Abstract
Fabrication of dentures aided with implants has become a preferred treatment option for rehabilitation of completely and partially edentulous patients when durability is concerned. Simulation to natural teeth in terms of esthetics and to a greater extent in function can be considered as key elements in the raise of implant dentistry worldwide. Despite its high success rate, therapy with osseointegrated dental implants is not free of complications. Implant failure can occur for other reasons, with implant fracture being one of the major reasons for late failure. Although the incidence of implant fractures may be low, it invariably effects the patient and also clinician. Thus, sound evidence based knowledge of cause of fracture is mandatory for that careful treatment that can reduce the incidence of fracture helping in a better treatment plan. The aim of this review is to enlighten the various causes of implant fracture.
Keywords: Bone resorption, implant fracture, occlusal loads, overloading, parafunctional forces, screw loosening
INTRODUCTION
Dental implants have been a preferred treatment option for rehabilitation of completely and partially edentulous patients. One of the most important complications is the fracture of a dental implant that has undergone osseointegration by which the prosthesis is adversely affected by the loss of the supporting tissue. Although the success rate of this procedure is >90%, at times it was also reported with fractures at rare incidence.[1] Balshi et al.[2] reported fracture of eight implants out of 4045 implants scoring up to 0.2%. Rangert et al.[3] found 39 patients with fractured implants after placing 10,000 implants. He also indicated that most of the fractures were in posterior partially edentulous segments, in which the generated occlusal forces can be greater, as opposed to anterior segments. In general, it has been reported that the incidence of implant fractures as 0.16–1.5%.[4]
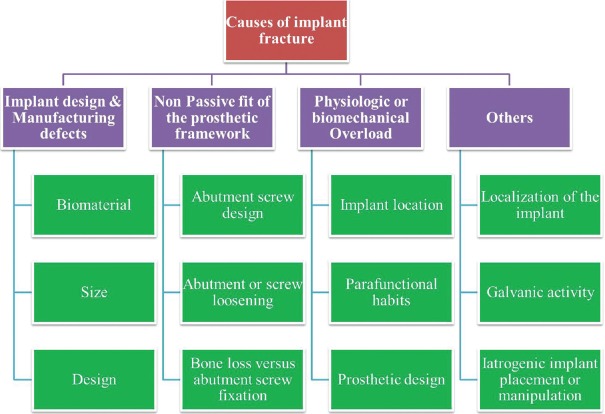
According to Goodacre et al.,[5] the risk of implant body fracture in early to intermediate period for implants of 3.75 mm in diameter is 1%, the abutment screw fracture risk is 2% and the prosthetic screw risk is 2%. According to Balshi, the cause of implant fracture may be broadly divided into [Figure 1].
Figure 1.
Flow chart depicting the causes of implant fractures
Implant design and manufacturing defects
Nonpassive fit of the prosthetic framework
Physiologic or biomechanical overload.[2]
Other possible causes of fracture can also include failure in the production and design of dental implants, bruxism or large occlusal forces, superstructure design, implant localization, implant diameter, metal fatigue, and bone resorption around the implant.[6] The risk of fracture also increases with time.[7]
Implant design and manufacturing defects
This may be considered as very unlikely reason for a fracture of implants. Balshi[8] and Piatelli et al.,[9] reported that microscopic evaluation of fractured fixtures revealed no porosity or defects in titanium surface minimizing implant design and manufacturing defect as a major cause. Conversely, Rangert et al.[3] documented that in the long term loading condition, 80% of all the failures may be related to implant body fracture. The key factors that influence the implant body fracture include the biomaterial, the size and the design of the implant.[7]
Biomaterial of the implant
There are many biocompatible materials that are unable to withstand the type and magnitude of parafunctional loads imposed on dental implants. Ceramic, which has an excellent biocompatibility, is susceptible to tension and bending loads and so unsuitable in implant body applications. Similarly, hydroxyapitite, though biocompatible with biological tissues lack the mechanical strength, biocompatibility and the potential for relative motion at the bone-implant interface. The biomaterials of choice related to implant failures are the vitreous carbon and Al2O3 ceramic implants. The vitreous carbon implants are good at modulus of elasticity, but without appropriate ultimate strength where it is vice versa with Al2O3 ceramic implants. However, the carbon body implants fail to withstand physiological loads within oral environment. Al2O3 ceramic implants are brittle in nature and susceptibility of failure in tension and shear, require geometric designs of the implant that may not be compatible with the anatomical dimensional limitations of the jaw. The geometric design of the implant is of equal importance in resisting occlusal loads. Microcracks in the body create a pathway of biological fluid into internal stainless steel post, which is then subjected to dramatic corrosion of the implant surface.[7]
Goodacre et al.,[5] and Morgan et al.,[10] reported that implant bodies and components are prone to fatigue fractures with incidence between 1% and 4%. The most common cause of long-term failure occurred when grade I titanium implant body was used and coupled with less than ideal treatment that has high stresses, as depicted with 80% of failures from Branemark implant body.[3]
Of all the materials available, titanium alloy represents the best compromise between biomechanical strength, biocompatibility and the potential for relative motion at the bone - implant interface.[11]
Size
Implants with smaller diameter tend to fracture more easily than those with larger diameters, especially when placed in the posterior region.[7] The large occlusal surfaces supported by a single implant will create forces that are not in line with the long axis of the implant.[5]
The fracture resistance ability of the implants and components against bending loads is directly proportional to the component's moment of inertia.
I (Moment of inertia)solid cylinder = ¼ × π × (radius)4.
Thus, if an implant or its component is 2 times wide, it is 16 times more resistant to fracture. Thus, smaller diameter implant tends to fracture more easily.[7] Shemtov-Yona et al.[12] found that 3.3 mm diameter implants did not exhibit a typical fatigue behavior like 5 mm and 3.75 mm implants. They reported that in 3.3 mm implants, 52% were fractured at the second thread and 48% at the third thread.
The overall fracture resistance is related as stress (σ) = My/I
M = Moment load caused by eccentric loading and cantilevers
y = Point in the center of the part
I = Moment of inertia.
Thus, it can be shown that an abutment screw, which has smaller cross sectional area, is more susceptible to fracture. Therefore, optimizing the implant body geometry within the anatomical dimensional limitation, reduces the overall stress in the impact.[7]
Design
In the search for superior stability, the general shape of the implants has changed dramatically from threaded parallel walls with external prosthetic connections to tapered internal prosthetic connections over the past decades. Anecdotal reports of internal hex failure with fracture of vertical walls of the implant are reported.[13] Even though, the literature reports are not available on incidence of implant fractures with internal connections, it is certainly possible that failure of an internal connection could pose a risk similar to or even greater than that seen with the externally hexed implants, depending on the thickness of the implant walls and the presence or absence of a bevel at the restorative platform. The abutment screw length is shorter than the receptor site within the implant. This permits the receptor site within the implant to be machined and allows the abutment screw to tighten the abutment, without the risk of “bottoming” out before the screw is completely tightened. The cross section of this portion of the implant body can be modeled as an annulus or hallow cylinder, similar to the cross section of a pipe. The wall thickness of the implant body in the region below the abutment screw controls the resistance to fatigue fracture.[7]
Nonpassive fit of the prosthetic framework
The stress caused by retaining screws of prostheses with a nonpassive fit may result in constant tension on the implant, predisposing it to fracture.[6] Schwarz stated that on the trail of screw loosening, metal fatigue occurs, which may result in implant fracture.[14]
Abutment screw design
In the screw design which is used presently, upon application of the torque to the screws, the most coronal two or three implant threads are placed in compression, resulting in stress concentration to the platform of the implant.[13] This should be taken into consideration when the implant is placed under repeated function.
Abutment or screw loosening
Screw loosening precedes implant fracture and may be a warning sign that the prosthetic structure needs to be reassessed. Screw loosening may be caused by framework misfit, excessive occlusal force, poor prosthetic component design, unfavorable leverage or parafunctional activities. In addition, improper prosthesis connection could result in insufficient or excessive torque to the retaining screws.[15] Abutment screw loosening varied dramatically from one study to another, ranging from 2% to 45%. The highest rate of abutment screw loosening was associated with single crowns which are around 25%.[16] Recently it was quoted that this ratio got declined to 8% with multiple unit fixed prosthesis at 5%. First, with an increase in stress applied to the prosthesis, the risk of abutment screw loosening increases. Second, with an increase in crown height attachment to the abutment increases the forces applied, resulting in increased screw loosening.[17] The height or depth of an anti-rotational component of the implant body also affects the amount of force applied to the abutment screw. The higher the hex height, the less stress applied to the screw and lower the risk of screw loosening.[18]
Screws may also become loose because of imprecise component fit and varying implant manufacturing tolerances.[19] Different implant manufacturing companies have different manufacturing tolerances. As the interface gap increases or tolerance becomes imprecise, the amount of possible rotation of components around the implant increases by the ratio of approximately 2:1.[20,21,22,23,24,25]
Screw-joint stability involves a number of critical factors, the most important being: (1) Adequate preload (2) the precision of the fit of the mating implant components and (3) the basic anti-rotational characteristics of the implant-to-abutment interface. Application of the correct torque to an implant screw is translated into a preload that holds the components together. With an external hex screw coupling system, the preload is the only force that will resist the patient's functional occlusal forces in order to keep the abutment from separating from the implant. If the preload is exceeded by the occlusal force, and especially if the docking mechanism has no precise anti-rotational feature, the screws will loosen. Even when an anti-rotational feature is present, problems can arise when the machining tolerances of the mating parts allow for rotational movements. Machined freedom between the mating surfaces of a “slip joint” such as the external hex results in vibration and micro-movement during functional loading, resulting in a loss of preload until screw-joint failure occurs.[26] This problem is compounded when mechanical torque drives are not used to tighten screws, as even experienced clinicians under-tighten them by 30–50%.[27] Precise fit of the mating components is very important when considering screw loosening.[28] Rotational motion of the abutment on the implant, which results from machining tolerances of the external hexagonal and their abutment counterparts, ultimately leads to failure of the screw. In a report on machining accuracy of several different external hex implants, all systems tested had rotational movement in excess of 4°.[22] With the ITI system, abutment rotation is eliminated through the mechanical friction fit.[29] After screws loosen, metal fatigue may result in screw fracture.[14]
The common practice of using a large retaining screw and small retaining screws as the fail safe mechanism has been called the weak link theory. This theory states that, as the implant is ascended there is descended strength in the screws. In other words if a screw were to break in a component stack (with the abutment screw in the implant and retaining screw in the abutment screw), the retaining screw would break because it is the smallest. This was considered a fail-safe mechanism. However, this theory has been disproved. Different studies have shown that either the screw or the implant can break, depending on where the forces are placed on restoration – abutment-implant interface.[30,31]
Screw loosening is often observed before implant fracture and usually the abutment screw fractures at the height of external helix.[19] If the implant axis is placed at a certain distance from the center of the prosthetic crown, forces created by this distance from the occlusal contact point to the implant axle may cause screw loosening or component fracture.[6] Peri-implant surface lacks the natural periodontal ligament. This absence of the periodontal ligament and direct opposition to bone does not allow the implant to move under loads to accommodate the occlusal forces. This may lead to excessive overloading affecting the weak link in implant system that is, ending up with screw loosening and/or fracture.[32]
The other contributing factor may be ill-fitting components leading to faulty abutment seating and/or inaccurate impression procedures leading to screw loosening. These loose screws not only cause fracture of implants and restorations, but also leads to breakage of the components, damage to the abutment or implants. If the restoration has multiple screws, a loose screw may cause breakage, but it often does not fracture itself. Usually, it is the adjacent screw, which is maintaining most of the load that fractures. This happens because the tight screw has a greater load placed on it and eventually the screw reaches its elastic limit and breaks.[19] Abutment screws were previously made with titanium, which do not offer the clamping forces. Newer abutment designs and improved abutment screws allow for an increased clamping force to be achieved without exercise torque levels, which have helped to reduce the rate of screw loosening.
Bone loss versus abutment screw fixation
Quirynen et al.[33] and Hoshaw et al.[34] documented that overload of an implant may lead to marginal bone resorption. A specific pattern of bone loss described as “cupping” that is, a rounded radiographic appearance of bone loss is observed in the patients with implant fractures. This may be due to the reaction to percolation of inflammatory infiltrate from repeated micro openings of initial fatigue cracks.[3] Bone loss causes the implant to high stress forces caused by support tissue loss, normally located at the end of the abutment screw level, where resistance to tension forces is reduced. This bone loss may be preceded by the fracture. Sometimes fracture may precede the bone loss where one cannot determine the exact pioneer of the two.
The other possibility of bone loss can be secondary to microfracture of the alloy microstructure and be a sequel of the fracture itself. This bone loss may also be contributed to the disruption of the biomechanical imbalance between host and parasite leading to peri-implant mucositis and peri-implantitis.[6] Bone resorption exceeding three threads in the apical direction will expose the weaker portion of the implant below the abutment screw engagement and thus contribute to overload of the implant leading to its fracture.[10] The commercially pure titanium will tear under chronic cyclic overload and create notch. If a defect notch starts at the interface and has a degree of micromotion or acts as a conduit for inflammatory mediators, it would be evident that bone loss would ensue. As a result of further propagation, the bone loss becomes a secondary factor for initiation by the microfracture.[13]
Physiologic or biomechanical overload
Biomechanical and physiologic overload seems to be the most common cause of dental implant fracture. Typical mechanical failures are due to either static overload where the stress in the material to exceed its ultimate strength after one load application or fatigue load where the material is subjected to lower loads but of repeated cycles. These overloads may be caused primarily by two factors: Parafunctional habits like bruxism and clenching and prosthesis design like presence of cantilevers.[6]
Implant location
Load factors are mainly related to the magnitude and direction of occlusal forces. Rangert et al.[3] stated that 90% of implant fractures are located in the molar and premolar regions of the mouth, where chewing forces and lateral movements associated with cusp inclination generate undesirable forces. Gargallo Albiol et al.[35] reported similar results with 80.9% fractured implants within 3–4 years after loading in the posterior region. Chewing occlusal forces when in function are 3 times more intense in the posterior region than in the anterior region.[36] Chewing involves mainly the vertical forces; however horizontal movement of the mandible and the inclination of the dental cusps create lateral forces that are transferred to the implant and to the bone.[31]
Balshi[8] also documented that all implant fractures occur in the region of premolars and molars, and no distinction has been made between the maxilla and mandible. In contrast Rangert et al.[3] stated that there is a higher predisposition for fracture in maxilla due to the presence of weaker bone. Even the proximity of the molar to the temporomandibular joint creates a mechanically unfavorable situation due to high magnitude of force transmission to that area.[15] These forces increase in magnitude in patients with parafunctional habits. Rangert et al.[3] reported that around 56% of patients with fractured dental implants presented with bruxism and marked occlusal forces. Parafunctional habits have been identified as the major causative factor associated with fixture fractures. Majority of fractures that occur in posterior quadrants are associated with bending overload created by a combination of parafunctional forces, cantilevers and possibly framework misfit.
Parafunctional habits
Under ideal conditions, the total time when teeth come into contact is about 30 min/day.[37] This value shoots up to several hours per day in bruxers and patients exhibiting clenching, parafunctional habits, which are the reason to create fatigue load on the implant resulting in fracture.[7] Balshi[8] analyzed eight fractured implants and identified five out of them belong to the patients who were exhibiting extreme bruxism and clenching habits having moderate to very high levels of stress in their daily routines.
A bruxing patient is at greater risk of fatigue fractures for two reasons. First, the magnitude of the forces increases over time as the muscles get stronger and secondly the number of cycles increases on the prosthetic component. In the case of clenching patients, they exhibit another phenomenon called “creep,” which results in fracture of the components. Creep occurs in the material when an increasing deformation is expressed as a function of time, when subjected to a constant load. Even though the cycles of load may not be present to affect the deformation of the material, the constant force is still able to cause fracture.[18]
Prosthetic design
According to Shackleton and Slabbert,[38] short cantilevers provided longer survival rates and recommended the use of mandibular extensions for a maximum of 15 mm. Rangert et al.[31] stated that good mandibular bone quality allows the use of cantilevers that measure 15–20 mm in length, whereas porous maxillary bone should not support cantilevers longer than 10 mm. It has been suggested that posterior cantilevers should be avoided or minimized, especially in partially edentulous patients. In a three unit posterior prosthesis, if it is supported by two implants and has a cantilever tooth, the bending moment may be doubled when compared with a prosthesis in which both ends are supported. The addition of the third implant offset to the other two will reduce the bending by approximately two-thirds.[39]
Other causes
Localization of implant
Fractures have been frequently documented in partially edentulous jaw when compared to edentulous jaw indicating the arrangement of implants is of more curvilinear fashion in latter and rectilinear fashion in the former. However, this arrangement can be limited by anatomical limitations at times.
Galvanic activity
Green et al.[40] has found that cytotoxic substances released from nonprecious metal alloys in the presence of oral fluids produces galvanic currents, which result in corrosion of nonnoble metals intensifying the bone loss around the implant surface.
Iatrogenic implant placement or manipulation
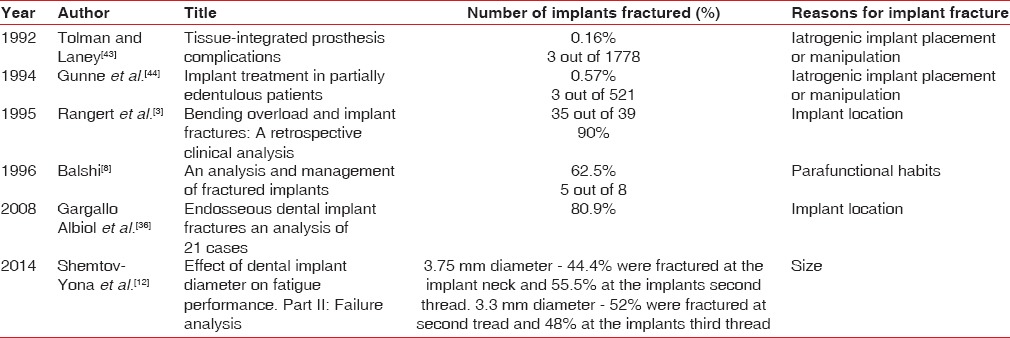
The incidence of fractures may also be correlated with the experience of the clinician apart from other clinical conditions. Zarb and Schmitt[41] reported no implant fracture after placing 274 implants. Tolman and Laney[42] documented a report of fractured implants being 3 out of 1778 implants. Gunne et al.[43] reported three fractured implants after placing 521 implants. Various reasons for fracture of implants were sited in Table 1.
Table 1.
Presenting the studies on implant fractures
TREATMENT
As suggested by Balshi,[8] there are three methods for treating fractures of dental implants:
Removal of the fractured implant (replace the implant and manufacture a new prosthesis),
Alteration of the existing prosthesis and maintenance of the osseointegrated fractured part, and
Alteration of the fractured implant and remanufacturing of the prosthetic portion.
Treatment of fractured implants represents a definite clinical challenge. First, the fractured fragment must be atraumatically removed with minimum bone removal. A new fixture is placed and the time to osseointegration must pass; only after that, the prosthetic phase begins.[44] It is suggested that, for removal of the intraosseous portion of a dental implant, a trephine bur should be used, and if possible, another implant with a larger diameter should be installed immediately.[44,45]
CONCLUSION
The fracture of osseointegrated implant is a late complication which can be due to multifactorial etiology. Even though, the frequency of implant fractures is low, appropriate treatment planning always plays a key role in its prevention.
Treating fractures can be accomplished either by replacing the implant and fabricating a new prosthesis or by refacing the fractured implant and modifying or refabricating a new prosthesis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11–25. [PubMed] [Google Scholar]
- 2.Balshi TJ, Hernandez RE, Pryszlak MC, Rangert B. A comparative study of one implant versus two replacing a single molar. Int J Oral Maxillofac Implants. 1996;11:372–8. [PubMed] [Google Scholar]
- 3.Rangert B, Krogh PH, Langer B, Van Roekel N. Bending overload and implant fracture: A retrospective clinical analysis. Int J Oral Maxillofac Implants. 1995;10:326–34. [PubMed] [Google Scholar]
- 4.Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002;29(Suppl 3):197–212. doi: 10.1034/j.1600-051x.29.s3.12.x. [DOI] [PubMed] [Google Scholar]
- 5.Goodacre CJ, Bernal G, Rungcharassaeng K, Kan JY. Clinical complications with implants and implant prostheses. J Prosthet Dent. 2003;90:121–32. doi: 10.1016/S0022-3913(03)00212-9. [DOI] [PubMed] [Google Scholar]
- 6.Gealh WC, Mazzo V, Barbi F, Camarini ET. Osseointegrated implant fracture: Causes and treatment. J Oral Implantol. 2011;37:499–503. doi: 10.1563/AAID-JOI-D-09-00135.1. [DOI] [PubMed] [Google Scholar]
- 7.Misch CE, Strong JT, Bidez MW. Scientific rationale for dental implant design. In: Misch CE, editor. Contemporary Implant Dentistry. 3rd ed. New Delhi: Elsevier; 2008. pp. 220–9. [Google Scholar]
- 8.Balshi TJ. An analysis and management of fractured implants: A clinical report. Int J Oral Maxillofac Implants. 1996;11:660–6. [PubMed] [Google Scholar]
- 9.Piattelli A, Piattelli M, Scarano A, Montesani L. Light and scanning electron microscopic report of four fractured implants. Int J Oral Maxillofac Implants. 1998;13:561–4. [PubMed] [Google Scholar]
- 10.Morgan MJ, James DF, Pilliar RM. Fractures of the fixture component of an osseointegrated implant. Int J Oral Maxillofac Implants. 1993;8:409–14. [PubMed] [Google Scholar]
- 11.Williams DF. Biocompatibility of Clinical Implant Materials. Vol. 1. Boca Raton, Fla: CRC Press; 1981. [Google Scholar]
- 12.Shemtov-Yona K, Rittel D, Machtei EE, Levin L. Effect of dental implant diameter on fatigue performance. Part II: Failure analysis. Clin Implant Dent Relat Res. 2014;16:178–84. doi: 10.1111/j.1708-8208.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 13.Eckert ES, Salinas TJ. Implant Fractures: Etiology, prevention, and treatment. In: Stuart J, editor. Forum Dental Implant Complications Etiology, Prevention, and Treatment. 1st ed. Singapore: Blackwell Publishing; 2010. pp. 100–9. [Google Scholar]
- 14.Schwarz MS. Mechanical complications of dental implants. Clin Oral Implants Res. 2000;11(Suppl 1):156–8. doi: 10.1034/j.1600-0501.2000.011s1156.x. [DOI] [PubMed] [Google Scholar]
- 15.Steven EE, Stephen JM, Ebru C, Richard KO. Analysis of incidence and associated factors with fractured implants: A retrospective study. Int J Oral Maxillofac Implants. 2000;15:662–7. [PubMed] [Google Scholar]
- 16.Goodacre CJ, Kan JY, Runcharassaeng K. Clinical complications of osseointegrated implants. J Prosthet Dent. 1999;81:537–52. doi: 10.1016/s0022-3913(99)70208-8. [DOI] [PubMed] [Google Scholar]
- 17.Kallus T, Bessing C. Loose gold screws frequently occur in full-arch fixed prostheses supported by osseointegrated implants after 5 years. Int J Oral Maxillofac Implants. 1994;9:169–78. [PubMed] [Google Scholar]
- 18.Misch CE. Stress treatment theorem for implant dentistry. In: Misch CE, editor. Contemporary Implant Dentistry. 3rd ed. New Delhi: Elsevier; 2008. pp. 68–91. [Google Scholar]
- 19.Jansen CE. Repair and replacement of damaged or fractured implant components. In: Zinner ID, Panno FV, Small SA, Landa LS, editors. Implant Dentistry: From Failure to Success. 1st ed. Illinois: Quintessence Publishing Co; 2004. pp. 149–60. [Google Scholar]
- 20.Davis DM, Zarb GA, Chao YL. Studies on frameworks for osseointegrated prostheses: Part 1. The effect of varying the number of supporting abutments. Int J Oral Maxillofac Implants. 1988;3:197–201. [PubMed] [Google Scholar]
- 21.Binon PP. Implants and components: Entering the new millennium. Int J Oral Maxillofac Implants. 2000;15:76–94. [PubMed] [Google Scholar]
- 22.Binon PP. Evaluation of machining accuracy and consistency of selected implants, standard abutments, and laboratory analogs. Int J Prosthodont. 1995;8:162–78. [PubMed] [Google Scholar]
- 23.English CE. Externally hexed implants, abutments, and transfer devices: A comprehensive overview. Implant Dent. 1992;1:273–82. doi: 10.1097/00008505-199200140-00009. [DOI] [PubMed] [Google Scholar]
- 24.Binon PP, McHugh MJ. The effect of eliminating implant/abutment rotational misfit on screw joint stability. Int J Prosthodont. 1996;9:511–9. [PubMed] [Google Scholar]
- 25.Binon PP, Weir D, Watanabe L, Walker L. Implant component compatibility. In: Laney WR, editor. Tissue integration in Oral, Orthopedic, and Maxillofacial Reconstruction. Chicago: Quintessence; 1992. pp. 218–26. [Google Scholar]
- 26.Binon P. The role of screws in implant systems. Int J Oral Maxillofac Implants. 1994;9(Suppl):48–51. [Google Scholar]
- 27.Goheen KL, Vermilyea SG, Vossoughi J, Agar JR. Torque generated by handheld screwdrivers and mechanical torquing devices for osseointegrated implants. Int J Oral Maxillofac Implants. 1994;9:149–55. [PubMed] [Google Scholar]
- 28.Beaty K. The role of screws in implant systems. Int J Oral Maxillofac Implants. 1994;9(Suppl):52–4. [Google Scholar]
- 29.Sutter F. The role of screws in implant systems. Int J Oral Maxillofac Implants. 1994;9(Suppl):51–2. [PubMed] [Google Scholar]
- 30.Rangert B, Jemt T, Jörneus L. Forces and moments on Branemark implants. Int J Oral Maxillofac Implants. 1989;4:241–7. [PubMed] [Google Scholar]
- 31.Binon P, Sutter F, Beaty K, Brunski J, Gulbransen H, Weiner R. The role of screws in implant systems. Int J Oral Maxillofac Implants. 1994;9:52–4. [Google Scholar]
- 32.Firas AM, Bashar AR, Ziad NA. Management of dental implant fractures. A case history. J Oral Implantol. 2009;35:210–4. doi: 10.1563/1548-1336-35.4.210. [DOI] [PubMed] [Google Scholar]
- 33.Quirynen M, Naert I, van Steenberghe D. Fixture design and overload influence marginal bone loss and fixture success in the Brånemark system. Clin Oral Implants Res. 1992;3:104–11. doi: 10.1034/j.1600-0501.1992.030302.x. [DOI] [PubMed] [Google Scholar]
- 34.Hoshaw S, Brunski J, Cochran G. Mechanical loading of Brånemark implants affects interfacial bone modeling and remodeling. Int J Oral Maxillofac Implants. 1994;9:345–60. [Google Scholar]
- 35.Gargallo Albiol J, Satorres-Nieto M, Puyuelo Capablo JL, Sánchez Garcés MA, Pi Urgell J, Gay Escoda C. Endosseous dental implant fractures: An analysis of 21 cases. Med Oral Patol Oral Cir Bucal. 2008;13:E124–8. [PubMed] [Google Scholar]
- 36.Traini T, De Paoli S, Caputi S, Iezzi G, Piattelli A. Collagen fiber orientation near a fractured dental implant after a 5-year loading period: Case report. Implant Dent. 2006;15:70–6. doi: 10.1097/01.id.0000202420.49004.1e. [DOI] [PubMed] [Google Scholar]
- 37.Graf H. Bruxism. Dent Clin North Am. 1969;13:659–65. [PubMed] [Google Scholar]
- 38.Shackleton JL, Carr L, Slabbert JC, Becker PJ. Survival of fixed implant-supported prostheses related to cantilever lengths. J Prosthet Dent. 1994;71:23–6. doi: 10.1016/0022-3913(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 39.Rangert B. Mechanical and biomechanical guidelines for the use of Brånemark system. Aust Prosthet J. 1993;7(Suppl):39–49. [PubMed] [Google Scholar]
- 40.Tagger Green N, Machtei EE, Horwitz J, Peled M. Fracture of dental implants: Literature review and report of a case. Implant Dent. 2002;11:137–43. doi: 10.1097/00008505-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Zarb GA, Schmitt A. The longitudinal clinical effectiveness of osseointegrated dental implants: The Toronto study. Part III: Problems and complications encountered. J Prosthet Dent. 1990;64:185–94. doi: 10.1016/0022-3913(90)90177-e. [DOI] [PubMed] [Google Scholar]
- 42.Tolman DE, Laney WR. Tissue-integrated prosthesis complications. Int J Oral Maxillofac Implants. 1992;7:477–84. [PubMed] [Google Scholar]
- 43.Gunne J, Jemt T, Lindén B. Implant treatment in partially edentulous patients: A report on prostheses after 3 years. Int J Prosthodont. 1994;7:143–8. [PubMed] [Google Scholar]
- 44.Muroff FI. Removal and replacement of a fractured dental implant: Case report. Implant Dent. 2003;12:206–10. doi: 10.1097/01.id.0000084168.57434.f1. [DOI] [PubMed] [Google Scholar]
- 45.Krogh P. Surgical and biomechanical advantages of large-diameter implants. J Prosthet Dent. 1994;72:623–34. In: Siddiqui AA, Caudill R, editors. Proceedings of the Fourth International Symposium on Implant Dentistry: Focus on Esthetics. San Diego, CA: January 27, through 29; 1994. [Google Scholar]