PD, and CAL, were recorded 12 months postoperatively using previously used acrylic stents. Periapical radiographs were taken after 12 months using paralleling technique to maintain standardization. These radiographs were scanned with HP Epson scanner. Computer-assisted image analysis of the radiographs was done with the help of image analysis software (AutoCad Ver2004; Autodesk, California, United States).
Statistical analysis
Mean values and standard deviations were calculated for all clinical and radiographic parameters at baseline and 12 months. Taking into account the paired nature of the changes from baseline to 12 months in each group, the Wilcoxon signed rank matched pair test was performed for the pair-wise statistical analysis of these data. The Mann–Whitney U-test was applied to compare clinical and radiographic outcomes between three groups (two groups at a time) at baseline, 12 months postsurgery and differences due to the time interval. All the calculations were performed using Statistical Package of Social Sciences (IBM SPSS Statistics; New York, United States), version 10.
RESULTS
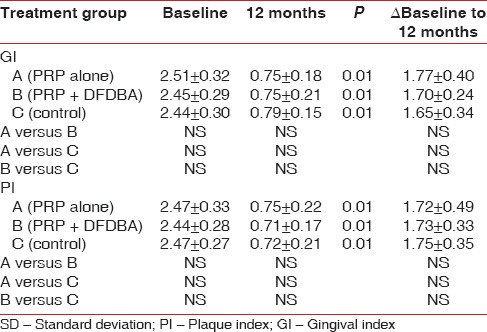
A total of 10 patients (30 sites) participated in the study. Two patients did not turn up for the third surgery making a total of 28 sites. Of these 10 sites were treated with PRP alone, 9 sites with PRP + DFDBA and 9 sites with OFD alone. The treatment groups were initially comparable. All treated cases showed uneventful wound healing. A statistically significant reduction in the PI and GI was observed in all three groups at 12 months postoperatively (P < 0.01) [Table 1].
Table 1.
Mean and SD of GI and PI at baseline, at 12 months and difference between pre- and post-measurements
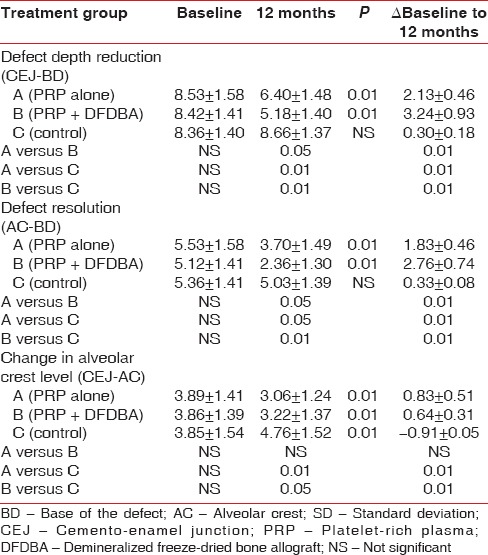
Mean values for pocket PD, CAL, and gingival recession at baseline and 12 months with mean changes are reported in Table 2. Both, PRP + DFDBA and PRP sites presented with a significantly greater PD reduction CAL gain than control sites at 12 months postoperatively. Defect depth reduction was found to be significantly greater (P < 0.01) in the PRP group (3.42 ± 0.62) and PRP + DFDBA group (3.89 ± 0.68) than OFD alone (0.15 ± 0.28) [Table 2]. PRP + DFDBA treated group showed greater defect depth reduction than PRP alone (P < 0.01) [Table 3]. In terms of percentage, defect depth reduction in PRP group (40.19%) was significantly <PRP + DFDBA group (47.18%), but significantly greater than control group (1.75%) [Table 3]. Similar results were obtained for defect resolution. PRP + DFDBA showed significantly greater resolution (2.37 ± 0.65) than PRP alone (1.90 ± 0.51) at P < 0.05. The results of PRP were significantly greater than control group (0.13 ± 0.52) at P < 0.01 [Table 3].
Table 2.
Mean and SD of PD, CAL and amount of recession at baseline, at 12 months and difference between pre- and post-measurements
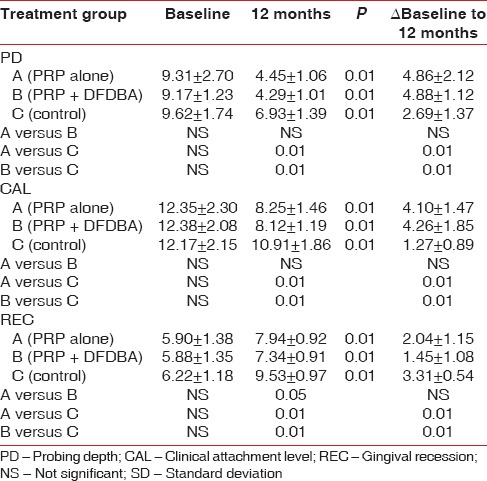
Table 3.
Mean and SD of amount of defect depth reduction, bone resolution and crestal bone loss at baseline, at 12 months and difference between pre- and post-measurements
DISCUSSION
The present study was designed to assess the outcome of the two regenerative techniques, that is, PRP alone and PRP in conjunction with DFDBA in treating periodontal intrabony defects and also compares their effectiveness using clinical and radiographic parameters.
Only three-wall intrabony periodontal defects (IBDs) are included in the present study because the number of bony walls remaining has been found to be positively correlated with regeneration potential when grafting procedures were used.[30,31,32] Three-wall-containing defects have been shown to provide the best spatial relationship for defect bridging by vascular and cellular elements from the periodontal ligament and adjacent osseous wall.[33] Statistically, clinical and radiographic parameters of the subjects were similar in all the three groups at baseline. All participants maintained an acceptable oral hygiene.
In this study, a significant reduction in PD and CAL gain is found in all three groups when compared with baseline and 12 months. However, there was more PD reduction (4.88 ± 1.18 mm) in the PRP + DFDBA-treated and PRP-treated groups (4.86 ± 1.18 mm) compared with the participants treated with conventional periodontal flap surgery alone. Similarly, CAL gain was more in PRP + DFDBA-treated (4.26 ± 1.85 mm) and PRP-treated groups (4.10 ± 1.47 mm) than OFD alone.
Richardson et al.[34] and Bender et al.[35] evaluated the treatment of human vertical intrabony defects with DFDBA at 6 months postsurgery; they found a PD reduction of 2.0 and 2.8 mm and a CAL gain of 2.6 and 2.4 mm, respectively.
The greater CAL gain and PD reduction observed in the DFDBA + PRP group in the present study may be explained by the additional biologic effects of PRP. The mechanism of action of PRP is attributed to several of its cellular effects. Marx et al.[12] demonstrated that the platelet stored growth factors and its specific cell receptors. PRP fractions promote important cell responses involved in tissue repair including fibroblast migration, cell adhesion, and myeloblastic differentiation of gingival fibroblasts[36] resulting in accelerated soft and hard tissue healing.[37] PRP has a favorable effect on human osteoclast-like cells, and acts both to enhance bone regeneration and as an activator in wound healing.[38]
Only, a few studies have been conducted to evaluate the influence of PRP alone on bone healing. The treatment of bony defects with PRP alone, in the femurs or calvaria of rabbits or the mandibles of dogs, showed no improvement of bone formation.[39,40,41] Conversely, favorable results were reported by Anitua,[42] Sammartino et al.,[43] and Simon et al.[44] in the bone healing of extraction sockets treated with PRP only. In intrabony defects, only few studies have been conducted using PRP alone[24,28,32,45] and the results are inconclusive.
In our study, mean intrabony defect depth reduction in the PRP group was 3.42 ± 0.62 mm. although it was significantly <PRP + DFDBA group (3.89 ± 0.68 mm), but it was significantly higher than OFD alone (0.15 ± 0.28 mm). Percentage reduction in defect depth obtained in our study for PRP group (40.18 ± 1.29) was although lesser than previous studies by Pradeep et al.,[27,32] but it was comparable with that obtained with PRP + DFDBA (47.19 ± 0.97). Although numerous studies have reported the treatment of periodontal intrabony defects using PRP, this study compares the effectiveness of PRP with OFD on one hand and with allograft combined with PRP, which has established high efficacy, on the other hand.
The discrepancy between the clinical (PD and CAL) outcomes and radiographic IBD in the control group may be attributable to the fact that OFD may have resulted in the presence of a long junctional epithelium between the newly regenerated tissues and the root surface and not true bone regeneration. This was similar to results obtained by Pradeep et al.[32] in their study, where CAL gain in OFD group was 2.83 ± 0.91 and defect depth reduction was only 0.13 ± 1.46.
The aim of periodontal therapy is to achieve a gain in CAL and return the periodontal tissues to a healthy state. The results of our study showed that PRP + DFDBA treated group showed most favorable clinical and radiographic outcomes. This was substantiated by various studies in the literature. Hanna et al.[46] reported a CAL gain of 3.15 mm for the PRP/bovine-derived xenograft, group. Okuda et al.[47] demonstrated a mean CAL gain of 3.4 ± 1.7 mm and a mean defect fill of 3.5 ± 1.5 mm with PRP/porous HA and concluded that adding PRP to HA led to a significantly more favorable clinical improvement in the treatment of IBDs. Lekovic et al.[17] demonstrated a defect fill of 4.82 ± 1.34 mm at 21 sites treated with PRP/bovine porous bone mineral. The varying results from different studies may derive from the use of different graft materials.
Although PRP preparation is autologous and economical, addition of an extra step to the surgical procedure and increase in time should also be considered.
CONCLUSION
Majority of the studies in the past have been conducted using PRP as an adjunct to various bone substitutes. This has masked the evaluation of actual regenerative potential of PRP. Hence, this study is one of the few studies that compare the use of PRP alone with control that is, OFD. The study demonstrates that use of autologous PRP was effective in the treatment of intrabony defects. However, amount of defect depth reduction was lesser than that obtained with PRP + DFDBA, but it was significantly higher than OFD alone. Thus, considering its autologous nature and decrease in the cost, PRP offers an excellent option to the clinician for regenerative approach. Further, long-term clinical trials are required to substantiate this finding.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Polimeni G, Xiropaidis AV, Wikesjö UM. Biology and principles of periodontal wound healing/regeneration. Periodontol 2000. 2006;41:30–47. doi: 10.1111/j.1600-0757.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 2.Bartold PM, Shi S, Gronthos S. Stem cells and periodontal regeneration. Periodontol 2000. 2006;40:164–72. doi: 10.1111/j.1600-0757.2005.00139.x. [DOI] [PubMed] [Google Scholar]
- 3.Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984;11:494–503. doi: 10.1111/j.1600-051x.1984.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 4.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19(1 Suppl):23S–37S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 5.Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74:1282–92. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- 6.Lynch SE, Wisner-Lynch L, Nevins M, Nevins ML. A new era in periodontal and periimplant regeneration: Use of growth-factor enhanced matrices incorporating rhPDGF. (679-80).Compend Contin Educ Dent. 2006;27:672–8. [PubMed] [Google Scholar]
- 7.McGuire MK, Kao RT, Nevins M, Lynch SE. rhPDGF-BB promotes healing of periodontal defects: 24-month clinical and radiographic observations. Int J Periodontics Restorative Dent. 2006;26:223–31. [PubMed] [Google Scholar]
- 8.Graves DT, Valentin-Opran A, Delgado R, Valente AJ, Mundy G, Piche J. The potential role of platelet-derived growth factor as an autocrine or paracrine factor for human bone cells. Connect Tissue Res. 1989;23:209–18. doi: 10.3109/03008208909002419. [DOI] [PubMed] [Google Scholar]
- 9.Lynch SE, Nixon JC, Colvin RB, Antoniades HN. Role of platelet-derived growth factor in wound healing: Synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987;84:7696–700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka T, Taniguchi Y, Gotoh K, Satoh R, Inazu M, Ozawa H. Morphological study of recombinant human transforming growth factor beta 1-induced intramembranous ossification in neonatal rat parietal bone. Bone. 1993;14:117–23. doi: 10.1016/8756-3282(93)90237-5. [DOI] [PubMed] [Google Scholar]
- 11.Okuda K, Murata M, Sugimoto M, Saito Y, Kabasawa Y, Yoshie H, et al. TGF-beta1 influences early gingival wound healing in rats: An immunohistochemical evaluation of stromal remodelling by extracellular matrix molecules and PCNA. J Oral Pathol Med. 1998;27:463–9. doi: 10.1111/j.1600-0714.1998.tb01913.x. [DOI] [PubMed] [Google Scholar]
- 12.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 13.Garrett S, Bogle G. Periodontal regeneration with bone grafts. Curr Opin Periodontol. 1994;12:168–77. [PubMed] [Google Scholar]
- 14.Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res. 2002;37:300–6. doi: 10.1034/j.1600-0765.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- 15.Lekovic V, Camargo PM, Weinlaender M, Nedic M, Aleksic Z, Kenney EB. A comparison between enamel matrix proteins used alone or in combination with bovine porous bone mineral in the treatment of intrabony periodontal defects in humans. J Periodontol. 2000;71:1110–6. doi: 10.1902/jop.2000.71.7.1110. [DOI] [PubMed] [Google Scholar]
- 16.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Djordjevic M, Kenney EB. The use of bovine porous bone mineral in combination with enamel matrix proteins or with an autologous fibrinogen/fibronectin system in the treatment of intrabony periodontal defects in humans. J Periodontol. 2001;72:1157–63. doi: 10.1902/jop.2000.72.9.1157. [DOI] [PubMed] [Google Scholar]
- 17.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Kenney EB. Comparison of platelet-rich plasma, bovine porous bone mineral, and guided tissue regeneration versus platelet-rich plasma and bovine porous bone mineral in the treatment of intrabony defects: A reentry study. J Periodontol. 2002;73:198–205. doi: 10.1902/jop.2002.73.2.198. [DOI] [PubMed] [Google Scholar]
- 18.Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Multi-center clinical comparison of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and ABM in human periodontal osseous defects. Six month results. J Periodontol. 2000;71:1671–9. doi: 10.1902/jop.2000.71.11.1671. [DOI] [PubMed] [Google Scholar]
- 19.Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I, Sculean A. Effect of platelet-rich plasma on the healing of intra-bony defects treated with a natural bone mineral and a collagen membrane. J Clin Periodontol. 2007;34:254–61. doi: 10.1111/j.1600-051X.2006.01044.x. [DOI] [PubMed] [Google Scholar]
- 20.Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I, Sculean A. Effect of platelet-rich plasma on the healing of intrabony defects treated with an anorganic bovine bone mineral and expanded polytetrafluoroethylene membranes. J Periodontol. 2007;78:983–90. doi: 10.1902/jop.2007.060349. [DOI] [PubMed] [Google Scholar]
- 21.Christgau M, Moder D, Wagner J, Glässl M, Hiller KA, Wenzel A, et al. Influence of autologous platelet concentrate on healing in intrabony defects following guided tissue regeneration therapy: A randomized prospective clinical split-mouth study. J Clin Periodontol. 2006;33:908–21. doi: 10.1111/j.1600-051X.2006.00999.x. [DOI] [PubMed] [Google Scholar]
- 22.de Obarrio JJ, Araúz-Dutari JI, Chamberlain TM, Croston A. The use of autologous growth factors in periodontal surgical therapy: Platelet gel biotechnology – Case reports. Int J Periodontics Restorative Dent. 2000;20:486–97. [PubMed] [Google Scholar]
- 23.Piemontese M, Aspriello SD, Rubini C, Ferrante L, Procaccini M. Treatment of periodontal intrabony defects with demineralized freeze-dried bone allograft in combination with platelet-rich plasma: A comparative clinical trial. J Periodontol. 2008;79:802–10. doi: 10.1902/jop.2008.070436. [DOI] [PubMed] [Google Scholar]
- 24.Ilgenli T, Dündar N, Kal BI. Demineralized freeze-dried bone allograft and platelet-rich plasma vs platelet-rich plasma alone in infrabony defects: A clinical and radiographic evaluation. Clin Oral Investig. 2007;11:51–9. doi: 10.1007/s00784-006-0083-y. [DOI] [PubMed] [Google Scholar]
- 25.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(Suppl):610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 27.Pradeep AR, Shetty SK, Garg G, Pai S. Clinical effectiveness of autologous platelet-rich plasma and Peptide-enhanced bone graft in the treatment of intrabony defects. J Periodontol. 2009;80:62–71. doi: 10.1902/jop.2009.080214. [DOI] [PubMed] [Google Scholar]
- 28.Pradeep AR, Pai S, Garg G, Devi P, Shetty SK. A randomized clinical trial of autologous platelet-rich plasma in the treatment of mandibular degree II furcation defects. J Clin Periodontol. 2009;36:581–8. doi: 10.1111/j.1600-051X.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 29.Su CY, Chiang CC, Lai WF, Lin KW, Burnouf T. Platelet-derived growth factor-AB and transforming growth factor-beta 1 in platelet gels activated by single-donor human thrombin. Transfusion. 2004;44:945. doi: 10.1111/j.1537-2995.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 30.Schallhorn RG, Hiatt WH, Boyce W. Iliac transplants in periodontal therapy. J Periodontol. 1970;41:566–80. doi: 10.1902/jop.1970.41.41.566. [DOI] [PubMed] [Google Scholar]
- 31.Perichard JF. The intrabony technique as a predictable procedure. J Periodontol. 1957;28:202–16. [Google Scholar]
- 32.Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J Periodontol. 2012;83:1499–507. doi: 10.1902/jop.2012.110705. [DOI] [PubMed] [Google Scholar]
- 33.Blumenthal NM, Alves ME, Al-Huwais S, Hofbauer AM, Koperski RD. Defect-determined regenerative options for treating periodontal intrabony defects in baboons. J Periodontol. 2003;74:10–24. doi: 10.1902/jop.2003.74.1.10. [DOI] [PubMed] [Google Scholar]
- 34.Richardson CR, Mellonig JT, Brunsvold MA, McDonnell HT, Cochran DL. Clinical evaluation of Bio-Oss: A bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Clin Periodontol. 1999;26:421–8. doi: 10.1034/j.1600-051x.1999.260702.x. [DOI] [PubMed] [Google Scholar]
- 35.Bender SA, Rogalski JB, Mills MP, Arnold RM, Cochran DL, Mellonig JT. Evaluation of demineralized bone matrix paste and putty in periodontal intraosseous defects. J Periodontol. 2005;76:768–77. doi: 10.1902/jop.2005.76.5.768. [DOI] [PubMed] [Google Scholar]
- 36.Cáceres M, Hidalgo R, Sanz A, Martínez J, Riera P, Smith PC. Effect of platelet-rich plasma on cell adhesion, cell migration, and myofibroblastic differentiation in human gingival fibroblasts. J Periodontol. 2008;79:714–20. doi: 10.1902/jop.2008.070395. [DOI] [PubMed] [Google Scholar]
- 37.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. J Oral Maxillofac Surg. 2005;63:362–9. doi: 10.1016/j.joms.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Dallari D, Fini M, Stagni C, Torricelli P, Nicoli Aldini N, Giavaresi G, et al. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. J Orthop Res. 2006;24:877–88. doi: 10.1002/jor.20112. [DOI] [PubMed] [Google Scholar]
- 40.Aghaloo TL, Moy PK, Freymiller EG. Evaluation of platelet-rich plasma in combination with freeze-dried bone in the rabbit cranium. A pilot study. Clin Oral Implants Res. 2005;16:250–7. doi: 10.1111/j.1600-0501.2004.01075.x. [DOI] [PubMed] [Google Scholar]
- 41.Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: Tissue-engineered bone regeneration. Tissue Eng. 2004;10:955–64. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 42.Anitua E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–35. [PubMed] [Google Scholar]
- 43.Sammartino G, Tia M, Marenzi G, di Lauro AE, D’Agostino E, Claudio PP. Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg. 2005;63:766–70. doi: 10.1016/j.joms.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Simon D, Manuel S, Geetha V, Naik BR. Potential for osseous regeneration of platelet-rich plasma – A comparative study in mandibular third molar sockets. Indian J Dent Res. 2004;15:133–6. [PubMed] [Google Scholar]
- 45.Markou N, Pepelassi E, Vavouraki H, Stamatakis HC, Nikolopoulos G, Vrotsos I, et al. Treatment of periodontal endosseous defects with platelet-rich plasma alone or in combination with demineralized freeze-dried bone allograft: A comparative clinical trial. J Periodontol. 2009;80:1911–9. doi: 10.1902/jop.2009.090216. [DOI] [PubMed] [Google Scholar]
- 46.Hanna R, Trejo PM, Weltman RL. Treatment of intrabony defects with bovine-derived xenograft alone and in combination with platelet-rich plasma: A randomized clinical trial. J Periodontol. 2004;75:1668–77. doi: 10.1902/jop.2004.75.12.1668. [DOI] [PubMed] [Google Scholar]
- 47.Okuda K, Tai H, Tanabe K, Suzuki H, Sato T, Kawase T, et al. Platelet-rich plasma combined with a porous hydroxyapatite graft for the treatment of intrabony periodontal defects in humans: A comparative controlled clinical study. J Periodontol. 2005;76:890–8. doi: 10.1902/jop.2005.76.6.890. [DOI] [PubMed] [Google Scholar]


