SUMMARY
The noncanonical inflammasome induced by intracellular lipopolysaccharide (LPS) leads to caspase-11-dependent pyroptosis which is critical for induction of endotoxic shock in mice. However, the signaling pathway downstream of caspase-11 is unknown. We found that cytosolic LPS stimulation induced caspase-11-dependent cleavage of the pannexin-1 channel and ATP release, which in turn activated the purinergic P2X7 receptor to mediate cytotoxicity. In the absence of P2X7 or pannexin-1, pyroptosis induced by LPS transfection or treatment with cholera toxin B and LPS was abrogated. Cleavage of pannexin-1 required the catalytic activity of caspase-11 and was essential for ATP release and P2X7-mediated pyroptosis. Priming the caspase-11 pathway in vivo with LPS or toll-like receptor-3 (TLR3) agonist resulted in high mortality in wild-type mice after secondary LPS challenge, but not in Casp11−/−, Panx1−/− or P2x7−/− mice. These results reveal a critical role for pannexin-1 and P2X7 downstream of caspase-11 for pyroptosis and susceptibility to sepsis induced by the noncanonical inflammasome.
INTRODUCTION
Inflammasomes are intracellular protein complexes that drive the activation of inflammatory caspases and induction of immune responses and pyroptosis, a proinflammatory form of cell death (Martinon and Tschopp, 2007). In response to diverse microbial and endogenous stimuli, innate immune cells including macrophages and neutrophils induce the activation of the inflammasome, a multi-protein platform that activates the protease caspase-1 (Schroder and Tschopp, 2010). To date, four canonical inflammasomes NLRP1, NLRP3, NLRC4 and AIM2 have been described that activate caspase-1, leading to the secretion of mature interleukin-1β (IL-1β) and IL-18 as well as the induction of pyroptosis (Schroder and Tschopp, 2010; Bauernfeind et al., 2009). Activation of the NLRP3 inflammasome is mediated by two signals. The first signal, referred as priming, induces NLRP3 and pro-IL-1β expression after stimulation by Toll-like receptor (TLR) agonists or certain cytokines such as TNF-α or IL-1β (Bauernfeind et al., 2009; Franchi et al., 2009). The second signal leads to the assembly of the NLRP3 inflammasome in response to various stimuli including ATP, bacterial pore-forming toxins, or particulate matter (Hornung et al., 2008; Mariathasan et al., 2006). Exogenous ATP activates the purinergic receptor P2X ligand-gated ion channel (P2X7) to allow the passage of small cations including K+ and Na+ across the plasma membrane (Bartllet et al., 2014). In addition, ATP stimulation via P2X7 leads to the opening a larger reversible pore that allows the uptake of organic ions such as the Yo-Pro-1 probe (Surprenant et al., 1996; North et al., 2002). All of the NLRP3-activating stimuli perturb the permeability of the plasma membrane to K+ leading to reduction in cytosolic K+ concentration, a step that is necessary and sufficient for NLRP3 activation (Muñoz-Planillo et al., 2013).
The pannexin-1 channels are non-selective, large-pore channels that release nucleotides including ATP (D’hondt et al., 2009). In response to apoptotic stimuli, the pannexin-1 channel can be functionally activated by caspase-3-mediated cleavage of the distal end of its autoinhibitory intracellular domain (Sandilos et al., 2012; Boyd-Tressler et al., 2014). Although pannexin-1 has been originally implicated in the formation of the large pore responsible for the enhanced permeation state of P2X7 (Pelegrin and Surprenant, 2006), recent studies reveal that pannexin-1 is dispensable for dye uptake mediated by the P2X7-associated pore and NLRP3 activation in response to ATP (Qu et al., 2011).
The noncanonical inflammasome activates another class of inflammatory caspases including mouse caspase-11 to induce pyroptosis independently of NLRP3, NLRC4, ASC and caspase-1 (Kayagaki et al., 2011). The noncanonical inflammasome, triggered by intracellular lipopolysaccharide (LPS), is critical for the cytosolic sensing of several gram-negative pathogens as well as induction of mouse mortality by LPS independently of the TLR4 receptor (Kayagaki et al., 2013; Hagar et al., 2013). Activation of caspase-11 also relies on guanylate-binding proteins (small interferon (IFN)-inducible GTPases) that mediate phagosomal lysis, allowing bacterial LPS to be sensed in the cytosol (Pilla et al., 2014; Meunier et al., 2014). In addition to pyroptosis, the noncanonical inflammasome can indirectly promote IL-1β secretion by triggering the canonical NLRP3 inflammasome (Kayagaki et al., 2011), but the link between caspase-11 and NLRP3 activation remains poorly understood. Activation of the noncanonical inflammasome is induced by direct binding of LPS to the mouse caspase-11 and related human caspase-4 and caspase-5 (Shi et al., 2014).
However, the signaling events that mediate pyroptosis downstream of caspase-11 remain unknown. In the current work, we provide evidence that upon intracellular LPS stimulation, caspase-11 cleaves the pannexin-1 channel leading to ATP release, which in turn activates P2X7 to induce cytotoxicity. Furthermore, the pannexin-1 and /P2X7 are required for susceptibility to endotoxic shock induced via the noncanonical inflammasome pathway.
RESULTS
Activation of caspase-11 by intracellular LPS induces pyroptosis via ATP-mediated P2X7 signaling
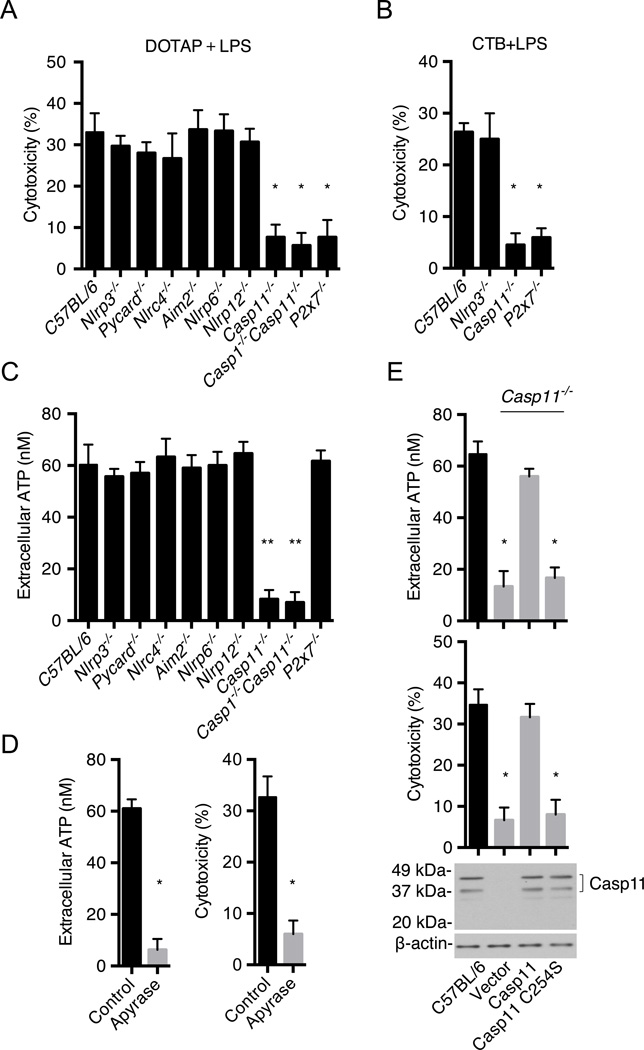
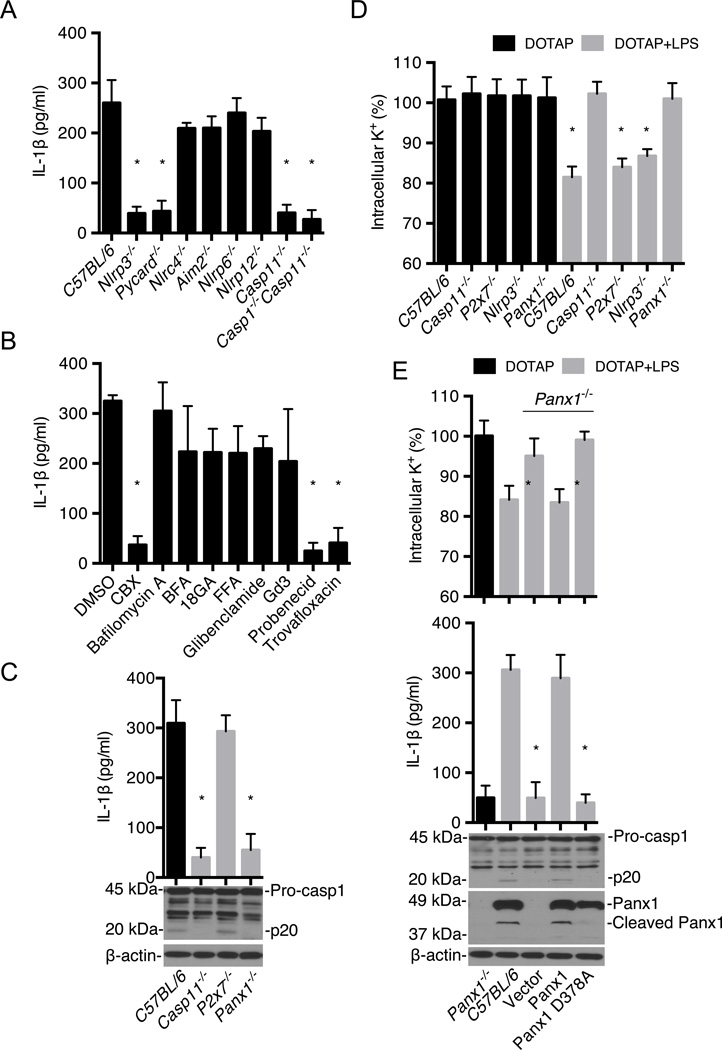
The signaling events that mediate pyroptosis downstream of caspase-11 remain unknown. To identify components of the noncanonical signaling pathway, bone marrow-derived macrophages (BMMs) from wild-type and a panel of mutant mice were primed with LPS and then transfected with ultrapure LPS from Salmonella minnesota RE595 (Hagar et al., 2013) and pyroptosis was monitored by a cell death assay. As expected from previous studies (Hagar et al., 2013), caspase-11 was important for cytotoxicity whereas components of the canonical inflammasomes, NLRP6 and NLRP12 were not (Fig. 1A). Notably, P2X7 was required for pyroptosis induced by intracellular LPS (Fig. 1A). In addition, pyroptosis induced by stimulation of LPS-primed BMMs with cholera toxin B (CTB) and LPS, another stimulus that activates the noncanonical inflammasome (Kayagaki et al., 2011 and 2013) was also dependent on caspase-11 and P2X7, but not NLRP3 (Fig. 1B). Because P2X7 is activated by extracellular ATP (Bartllet et al., 2014; Surprenant et al., 1996), we assessed the amount of extracellular ATP before and after stimulation with transfected LPS. There was a rapid and transient release of ATP upon LPS transfection in wild-type and P2x7−/− BMMs, but not in cells from Casp11−/− and Casp1−/−/Casp11−/− mice (Fig. 1C and Fig. S1A). Extracellular ATP is rapidly hydrolyzed to ADP by cell surface ATPases (Trentham et al., 1976). Consistently, the accumulation of extracellular ATP induced by LPS transfection was enhanced by treatment with the ATPase inhibitor ARL67156 (Lévesque et al., 2007) (Fig. S1B). In line with a role for ATP-induced P2X7 activation, the increase in extracellular ATP and pyroptosis induced by LPS transfection was blocked by addition of apyrase, an enzyme that hydrolyzes extracellular ATP (Fig. 1D). To determine whether the catalytic activity of caspase-11 is required for ATP release and pyroptosis, we expressed wild-type or mutant caspase-11 with replacement of the critical cysteine for serine at position 254 (C254S) in Casp11−/− BMMs by lentivirus-mediated transduction (Fig. 1E). Importantly, the ability of intracellular LPS to induce ATP release and pyroptopsis was impaired in Casp11−/− BMMs reconstituted with C254S–caspase-11 when compared to BMMs reconstituted with wild-type caspase-11 (Fig. 1E). Collectively, these results indicate that activation of caspase-11 by intracellular LPS induces pyroptosis via ATP-mediated P2X7 signaling.
Figure 1. Activation of caspase-11 by intracellular LPS induces pyroptosis via ATP-mediated P2X7 signaling.
(A–E) BMMs were primed overnight with LPS and then transfected with ultrapure S. minnesota RE595 LPS packaged with DOTAP or simulated with CTP plus LPS.
(A) Cytotoxicity in C57BL/6, Nlrp3−/−, Pycard−/−, Nlrc4−/−, Aim2−/−, Nlrp6−/−, Nlrp12−/−, Casp11−/−, Casp1−/−/Casp11−/− and P2X7−/− BMMs was examined 2 hrs after transfection.
(B) Cytotoxicity in C57BL/6, Nlrp3−/−, Casp11−/− and P2X7−/− BMMs was examined 8 hrs after CTB plus LPS stimulation.
(C) Extracellular ATP in BMMs culture medium was measured 15 min after LPS transfection.
(D) Wild-type BMMs were treated with apyrase (25 U/mL) or left untreated (control) and the amounts of extracellular ATP and cytotoxicity were measured after LPS transfection.
(E) Extracellular ATP and cytotoxicity after LPS transfection in Casp11−/− BMMs reconstituted with lentiviral vectors expressing wild-type caspase-11, caspase-11 C254S or vector alone. Immunoblotting for caspase-11 in wild-type BMMs is shown as a control.
(A–E) Data are representative of at least 3 experiments. Graphs show mean ± SD of triplicate cultures, * p<0.05, ** p<0.01. See also Figure S1.
Cytoplasmic LPS induces opening of the large P2X7-associated pore
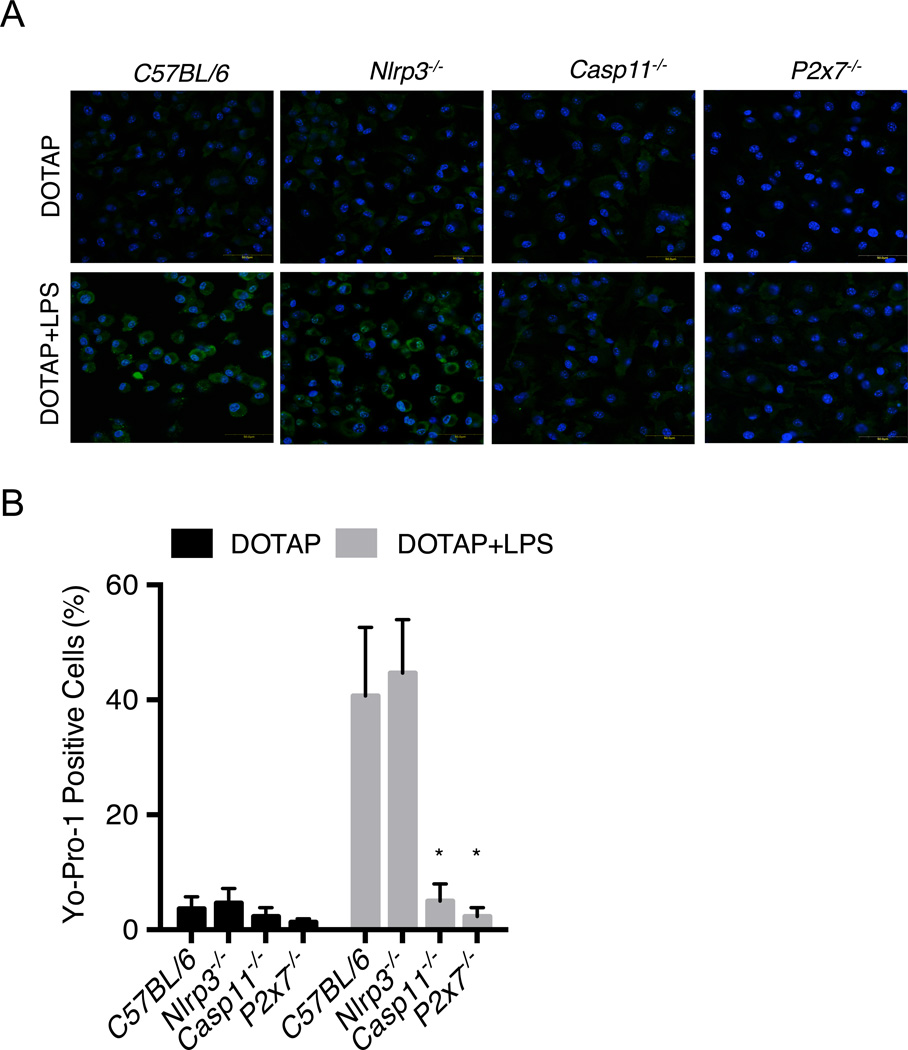
The EC50 concentration to activate P2X7 in naïve BMMs is in the 300–500 µM range (North and Surprenant, 2000), which is much higher than the extracellular concentration of ~80 nM triggered by cytosolic LPS. Stimulation of P2X7 with ATP induces a rapid opening of the ion-selective channel (Mackenzie et al., 2005), followed by the gradual opening of a larger pore that can be evaluated by uptake of the fluorescent dye such as Yo-Pro-1 (Surprenant et al., 1996; North et al., 2002). Thus, we assessed whether cytoplasmic LPS stimulation induces the opening of the P2X7-associated pore by measuring the uptake of Yo-Pro-1 in LPS-primed BMMs stimulated with DOTAP plus LPS or with DOTAP alone as a control. There was marked Yo-Pro-1 uptake in wild-type and Nlrp3−/− BMMs, but not in Casp11−/− or P2x7−/− BMMs transfected with DOTAP plus LPS or wild-type BMMs treated with DOTAP alone (Fig. 2A and B), indicating that cytosolic LPS induces the opening of a large pore which required caspase-11 and P2X7. To address whether LPS transfection increases the sensitivity to P2X7 activation, we assessed the response to ATP of Casp11−/− and P2x7−/− BMMs that are protected from cytotoxicity induced by cytosolic LPS (Fig. 1A). Stimulation of Casp11−/− BMMs with 50–100 nM of ATP induced P2X7-dependent Yo-Pro-1 uptake and pyroptosis in LPS-primed BMMs pre-stimulated by LPS transfection, whereas ~500 µM of ATP was required in LPS-primed BMMs in the absence of LPS transfection (Fig. S2A and B and Fig. S3). Thus, cytoplasmic LPS stimulation reduces the amount of ATP required for opening of the P2X7-associated pore.
Figure 2. Yo-Pro-1 uptake is induced after transfection with DOTAP or DOTAP plus LPS in LPS-primed BMMs.
(A–B) BMMs were primed overnight with LPS and then transfected with ultrapure S. minnesota RE595 LPS packaged with DOTAP.
(A) Yo-Pro-1 uptake was measured 2 hrs after treatment with DOTAP or DOTAP plus LPS in BMMs from indicated wild-type and mutant mice by fluorescence microscopy. Representative microscopic images are shown.
(B) Percentage of Yo-Pro-1 positive cells was calculated from different fields with 25–30 cells per field at each condition.
(A–B) Graph show mean ± SD of six fields. Data are representative of at least 3 experiments. * p<0.05. See also Figure S2 and S3.
Pannexin-1 is critical for ATP-induced pyroptosis in response to cytosolic LPS
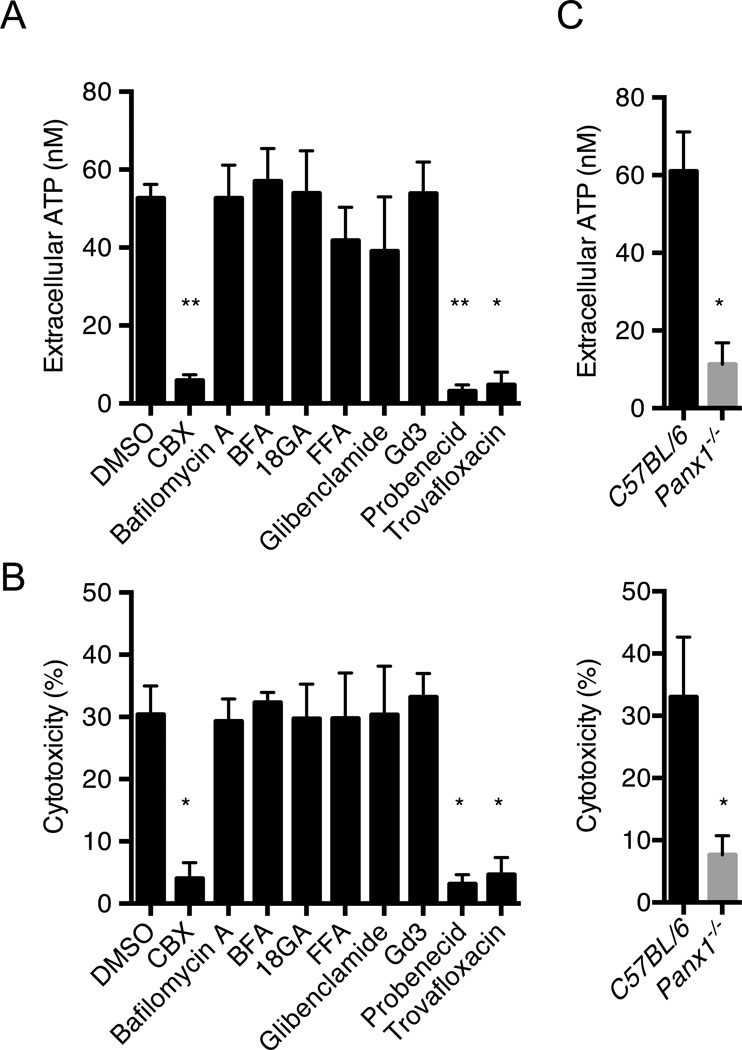
We next addressed the mechanism by which caspase-11 induces release of ATP. In initial experiments, we assessed the ability of compounds that inhibit channels or cellular processes that mediate ATP efflux. ATP release and pyroptosis induced by LPS transfection were effectively inhibited by carbenoxolone (CBX) (D’hondt et al., 2009), probenecid (Silverman, et al., 2008) and trovafloxacin (Poon et al., 2014), three inhibitors of pannexin-1, a plasma membrane channel that facilitates ATP export (Sandilos et al., 2012) (Fig. 3A and B). In contrast, bafilomycin A (an inhibitor of vesicular H+-ATPase), brefeldin A (BFA, an inhibitor of vesicular trafficking), 18-glycyrrhetinic (18GA, a connexin hemichannel inhibitor), flufenamic acid (FFA, an anion transporter inhibitor), glibenclamide (an anion transporter inhibitor) and gadolinium III (Gd3, a maxi-anion channel inhibitor) (Sakaki et al., 2013) did not impair either ATP release or pyroptosis (Fig. 3A and B). To validate the role of pannexin-1 in intracellular LPS-induced ATP release and pyroptosis, we assessed BMMs from mice deficient in pannexin-1 that express functional caspase-11 (Qu et al., 2011), and found that ATP release and pyroptosis induced by LPS transfection were effectively abolished in Panx1−/− BMMs (Fig. 3C). In line with these results, pannexin-1, caspase-11, and P2X7, but not NLRP3, were also required for cytotoxicity induced by CTB plus LPS (Fig. S4A). The non-canonical pathway is also activated by infection of BMMs with Salmonella mutant deficient in Flagellin (Broz et al. 2012). Consistently, infection of BMMs with Δflag-Salmonella induced cytotoxicity which required Caspase-11, Pannexin-1, and P2X7, but not NLRP3 (Fig. S4B). Stimulation of Panx1−/− BMMs with 50–100 nM of ATP induced Yo-Pro-1 uptake and pyroptosis in LPS-primed BMMs pre-stimulated by LPS transfection, whereas ~500 M of ATP was required in LPS-primed BMMs in the absence of LPS transfection (Fig. S5). Thus, the lowering of the threshold for P2X7 pore opening induced cytosolic LPS is independent of pannexin-1. Collectively, these results indicate that pannexin-1 plays a critical role in inducing ATP release and ATP-induced P2X7 pyroptosis in response to cytosolic LPS stimulation.
Figure 3. Pannexin-1 is critical for ATP-induced pyroptosis induced by cytosolic LPS.
(A–C) BMMs were primed overnight with LPS and then transfected with ultrapure S. minnesota RE595 LPS packaged with DOTAP.
(A) Wild-type BMMs were treated with DMSO, CBX (50 µM), bafilomycin A (50 nM), BFA (1 µM), 18GA (50 µM), FFA (50 µM), glibenclamide (100 µM), Gd3 (100 µM), probenecid (100 µM) and trovafloxacin (100 µM), and extracellular ATP was measured 15 min after LPS transfection.
(B) Cytotoxicity was measured 2 hrs after LPS transfection. Treatment with inhibitors was as in (A).
(C) ATP release was measured 15 min after LPS transfection, and cytotoxicity was determined 2 hrs after transfection in wild-type and Panx1−/− BMMs.
(A–C) Data are representative of at least 3 experiments. Graphs show mean ± SD of triplicate cultures, * p<0.05, ** p<0.01. See also Figure S4 and S5.
Activation of caspase-11 leads to pannexin-1 cleavage and ATP release
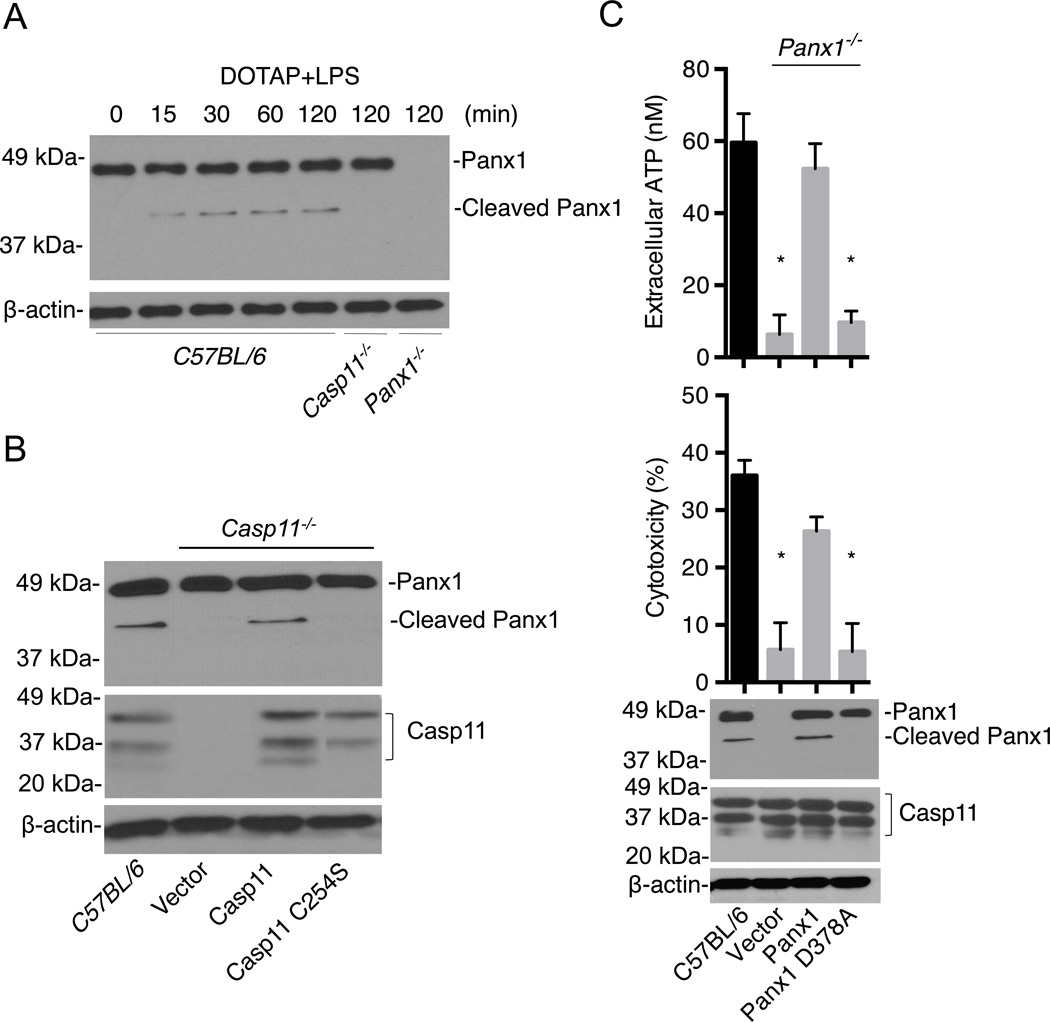
The pannexin-1 channel can be activated by cleavage of its COOH-terminal autoinhibitory domain at a caspase cleavage site by caspase-3 in response to apoptotic stimuli or purified caspase-3 (Sandilos et al., 2012; Boyd-Tressler et al., 2014; Chekeni et al., 2010). We examined whether activation of the noncanonical inflammasome pathway can induce processing of pannexin-1. Transfection of BMMs with LPS induced rapid processing of pannexin-1 into the predicted ~42 kDa fragment lacking its 46-amino acid COOH-terminal segment, and this cleavage were abolished in Casp11−/− BMMs (Fig. 4A). Furthermore, cytosolic delivery of LPS by CTB also induced detectable pannexin-1 cleavage, which required caspase-11 (Fig. S6). To determine whether the protease activity of caspase-11 was important for LPS-induced pannexin-1 cleavage, we expressed wild-type and catalytic-inactive caspase-11 (C254S) in Casp11−/− BMMs by lentivirus-mediated transduction. The cleavage of pannexin-1 induced by LPS transfection was restored by expression of wild-type, but not catalytic-inactive caspase-11 (Fig. 4B). To determine whether pannexin-1 cleavage is important for ATP release and induction of pyroptosis, Panx1−/− BMMs were reconstituted with either wild-type or pannexin-1 with a mutation in the critical aspartic acid residue at the predicted caspase cleavage site (Sandilos et al., 2012; Boyd-Tressler et al., 2014; Chekeni et al., 2010). Expression of wild-type pannexin-1, but not mutant pannexin-1 (D378A), restored LPS-induced ATP release and pyroptosis (Fig. 4C). In line with these findings, immunoblotting analysis revealed that upon LPS transfection, pannexin-1 cleavage was detected in Panx1−/− BMMs reconstituted with plasmid producing wild-type pannexin-1, but not pannexin-1 (D378A) (Fig. 4C). These results indicate that activation of caspase-11 in the noncanonical inflammasome pathway leads to cleavage of the pannexin-1 channel and pannexin-1-dependent ATP release, which mediates pyroptosis.
Figure 4. Activation of caspase-1 leads to pannexin-1 cleavage and ATP release.
(A–C) BMMs were primed overnight with LPS and then transfected with ultrapure S. minnesota RE595 LPS packaged with DOTAP.
(A) Immunoblotting for pannexin-1 before and after LPS transfection in wild-type (C57BL/6), Casp11−/− and Panx1−/− BMMs.
(B) Pannexin-1 cleavage after LPS transfection in Casp11−/− BMMs reconstituted with lentiviral vectors expressing wild-type caspase-11, caspase-11 C254S and vector alone. Immunoblotting for caspase-11 and β-actin are also shown.
(C) ATP release (upper panel), cytotoxicity (middle panel) and pannexin-1 cleavage (lower panel) were measured after LPS transfection in Panx1−/− BMMs reconstituted with wild-type pannexin-1, pannexin-1 D378A lentiviruses or control vector. Immunoblotting for pannexin-1 and caspase-11 in wild-type BMMs is also shown as a control.
(A–C) Data are representative of at least 3 experiments. Graphs show mean ± SD of triplicate cultures, * p <0.05. See also Figure S6.
Caspase-11 induces the cleavage of pannexin-1
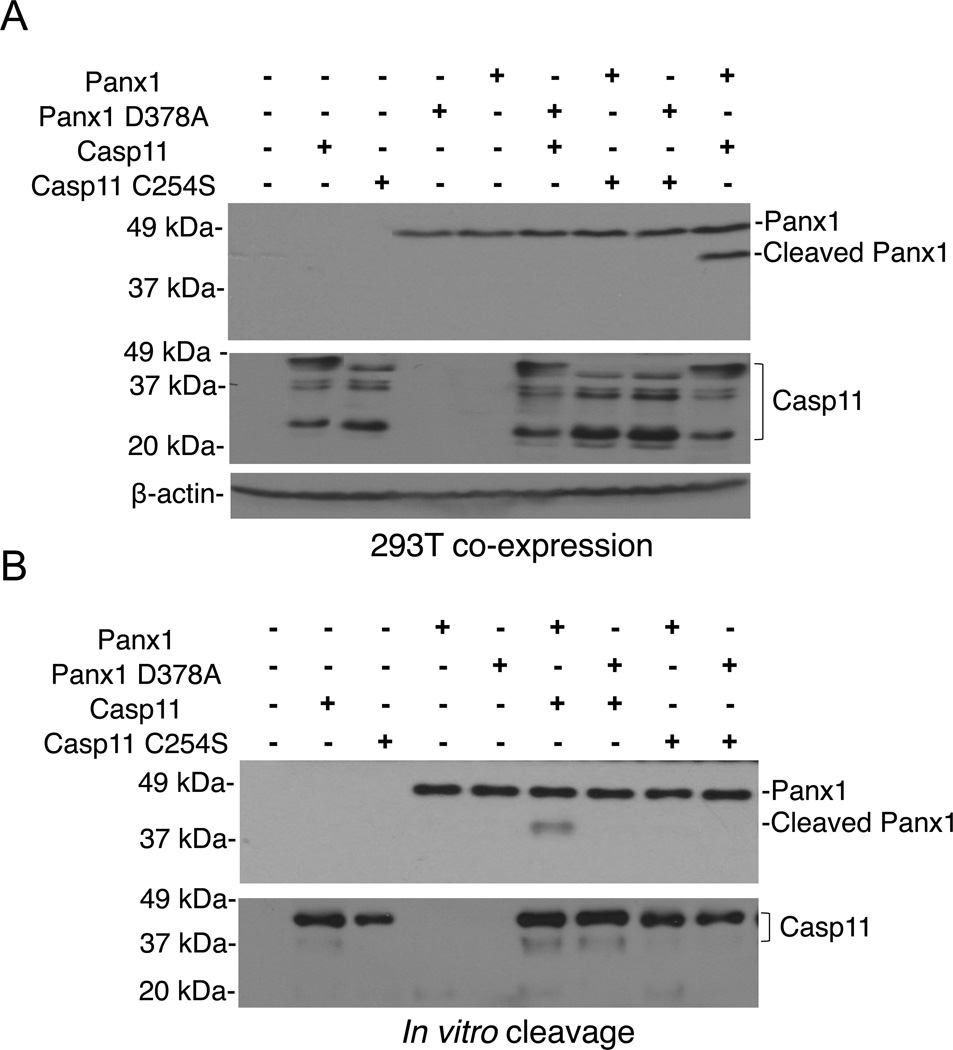
To determine whether caspase-11 can induce the cleavage of pannexin-1, we co-expressed wild-type or mutant caspase-11 with pannexin-1 proteins in 293T cells that lack caspase-11 expression (Shi et al., 2014). Expression of wild-type, but not the catalytic-inactive caspase-11 mutant, induced cleavage of wild-type pannexin-1, and this cleavage was abolished when pannexin-1 (D378A) was co-expressed with caspase-11 (Fig. 5A). Furthermore, mixture of purified pannexin-1 and caspase-11 proteins revealed that wild-type caspase-11, but not catalytic-inactive caspase-11, induced the cleavage of wild-type pannexin-1, which required the presence of the caspase cleavage site at the position of 378 amino acid (Fig. 5B). These results indicate that caspase-11 cleaves pannexin-1.
Figure 5. Caspase-11 cleaves pannexin-1.
(A) HEK293T cells were co-transfected with indicated pannexin-1 and caspase-11 expression vectors, cultured for 18–24 hrs, and pannexin-1 cleavage was assessed by immunoblotting.
(B) In vitro cleavage of purified pannexin-1 proteins after incubation with indicated caspase-11 proteins. Immunoblotting for pannexin-1 and caspase-11 is shown.
(A–B) Data are representative of at least 3 experiments.
Caspase-11 engages the pannexin-1 channel to induce NLRP3 activation
The noncanonical inflammasome can indirectly promote IL-1β secretion via the canonical NLRP3 inflammasome (Kayagaki et al., 2011 and 2013; Hagar et al., 2013), but the mechanism is poorly understood. Transfection of LPS into BMMs induced the release of IL-1β, which was specifically inhibited in cells deficient in NLRP3, ASC or caspase-11 (Fig. 6A). Notably, IL-1β secretion induced by LPS transfection was blocked by inhibitors of the pannexin-1 channel (CBX, probenecid and travofloxacin) (Fig 6B). Importantly, both IL-1β secretion and activation of caspase-1 were abolished in Casp11−/− or Panx1−/− BMMs, but not P2x7−/− BMMs (Fig. 6C). Previous experiments showed that pannexin-1 and P2X7 were dispensable for CTB plus LPS-induced IL-1β secretion when assessed at 16 hrs post-stimulation (Kayagaki et al., 2011). We therefore performed time course studies to assess IL-1β secretion induced after cytosolic LPS stimulation in wild-type, Panx1−/−, P2x7−/−, Nlrp3−/− and Casp11−/− BMMs. In agreement with previous results (Kayagaki et al., 2011), P2X7 was dispensable for IL-1β secretion induced by LPS transfection and CTB delivered LPS (Fig. S7). Furthermore, caspase-11, pannexin-1 and NLPR3 were required for IL-1β secretion induced by both LPS transfection and CTB-mediated LPS delivery (Fig. S7) in agreement with results shown in Fig. 6A, C and E. However, pannexin-1 was dispensable for IL-1β release at 16 hrs post-stimulation which is consistent with previous results (Kayagaki et al., 2011). Activation of the canonical NLRP3 inflammasome and pyroptosis are induced by several stimuli including nigericin and aluminiun hydroxide (alum), in addition to ATP (Franchi et al., 2009). Nigericin and alum, as well as poly(dA:dT) that activates the AIM2 inflammasome (Franchi et al., 2009), induced pyroptosis in the absence of caspase-11, P2X7 or pannexin-1 (data not shown). Furthermore, canonical inflammasome activation by ATP was independent of caspase-11 and pannexin-1, but relied on P2X7 and NLRP3 (data not shown), in agreement with previous studies (Qu et al., 2011). The efflux of K+ is a critical step in activation of the NLRP3 inflammasome (Muñoz-Planillo et al., 2013; Perregaux and Gabel, 1994). Importantly, transfection of LPS induced K+ release in wild-type, P2x7−/− and Nlrp3−/−, but not Casp11−/− BMMs (Fig. 6D). Furthermore, K+ efflux induced by LPS transfection also required pannexin-1 (Fig. 6D). In addition, expression of wild-type pannexin-1, but not pannexin-1 (D378A), restored cytosolic LPS-induced K+ release, IL-1β secretion and caspase-1 activation in Panx1−/− BMMs (Fig. 6E). Collectively, these results indicate that the noncanonical inflammasome pathway mediated by caspase-11 engages the pannexin-1 channel to induce K+ efflux and NLRP3 activation.
Figure 6. Noncanonical inflammasome pathway engages the pannexin-1 channel to induce K+ efflux and NLRP3 activation.
(A–E) BMMs were primed with LPS and then transfected with ultrapure S. minnesota RE595 LPS packaged with DOTAP.
(A) IL-1β release from wild-type (C57BL/6), Nlrp3−/−, Pycard−/−, Nlrc4−/−, Aim2−/−, Nlrp6−/− Nlrp12−/−, Casp11−/− and Casp1−/−/Casp11−/− BMMs was measured by ELISA.
(B) Wild-type BMMs were pre-treated with various inhibitors as in Fig 3 (A), IL-1β release was measured 2 hrs after LPS transfection.
(C) IL-1β release and caspase-1 processing in C57BL/6, Casp11−/−, P2X7−/− and Panx1−/− BMMs were measured by ELISA and immunoblotting.
(D) Intracellular K+ was measured 30 min after treatment of BMMs with DOTAP or DOTAP plus LPS.
(E) Intracellular K+, IL-1β release, caspase-1 and pannexin-1 processing were assessed after LPS transfection of Panx1−/− BMMs reconstituted with wild-type, pannexin-1 D378A or control vector. DOTAP-treated Panx1−/− BMMs and LPS-transfected wild-type (C57BL/6) BMMs are shown as controls.
(A–E) Data are representative of at least 3 experiments. Graphs show mean ± SD of triplicate cultures, * p<0.05. See also Figure S7.
The pannexin-1/P2X7 axis is critical for susceptibility to endotoxic shock
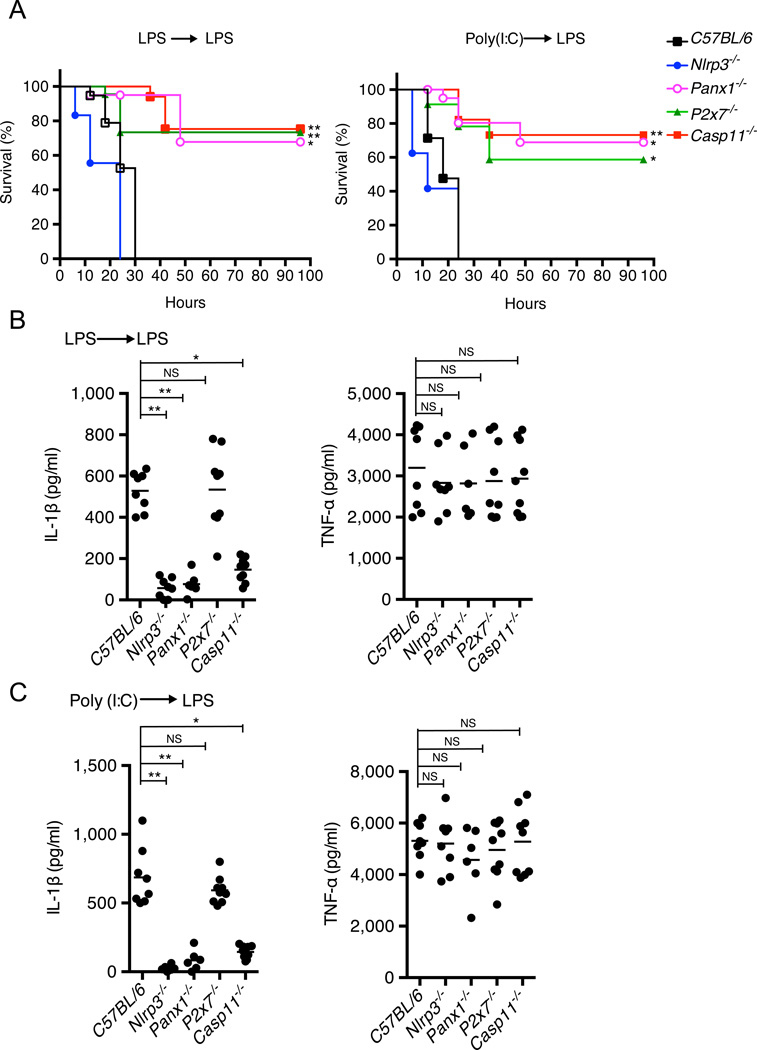
We next performed experiments to assess the role of pannexin-1 and P2X7 in endotoxic shock models that have been developed to assess the noncanonical inflammasome in vivo (Kayagaki et al., 2013; Hagar et al., 2013). In agreement with previous studies (Kayagaki et al., 2013; Hagar et al., 2013), wild-type mice primed with non-lethal doses of LPS or the TLR3 agonist polyinosinic-polycytidylic acid (poly(I:C)) and then challenged with LPS rapidly succumbed whereas Casp11−/− mice were protected (Fig. 7A). Importantly, LPS and poly(I:C)-primed P2x7−/− and Panx1−/− mice, but not Nlrp3−/− mice, were also resistant to secondary LPS challenge (Fig. 7A). Furthermore, production of IL-1β, but not TNF-α, in sera was reduced in Casp11−/−, Nlrp3−/− and Panx1−/− mice, but not P2x7−/− mice (Fig. 7B and C). Collectively, these results indicate that activation of caspase-11 by LPS in vivo induces a rapid onset of mortality via the pannexin-1/P2X7 signaling axis. Furthermore, the studies indicate that IL-1β production after activation of the noncanonical inflammasome pathway is independent of P2X7 receptor, but dependent on NLRP3 in vivo.
Figure 7. Critical role of pannexin-1 and P2X7 in endotoxic shock induced by activation of the noncanonical inflammasome.
(A) Survival of mice primed with 400 µg/kg E. coli O111:B4 LPS (left) or 10 mg/kg poly(I:C) (right), and then challenged with 10 mg/kg E. coli O111:B4 LPS 7 hrs later. N=15 mice in C57BL/6 or Nlrp3−/− groups; N=17 mice in P2X7−/− or Casp11−/− groups; N=12 mice in Panx1−/− group. Data shown are from at least 2 representative experiments, * p<0.05, ** p<0.01, NS=not significant.
(B–C) IL-1β and TNF-α levels in mouse sera were measured 2 hrs after re-challenge with LPS. N=8 mice in C57BL/6 or Nlrp3−/− groups; N=7 mice in P2X7−/− or Casp11−/− groups; N=6 mice in Panx1−/− group.
(A–C) Data are representative of at least 3 experiments. Error bars indicate SD of technical replicates, * p<0.05, ** p<0.01, NS=not significant.
DISCUSSION
A major gap in our knowledge of the noncanonical inflammasome pathway is the events downstream of caspase-11 that mediate pyroptosis and susceptibility to LPS in vivo (Kayagaki et al., 2011 and 2013; Hagar et al., 2013). Our study provides evidence for a signaling pathway involving the noncanonical inflammasome in which caspase-11 cleaves and activates the pannexin-1 channel to induce ATP release, which in turn activates the purinergic P2X7 receptor to induce cytotoxicity and to regulate susceptibility to endotoxic shock.
Upon intracellular LPS stimulation induced by cytosolic delivery of LPS with CTB or transfection, the pannexin-1 channels are proteolytically processed at a caspase-cleavage site at the distal end of the intracellular domain of pannexin-1. Cleavage of pannexin-1 is functionally important because reconstitution of Panx1−/− macrophages with an uncleavable mutant form of pannexin-1 impaired ATP release and pyroptosis in response to cytosolic LPS stimulation. Several results indicate that pannexin-1 is cleaved by caspase-11. First, pannexin-1 processing induced by cytosolic LPS stimulation was abrogated in Casp11−/− macrophages. Second, the catalytic activity of caspase-11 was required for pannexin-1 cleavage, ATP release and pyroptosis. Finally, purified caspase-11 cleaved pannexin-1 at the expected caspase-cleavage site, which required the catalytic activity of caspase-11. Pannexin-1 is also cleaved and activated by caspase-3 in apoptotic T cells and HEK293 cells (Sandilos et al., 2012; Boyd-Tressler et al., 2014; Chekeni et al., 2010). We found no evidence for caspase-3 activation in macrophages stimulated with cytosolic LPS unlike in cells stimulated with apoptotic stimuli. Thus, the particular caspases that regulate pannexin-1 cleavage and activation may reflect, at least in part, the cellular context involved including the stimulus and cell lineage.
Previous studies have shown that pyroptosis induced by the noncanonical inflammasome is independent of NLRP3, ASC and caspase-1 (Kayagaki et al., 2011 and 2013; Hagar et al., 2013), which is in line with our current results. Instead, macrophage cytotoxicity induced by intracellular LPS stimulation relies on pannexin-1 and ATP-mediated P2X7 signaling. An important observation was the marked increase in sensitivity of the P2X7-associated pore and pyroptosis to ATP stimulation after cytosolic LPS stimulation. The mechanism by which cytoplasmic LPS decreases the threshold for the opening of the P2X7-associated pore in response to ATP remains unclear. Because LPS priming does not increase the sensitivity of P2X7 to ATP, the presence of LPS in the cytosol appears to be required for the enhancing effect. Previous studies have identified a LPS-binding region with amino acid homology to the LPS binding site of LPS-binding protein (LBP) in the C-terminal intracellular domain of P2X7 (Denlinger et al., 2001). Thus, one possibility is that LPS binding to the cytosolic domain of P2X7 induces conformational changes responsible for increased sensitivity to ATP-induced opening of the P2X7 pore. The molecular basis of the conversion of P2X7 from an ion channel to a pore is not clear. Genetic experiments clearly showed that P2X7, but not pannexin-1, is required for the formation of the P2X7-associated pore induced by ATP stimulation (Qu et al., 2011). Our results are consistent with the latter and indicate that pannexin-1 acts upstream of P2X7 by mediating the release of ATP and subsequent opening of the P2X7-associated pore. Although the pannexin-1 channel releases ATP and pannexin-1 is required for K+ efflux-mediated NLRP3 activation, the latter event is not sufficient to drive cytolysis in that subsequent ATP-mediated P2X7 activation is required for cellular demise. Cytolysis is likely to be mediated by the opening of the P2X7-associated pore in response to nM concentrations of ATP. The activity of the P2X7 pore that mediates influx/efflux of large molecules has been linked to cytolytic activity and is distinct from that of the P2X7 channel that induces K+ efflux (Surprenant et al., 1996; Barlett et al., 2014). Furthermore, the channel and pore activities of P2X7 can be dissociated. For example, truncation of the cytosolic tail of P2X7 abolishes pore activity without affecting channel activity (Surprenant et al.; Smart et al., 2003). The lack of requirement for NLRP3 and caspase-1 for pyroptosis in response to cytosolic LPS is consistent with earlier studies (Kayagaki et al., 2011 and 2013; Hagar et al., 2013). One possibility is that stimuli that activate the canonical NLRP3 inflammasome such as ATP induce damaging events including mitochondrial dysfunction that are not required for caspase-1 activation (Munoz-Planillo et al., 2013; Allam et al. 2014) whereas the caspase-11/pannexin-1 axis may induce primarily K+ efflux which is sufficient for NLRP3 activation (Munoz-Planillo et al., 2013). Another possibility is that the opening of pannexin-1 channels is transient and/or partial and insufficient to trigger cytolysis in the absence of P2X7 signaling. The latter is consistent with the finding that the levels of extracellular ATP induced by cytosolic LPS are only in the 50–100 nM range and the increase in extracellular ATP is transient. Furthermore, the cleavage of pannexin-1 channels is partial based on immunoblotting analysis, suggesting that only a subset of pannexin-1 channels are activated in response to cytosolic LPS. Although more work is needed, the results suggest that P2X7 is required for organelle damage and cytolysis, events that are triggered by the opening of the P2X7-asociated pore (Mackenzie et al., 2005; Barlett et al., 2014).
Our results suggest that the caspase-11/pannexin-1 pathway bifurcates at pannexin-1, which also regulates the activation of the canonical NLRP3 inflammasome through the induction of K+ efflux independently of P2X7. In contrast to the noncanonical inflammasome, pannexin-1 was not required for pyroptosis induced by stimuli that activate the canonical NLRP3 or AIM2 inflammasomes. The finding that activation of the noncanonical inflammasome induces NLRP3-dependent caspase-1 activation and IL-1β release independently of P2X7 is in line with published work (Kayagaki et al., 2011). Previous results suggest that pannexin-1 is dispensable for IL-1β release in response to CTB plus LPS when assessed 16 hrs after stimulation (Kayagaki et al., 2011). However, our kinetic studies revealed that pannexin-1 was required for IL-1β release at 2 and 8 hrs post-stimulation with DOTAP plus LPS or CTB plus LPS. Unlike results observed at earlier timepoints, cytotoxicity at 16 hrs was independent of pannexin-1, which is consistent with previous results (Kayagaki et al., 2011). Although the reason for the differential role of pannexin-1 at early and late time points in vitro remain unclear, IL-1β production was impaired in Panx1−/− mice after secondary challenge with LPS suggesting that the early in vitro results are more relevant than those at 16 hrs post stimulation.
Earlier studies suggested that lethality induced by endotoxic shock in mice is induced primarily by caspase-11, but not caspase-1 (Kayagaki et al., 2011 and 2013; Hagar et al., 2013). Furthermore, LPS-induced lethality appears to be driven by caspase-11-dependent pyroptosis rather than caspase-1-dependent production of IL-1β (Kayagaki et al., 2011). In line with the latter results, we show that the pannexin-1/P2X7 axis activated by caspase-11 is critical for lethality induced by secondary LPS challenge. Consistently, P2x7−/− mice are protected from lethality induced in the cecal ligation and puncture sepsis model (Santana et al., 2015). In contrast, NLRP3 that regulates IL-1β in response to LPS was not required for lethality. Although clinical sepsis is a highly complex disorder in which multiple mediators are involved in morbidity, our studies suggest that inhibitors of the pannexin-1 channel and P2X7 receptor may benefit patients with gram-negative sepsis.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 mice were originally purchased from the Jackson Laboratory and bred in our animal facility. Casp1−/−/Casp11−/−, Casp11−/−, Nlrp3−/−, Nlrp6−/−, Nlrp12−/−, Nlrc4−/−, Pycard−/− and P2x7−/− mice on a C57BL/6 background have been described (He et al., 2013; Wang et al., 1998; Chen et al., 2011; Zaki et al., 2011). Panx1−/− and Aim2−/− mice (Qu et al., 2011; Jones et al., 2010) generated on a C57BL/6 background were provided by Vishva Dixit (Genentech Inc.) All animal experiments were conducted according to the U.S.A. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Animals were maintained in an AAALAC approved facility, and all animal studies were approved by the Animal Care and Use Committee at the University of Michigan (Ann Arbor, MI).
Reagents
DOTAP liposomal transfection reagent was from Roche (Mannheim, Germany). Cholera toxin B subunit (CTB) was from List Biological Laboratories, Inc. Ultra-pure lipopolysaccharide (E. coli O111:B4), ultra-pure lipopolysaccharide (S. minnesota RE595), poly(I:C) LMW and poly(dA:dT)/lyovec were from Invivogen. ATP, apyrase, carbenoxolone (CBX), bafliomycin A, brefeldin (BFA), 18-glycyrrhetinic (18GA), flufenamic acid (FFA), glibenclamide, gadolinium III (Gd3), probenecid, ARL67156 (an ecto-ATPase inhibitor) and trovafloxacin (a pannexin-1-selective antagonist) were from Sigma-Aldrich. Alum was from Thermo Scientific. Nigericin and Ac-DNLD-CHO (caspase-3/7 inhibitor) were from Calbiochem, and zVAD-FMK (pan-caspase-inhibitor) and zDEVD-FMK (caspase-3 inhibitor) was from R&D system. Fluorescent Yo-Pro-1 was from Life Technology.
Bacterial growth and conditions
Flagellin deficient Salmonella typhimurium (Δflag-Sal (fljAB::Kan, fliC::Cm)) was a gift of Denise Monack, Stanford University. The bacteria were grown to stationary phase overnight in LB medium at 37°C with aeration and the BMMs were infected as described below.
Macrophage culture, transfection, infection, and cytotoxicity assay
BMMs were cultured as previously described (Franchi et al., 2009). When indicated, BMMs were primed with LPS from E. coli O111:B4 (50 ng/mL) in Opti-MEM overnight. For LPS transfection, 75 ng of LPS (S. minnesota RE595) and 375 ng DOTAP were suspended in 2 µl of Opti-MEM for 5 min and then suspensions were mixed and incubated for 30 min at room temperature. Reaction volumes were then brought up to 100 µl with Opti-MEM as previously described before addition to 5×104 BMMs per well in 96-well plates (Hagar et al., 2013). For CTB treatment, primed cells were stimulated with 20 µg/ml CTB plus 1 µg/ml LPS (Kayagaki et al., 2011 and 2013). Flagellin deficient Salmonella enterica serovar Typhimurium (Δflag-Sal (fljAB::Kan, fliC::Cm)) was a gift from Denise Monack, Stanford University. The bacteria were grown to stationary phase overnight in LB medium at 37°C with aeration. For infections, culture media was replaced with Opti-MEM and then Salmonella (Δflag-Sal) were added to BMMs at a MOI of 20, gentamicin (20 µg/ml) was added 60 min after infection. For inhibitor assays, BMMs were pre-treated with drugs for 30 min before transfection, then transfected with LPS complexes. Cytotoxicity was measured using the CytoTox 96® Non-Radioactive Cytotoxicity Assay Kit (Promega) according to manufacturer’s instructions. The cytotoxicity was normalized to total lactate dehydrogenase activity in cell lysates.
In vitro caspase cleavage assay
A mouse Flagx3-tagged pannexin-1 expression plasmid was generated by PCR cloning of pannexin-1 cDNA into pcDNA3. Mutation of amino acid 378 was generated using a QuikChange® Site-Directed Mutagenesis Kit (Stratagene) and verified by sequencing. Caspase-11 and caspase-11 C254S plasmids were gifts from Amal Amer (Ohio State University). HEK293T cells were transfected with flag×3-tagged pannexin-1 or caspase-11 plasmids using Lipofectamine LTX® reagents (Invitrogen). After 24 hours, whole cell lysates were immunoprecipitated with GenScript Anti-DYKDDDDK G1 Affinity Resin (Cat. No. L00432), washed in buffer, and bound target protein was eluted by competitive binding with DYKDDDDK peptide. The purified pannexin-1 and caspase-11 proteins were re-suspended in reaction buffer (50 mM Tris, pH 8.0, 0.5 mM EDTA, 0.5 mM Sucrose, 10 mM DTT, 1×EDTA-free proteinase inhibitor mixture (Roche Applied Science), and in vitro caspase cleavage assay was carried out for 1 hr at 37°C and terminated by addition of SDS sample buffer and boiling at 95 °C for 5 min. The reactions were analyzed by SDS-PAGE and immunoblotting.
HEK293T cell transfection
2.5×106 HEK293T cells were plated in a 100-mm Petri dish 24 hrs before transfection. Cells were then co-transfected with pcDNA3 caspase-11 or caspase-11 (C254S) and pannexin-1 or pannexin-1 (D378A) using Lipofectamine LTX. Cells were lysed in 1.5 ml of ice-cold lysis buffer (50 mM Tris, pH 7.4, 2 mM EDTA, 150 mM NaCl, 0.5% Nonidet P-40, 1×EDTA-free proteinase inhibitor mixture (Roche)). Cell lysates were clarified by centrifugation (14, 000× g) at 4 °C for 10 min. Pannexin-1 cleavage was analyzed by SDS-PAGE and immunoblotting.
Reconstitution of BMMs with caspase-11 or pannexin-1
For caspase-11 reconstitution, Casp11−/− BMMs were transduced with pHIV (Addgene #21373) derived lentivirus producing caspase-11 or caspase-11 C254S. For reconstitution of pannexin-1, Panx1−/− BMMs were transduced with pHIV-derived lentivirus carrying pannexin-1 or pannexin-1 D378A. After 3–4 days, transduced cells were sorted and replated, the expression of reconstituted proteins was determined by immunoblotting.
ATP release assay
After transfection, the extracellular ATP concentration was measured using ENLITEN® rLuciferase/Luciferin Reagent (Promega). At indicated time points, each sample was centrifuged at 1500 rpm for 1 min and 10 µl of the supernatant was collected for ATP determination. The luciferin-luciferase reagent (100 µl) was injected into the supernatant and chemiluminescence was measured using a LMax Luminometer (Molecular Devices). The concentration of ATP in each sample was determined by comparing the luminescence of samples with signals of a standard in the range of 10−6 to 10−10 M.
K+ efflux assay
Intracellular K+ measurements were performed by inductively coupled plasma optical emission spectrometry (ICP-OES) with a Perkin-Elmer Optima 2000 DV spectrometer using yttrium as internal standard as described (Muñoz-Planillo et al., 2013).
Yo-Pro-1 uptake assay
BMMs treated under various conditions (indicated in figure legends) were stained with 1 µM Yo-Pro-1 for 15 min at room temperature, then washed with PBS and fixed by 4% paraformaldehyde solution in PBS for 20 min, and analysis was performed using a fluorescence Olympus FV 500 confocal microscope.
Immunoblotting
Caspase-1 was analyzed with rabbit anti-caspase-1 antibody as described (Franchi et al., 2009). Caspase-11 was analyzed using anti-caspase-11 antibody (17D9, Sigma-Aldrich). Pannexin-1 was assessed using rabbit anti-pannexin-1 antibody (Invitrogen). Caspase-3 and caspase-7 were analyzed with anti-caspase-3 antibody (Cell Signaling) and anti-caspase-7 antibody (R&D system). Blots were stripped and re-probed for β-actin as a loading control.
ELISA assay
The amounts of IL-1β and TNF-α in cell culture supernatants or mouse sera were measured by ELISA (R&D Systems) according to the manufacturer’s manual. Assays were performed in triplicate for each independent experiment.
Endotoxic shock model
6–8 week-old mice were primed with E coli O111:B4 LPS (400 µg/kg) or poly(I:C) (LMW, 10 mg/kg) by intra-peritoneal injections for 7 hrs. Mice were then challenged with 10 mg/kg of LPS (E coli O111:B4) i. p.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 program (GraphPad Software). Differences between two groups were evaluated using Student’s t- test. One way ANOVA test was used to analyze differences among multiple groups. Differences in mouse survival were assessed using the Long-rank (Mantel-Cox) test. Statistical significance was defined as *p<0.05, ** p <0.01.
Supplementary Material
Highlights.
-
-
Caspase-11 induces pyroptosis via ATP-mediated P2X7 signaling.
-
-
LPS-induced caspase-11 activation triggers \ activation of the pannexin-1 pore.
-
-
Pannexin-1 cleavage was required for NLRP3 activation in response to cytosolic LPS.
-
-
Pannexin-1 and P2X7 are essential for endotoxic shock.
Acknowledgments
We thank Junying Yuan for sharing caspase-11 knockout mice, and Vishva Dixit (Genentech Inc.) for sharing pannexin-1 and Aim2 knockout mice, Amal Amer for providing caspase-11 plasmids, and Melody Zeng, Grace Chen and Jessica Werner for critical reading of the manuscript. We also thank members of the Núñez lab for discussions and technical assistance, Lisa Burmeister for mouse husbandry, and Joel Whitfield from the University of Michigan Immunology Core for performing ELISA assays. D.Y. was supported by the State Scholarship Fund from China Scholarship Council (No. 201306740018). This work was supported by grants R01AI063331 and R01DK091191 from the National Institutes of Health to G.N..
Footnotes
AUTHOR CONTRIBUTIONS:
D.Y., Y.H. and G.N. conceived the study; D.Y. performed the majority of experiments, assisted by Y.H.; R.M. and D.Y. did the K+ efflux experiments; D.Y., Y.H., Q.L. and G.N. analyzed the data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Contributor Information
Qin Liu, Email: qinliu@ecust.edu.cn.
Gabriel Núñez, Email: gabriel.nunez@umich.edu.
REFERENCES
- Allam R, et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15(9):982–990. doi: 10.15252/embr.201438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartllet R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 2014;66(3):638–675. doi: 10.1124/pr.113.008003. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183(2):787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd-Tressler A, et al. Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism. J. Biol. Chem. 2014;289:27246–27263. doi: 10.1074/jbc.M114.590240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Liu MC, Wang F, Bertin J, Núñez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J. Immunol. 2011;186:7187–7194. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekeni FB, et al. Pannexin 1 channels mediated ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger LC, et al. Cutting Edge: The nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J. Immunol. 2001;167(4):1871–1876. doi: 10.4049/jimmunol.167.4.1871. [DOI] [PubMed] [Google Scholar]
- D’hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions. BioEssays. 2009;31:953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Núñez G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, et al. The inflammasome: a caspase-1 activation platform regulating immune responses and disease pathogenesis. Nat. Immunol. 2009;10(3):241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar JA, Powel DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Franchi L, Núñez G. TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J. Immunol. 2013;190:334–339. doi: 10.4049/jimmunol.1202737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. U.S.A. 2010;107(21):9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- Lévesque SA, Lavoie ÉG, Lecka J, Bigonnesse F, Sévigny J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br. J. Pharmacol. 2007;152(1):141–150. doi: 10.1038/sj.bjp.0707361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie AB, Young MT, Adinolfi E, Surprenant A. Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J. Biol. Chem. 2005;280:33968–33976. doi: 10.1074/jbc.M502705200. [DOI] [PubMed] [Google Scholar]
- Mackenzie AB, et al. Pseudoapoptosis inducd by brief activation of ATP-gated P2X7 receptors. J. Biol. Chem. 2005;280:33968–33976. doi: 10.1074/jbc.M502705200. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- Meunier E, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509(7500):366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- Muñoz-Planillo R, et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu. Rev. Pharmacol. Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- Pilla DM, et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl. Acad. Sci. U.S.A. 2014;111(20):7391–7396. doi: 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon IKH, et al. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature. 2014;507:329–334. doi: 10.1038/nature13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, et al. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 2011;186(11):6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- Sakaki H, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PLoS ONE. 2013;8:e59788. doi: 10.1371/journal.pone.0059778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilos JK, et al. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J. Biol. Chem. 2012;287(14):11303–11311. doi: 10.1074/jbc.M111.323378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilos JK, et al. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J. Biol. Chem. 2012;287:11303–11311. doi: 10.1074/jbc.M111.323378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasome. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin1 channels. Am. J. Physiol. Cell Physiol. 2008;295(3):C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart ML, et al. P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J. Biol. Chem. 2003;278(10):8853–8860. doi: 10.1074/jbc.M211094200. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2z receptor for extracellular ATP identified as a P2x receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Trentham DR, Eccleston JF, Bagshaw CR. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Wang S, et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- Zaki MH, et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.