Abstract
The aim of this study was to evaluate the effect of a diode laser with nonsurgical periodontal therapy on chronic periodontitis. The patient, a 37-year-old female, with chronic periodontitis reported to the private dental clinic. Her health history indicated that she had good general health. The periodontal examination included a gingival index and complete periodontal probing depth with William's graduated probe. She was treated with 940 nm diode laser and scaling and root planning. Assessment was done after 6 months following laser therapy; the probing depths improved; gain in clinical attachment levels; no inflammation; the tissue tone was good, showing increased stippling.
Keywords: Dental plaque, diode laser, periodontal pocket, periodontitis, sterilization
INTRODUCTION
Periodontal disease can have periods of intense activity and dormancy. It triggers a host-inflammatory response altering the metabolism of the connective tissue and supporting bone. It is essential to manage the disease by addressing the microbes responsible while protecting patient's health.[1] Soft tissue lasers reduce the microbial population while providing coagulation at the treatment site. The lasers are used adjunctively in periodontal therapy after the hard accretions have been removed from the tooth and root surfaces.[2]
The case report here presents a case of chronic generalized periodontitis that is managed by 940 nm diode laser with scaling and root planning.
CASE REPORT
The patient, a 37-year-old female, presented with generalized bleeding and gingival inflammation with swelling to the private dental clinic.
The periodontal examination included a gingival index, evaluation of probing depth with William's graduated probe. The examination showed generalized inflammation, supra- and sub-gingival calculus, pathologic migration with 31 and 41, bluish red gingiva; probing depth was 5–6 mm throughout and generalized bleeding on probing [Figure 1]. A diagnosis of chronic generalized periodontitis was made. After explaining both surgical and laser treatment, informed consent was taken. It was decided to manage the case using diode laser 940 nm (Ezlase 940, Biolase, Cromwell, USA). The area was anesthetized using Xylocaine spray (Warren Pharma, Maharashtra). Pocket was disinfected using a diode laser with a noninitiated tip of 300 µm. The settings were 1.5 W with a pulse interval of 1.00 ms and pulse length of 1.00 ms. The tip was moved from apical to the coronal direction and was not kept stationary for more than 5 s.[3] Pocket disinfection was followed by ultrasonic scaling (EMS Switzerland) and thorough root planning with Gracey Curettes (Hu-Friedy USA). The pocket was rinsed with normal saline to eliminate blood clots. Target tissue was inflamed epithelial lining of the pocket which was debrided using a sinusoidal movement of the tip. The fiber was moved both horizontally and vertically, “painting” the tissue on the wall of the sulcus with laser energy from the calibrated depth to gingival margin [Figure 2]. The fiber was inspected often; any accumulated debris was wiped off with dry gauze to avoid any inefficiency. The setting used were 3.5 W pulsed mode, pulse length 0.50 ms, and pulse interval of 0.20 ms. The pocket was again rinsed with normal saline to eliminate the debris followed by denuding and plasty of the outer surface epithelium to recontour the gingiva [Figure 3]. No postoperative dressing was applied. Postoperative instructions were given.
Figure 1.
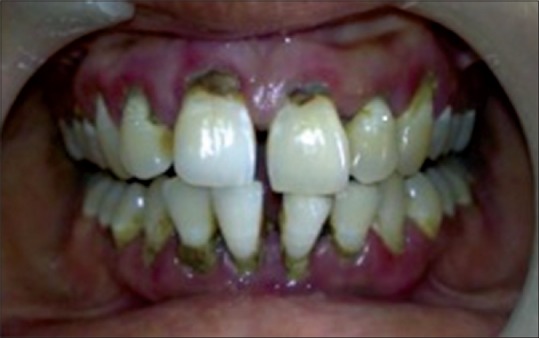
Chronic generalized periodontitis
Figure 2.
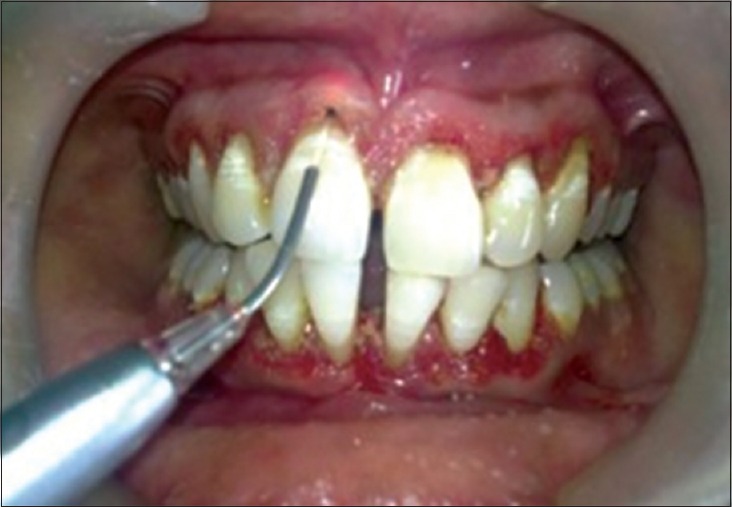
Debridement done with laser
Figure 3.
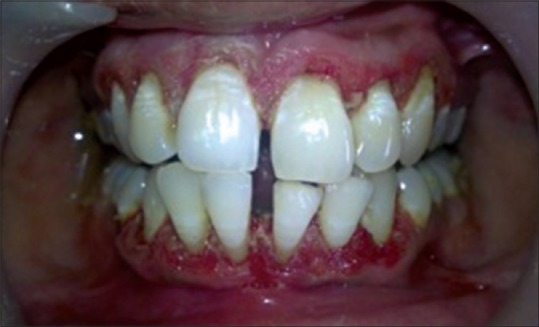
Epithelial denudation done
Treatment assessment
On recall visit, the patient did not report any postoperative pain, swelling or discomfort. Analgesics were needed only during initial 24 h. The patient was recalled after 1-month to check the healing. Tissue showed improvement with no inflammation. No probing was done. A definitive six-point probing was done after 6 months. This rationale for the probing schedule of laser-treated areas is suggested because the tissue at the bottom of the sulcus is healing. The fibers are fragile as they reattach to the root surface and could be damaged by introducing a probe too early.[4]
Assessment of treatment was done at 3 and 6 months following therapy [Figure 4], the probing depths improved with a gain in clinical attachment levels; no inflammation with good tissue tone, showing increased stippling.
Figure 4.
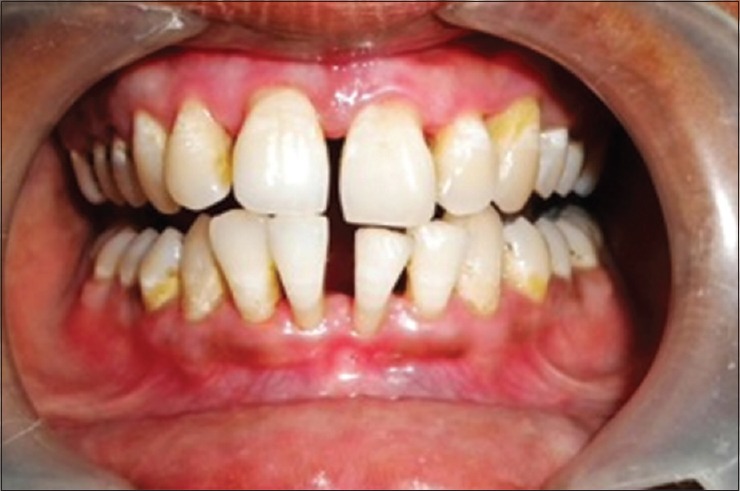
Six months post therapy
Prognosis
The long-term prognosis is good. Regular maintenance appointments are necessary to debride any calculus and biofilm accumulation, to evaluate the pocket depth, tissue tone and inflammation, and continue to monitor patient's oral hygiene skills.
DISCUSSION
Laser-assisted subgingival curettage is less invasive and less painful having a number of advantages over standard techniques. Operative and postoperative bleeding with laser surgery is significantly less than conventional method. It also improves operation area visibility, shortens treatment time, and increases patient comfort. Lasers accelerate wound healing, fibroblast proliferation, and collagen synthesis through biostimulation.[5] Laser beam causes vaporization of granulation and necrotic tissues as well as reduction and delayed recolonization of the subgingival bacterial flora. The absorption of ultraviolet or visible light by organic molecules leads to deformation of the binding angle, which weaken or break up chemical compounds. Laser irradiation has a bactericidal effect caused by changes in the cell wall. An indirect irradiation of ~ 1W leads to vesicle formation called as membrane blebbing which is caused by splitting of the inner layer from outer two layers.[6] Diode, thus has a bactericidal effect and is able to reduce inflammation in combination with scaling.
Similar results were reported by Moritz et al. in a study which showed that laser when used along with scaling supports healing of periodontal pocket by eliminating bacteria.[7] In another study, Neill and Mellonig checked the clinical efficacy of the neodymium-doped yttrium aluminum garnet (Nd: YAG) laser for combination periodontal therapy. Results of this limited clinical trial suggest that mechanical scaling and root planning therapy alone may not be the most effective mode of treatment for patients affected by moderate to severe adult periodontitis. However, they showed scaling and planning combined with laser therapy utilizing a low-powered pulsed Nd: YAG laser to be more successful in the elimination of the bacteria commonly associated with the development of this oral condition.[2] Sjostrom and Friskopp used laser treatment as an adjunct to debridement of periodontal pockets, which showed laser treatment diminished bleeding and enhanced visual control at debridement. Clinical significance of these findings is that mechanical SRP therapy alone may not be so effective and there are several additional areas where the adjunctive use of Nd: YAG may have an advantage over SRP alone as a mechanical approach to nonsurgical therapy.[8] But, the results of Liu et al. were in contrast to the above-stated study. They suggested that SRP is more effective than laser therapy at reducing gingival inflammation. They found no additional benefit when Nd: YAG was used secondary to SRP.[9]
Gingival curettage has been recommended as a means of eliminating chronically inflamed connective tissue, under assumption that toxic and heterolytic products would delay or interfere with wound healing. After curettage, there is a loss of clinical attachment level followed by a slight gain of 0.5 mm in 1-month. Lasers exhibit the bactericidal effect, detoxification effect, and removal of the epithelium lining and granulation tissue, which are desirable properties for the treatment of periodontal pockets. It is advised to irrigate the pocket with saline every time before we introduce the fiber in the pocket.
The tips were placed parallel to the root surface thereby avoiding damage to the root surface and the pocket were irrigated with saline each time before we introduced the fiber in the pocket.[4] Denuding helped sculpting of gingiva and prevented ingress of epithelium from marginal gingiva and formation of long junctional epithelial attachment.[10]
Use of conventional surgery has lot of limitations such as postoperative swelling and bacteremia, use of antibiotics, diet modification, need for suturing, and dental hypersensitivity. With the use of lasers as an alternative to flap surgery, all these postoperative sequelae were eliminated.
CONCLUSION
Laser-assisted first phase periodontal therapy can, thus, be used effectively to help achieve and maintain optimum levels of health. Studies of laser soft-tissue therapy and clinical observations of patients treated with the soft-tissue lasers are showing good results. With education and experience, lasers can be used as a powerful tool in the treatment of periodontal disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ishikawa I, Nakashima K, Koseki T, Nagasawa T, Watanabe H, Arakawa S, et al. Induction of the immune response to periodontopathic bacteria and its role in the pathogenesis of periodontitis. Periodontol 2000. 1997;14:79–111. doi: 10.1111/j.1600-0757.1997.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 2.Neill ME, Mellonig JT. Clinical efficacy of the Nd: YAG laser for combination periodontitis therapy. Pract Periodontics Aesthet Dent. 1997;9(6 Suppl):1–5. [PubMed] [Google Scholar]
- 3.Schoop U, Moritz A, Blum R, Romanos G, Schwarz F. Laser assisted periodontal therapy. In: Mortiz A, Beer F, Goharkhay K, Schoop U, editors. Oral Laser Application. Berlin: Quintessence; 2006. pp. 333–76. [Google Scholar]
- 4.Raffetto N. Lasers for initial periodontal therapy. Dent Clin North Am. 2004;48:923–36. doi: 10.1016/j.cden.2004.05.007. vii. [DOI] [PubMed] [Google Scholar]
- 5.White JM, Goodis HE, Rose CL. Use of pulsed Nd;YAG laser for intraoral soft tissue surgery. Lasers Surg Med. 1991;11:455–61. doi: 10.1002/lsm.1900110511. [DOI] [PubMed] [Google Scholar]
- 6.Moritz A, Schoop U, Klimscha J, Patruta S, Terpotiz I, Kluger W, et al. Lasers in endodontics. In: Mortiz A, Beer F, Goharkhay K, Schoop U, editors. Oral Laser Application. Berlin: Quintessence; 2006. pp. 241–314. [Google Scholar]
- 7.Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, et al. Treatment of periodontal pockets with a diode laser. Lasers Surg Med. 1998;22:302–11. doi: 10.1002/(sici)1096-9101(1998)22:5<302::aid-lsm7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Sjöström L, Friskopp J. Laser treatment as an adjunct to debridement of periodontal pockets. Swed Dent J. 2002;26:51–7. [PubMed] [Google Scholar]
- 9.Liu CM, Hou LT, Wong MY, Lan WH. Comparison of Nd: YAG laser versus scaling and root planing in periodontal therapy. J Periodontol. 1999;70:1276–82. doi: 10.1902/jop.1999.70.11.1276. [DOI] [PubMed] [Google Scholar]
- 10.Kreisler M, Al Haj H, Daubländer M, Götz H, Duschner H, Willershausen B, et al. Effect of diode laser irradiation on root surfaces in vitro. J Clin Laser Med Surg. 2002;20:63–9. doi: 10.1089/104454702753768034. [DOI] [PubMed] [Google Scholar]


