Abstract
Glycosyltransferase-catalyzed enzymatic and chemoenzymatic syntheses are powerful approaches for the production of oligosaccharides, polysaccharides, glycoconjugates, and their derivatives. Enzymes involved in the biosynthesis of sugar nucleotide donors can be combined with the glycosyltransferases in one pot for efficient production of target glycans from simple monosaccharides and accpetors. The identification of enzymes involved in the salvage pathway of sugar nucleotide generation has greatly facilitate the development of simplified and efficient one-pot multienzyme (OPME) systems for synthesizing major glycan epitopes in mammalian glycomes. The applications of OPME methods are steadily gaining popularity mainly due to the increasing availability of wild-type and engineered enzymes. Substrate promiscuity of these enzymes and their mutants allows OPME synthesis of carbohydrates with naturally occurring post-glycosylational modificiation (PGMs) and their non-natural derivatives using modified monosaccharides as precursors. The OPME systems can be applied in sequential for synthesizing complex carbohydrates. The sequence of the sequential OPME processes, the glycosyltransferase used, and the substrate specificities of glycosyltransferasese define the structures of the products. The OPME and sequential OPME strategies can be extended to diverse glycans in other glycomes when suitable enzymes with substrate promiscuity become available. The Perspective summariezes the work of the authors and collaborators on the development of glycosyltransferase-based OPME systems for carbohydrate synthesis. Future directions are also discussed.
Introduction
Carbohydrates play important roles in biological systems.1,2 They are valid leads3 and candidates4-7 for the development of new therapeutics. Because of their potential biomedical applications, naturally occurring carbohydrates and their non-natural derivatives have been attractive synthetic targets. Their synthesis, however, is not trivial. Advanced chemical synthetic strategies are being constantly developed which allow the access to many structurally different carbohydrates and glycoconjugates.8-10 Glycosynthases effective for glycosidic bond formation are being generated from glycosidases by mechanism-based rational design and directed evolution.11,12 On the other hand, glycosyltransferase-based enzymatic and chemoenzymatic methods are steadily gaining popularity due to the increasing availability of related enzymes in their native or engineered forms and better understanding of their functions and substrate specificities.6,12-16
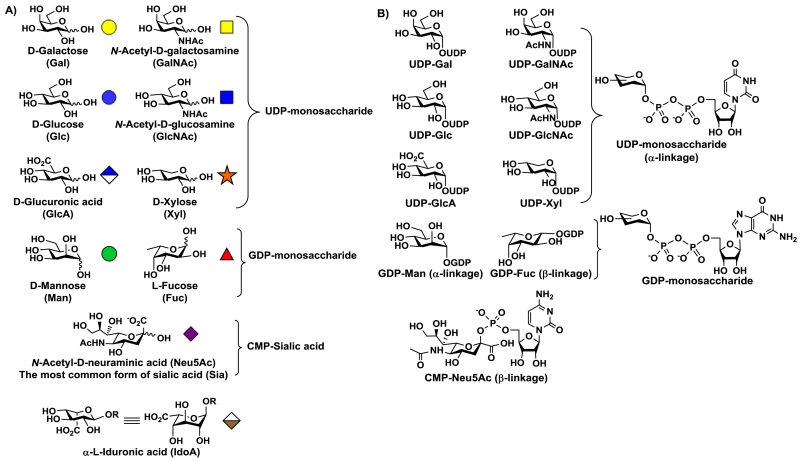
Sugar-nucleotide-dependent (Leloir type)17 glycosyltransferases are the main enzymes in nature that are responsible for the synthesis of divers carbohydrate-containing structures in a regio- and stereo-controlled manner. They can be categorized based on the types of monosaccharides being transferred. Mammalian glycomes are constituted by 10 common monosaccharide building blocks (Figure 1A) and their derivatives with modifications at various positions. Among these building blocks, L-iduronic acid (L-IdoA) has been found in some polysaccharides such as glycosaminoglycans (GAGs) including heparin/heparan sulfate (HS) and dermatan sulfate (DS). It is formed after the formation of the polysaccharide backbone by an epimerase-catalyzed reaction and can be considered one of the carbohydrate post-glycosylational modifications (PGMs) in nature.6,18 Other than L-IdoA, the free monosaccharide forms of other nine mammalian glycome building blocks can be activated and transferred to glycans and glycoconjugates via de novo or salvage pathways. These include D-glucose (Glc), D-galactose (Gal), N-acetyl-D-glucosamine (GlcNAc), N-acetyl-D-galactosamine (GalNAc), L-fucose (Fuc), D-mannose (Man), N-acetyl-D-neuraminic acid (Neu5Ac), D-xylose (Xyl), and D-glucuronic acid (GlcA).19 The corresponding sugar nucleotide donors used by the glycosyltransferases are uridine 5′-diphosphate-sugars (UDP-monosaccharides) for Glc, Gal, GlcNAc, GalNAc, GlcA and Xyl; guanosine 5′-diphosphate-sugars (GDP-monosaccharides) for Fuc and Man; and cytidine 5′-monophosphate sugar (CMP-monosaccharide) for Neu5Ac (Figure 1B).
Figure 1.
Common monosaccharide building blocks of mammalian glycomes (A) and their sugar nucleotides (B).
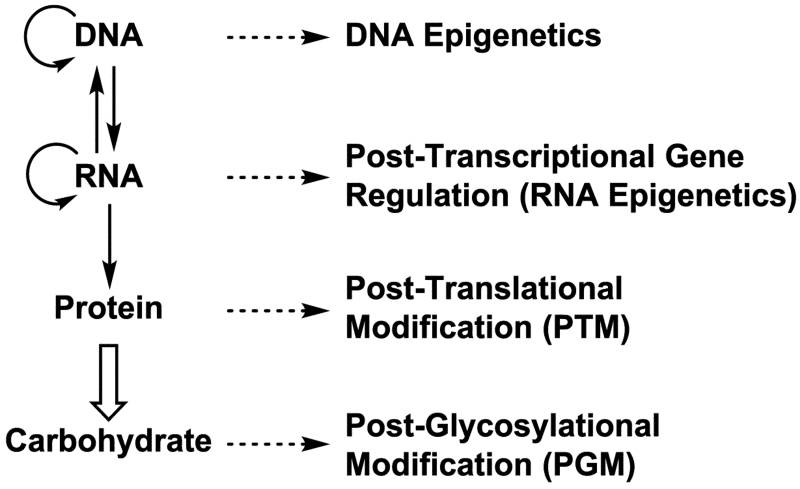
Glycosyltransferases catalyze the glycosidic bond formation with a high regio and stereo-control. After the formation of the glycosidic bond, additional modifications can take place on the glycans. We named these processes as carbohydrate postglycosylation modifications (PGMs)18 (Figure 2) in analogous to protein post-translational modifications (PTMs).20,21 Carbohydrate PGMs contribute to the recognition or the masking of carbohydrate-binding proteins.22,23 Common PGMs include O-sulfation, O-acetylation, O-methylation, O-lactylation, N-deacetylation, N-sulfation, and epimerization. These processes are catalyzed by enzymes and some processes require high energy donors such as 3′-phosphoadenosine-5′-phosphosulfate (PAPS), acetyl coenzyme A (acetyl-CoA), S-adenosyl methionine (SAM) etc. Some of these enzymes have not been identified. which possesses challenges for the biosynthesis of some complex carbohydrates with desired PGMs.18
Fig. 2.
An updated view of information flow in biological systems (solid arrows) and structural modifications of biomacromolecules (dashed arrows).18,20,21,24-26 The carbohydrate sequences are not template-driven but are defined by the activities of proteins (enzymes) encoded by the genetic information and a bold hollow arrow is used to represent the process. Carbohydrates presented on proteins are also considered as a type of protein post-translational modification (PTM).
One-pot multienzyme (OPME) systems for the synthesis of sugar nucleotides and carbohydrates
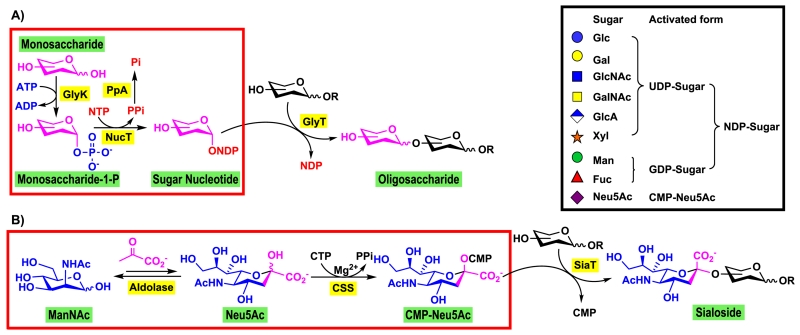
An effective route for the synthesis of complex glycans and glycoconjugates is to combine the sugar nucleotide biosynthetic process with glycosyltransferase-catalyzed reactions (Figure 3). Bacteria express carbohydrate structures mimicking those in mammals.27 Therefore, bacterial enzymes are well suited for synthesizing many glycans presented in the mammalian glycomes. For transferring a monosaccharide other than sialic acid in mammalian glycomes, glycosyltransferases use nucleoside diphosphate (NDP)-activated monosaccharides which can be formed from simple monosaccharides, adenosine 5′-triphosphate (ATP), and nucleoside 5′-triphosphate (NTP) via a suitable glycokinase (GlyK) and a sugar nucleotide pyrophosphorylase28 (or nucleotidyltransferase, NucT).13,29 An inorganic pyrophosphatase can also be added to drive the reaction towards the formation of the sugar nucleotides (Figure 3A).13 On the other hand, sialyltransferases use cytidine 5’-monophosphate (CMP) activated sialic acid (CMP-Sia) which can be formed from sialic acid (Sia) and cytidine 5′-triphosphate (CTP) via CMP-sialic acid synthetase (CSS)-catalyzed reaction. Sialic acids can be further obtained conveniently from their six-carbon precursors via a sialic acid aldolase-catalyzed reaction (Figure 3B).30 The glycosyltransferase and sugar nucleotide biosynthetic enzymes can be used in one-pot for the formation of desired glycans with specific glycosidic linkage from simple monosaccharides and suitable acceptors. As bacterial glycosyltransferases are less sensitive to nucleotide inhibition compared to mammalian glycosyltransferases27 and the cost of nucleoside triphosphates (substrates for the synthesis of sugar nucleotides) is continuously decreasing, it is not necessary to recycle the nucleotide 5′-diphosphate (NDP) or CMP generated in the glycosylation reaction as reported previously.31,32
Fig. 3.
The simplest routes for glycosyltransferase-catalyzed enzymatic synthesis of complex mammalian glycans with in situ generation of sugar nucleotides from (A) a monosaccharide other than sialic acid or (B) sialic acid (Sia). Enzyme abbreviations: GlyK, glycokinase; NucT, nucleotidyltransferase; GlyT, glycosyltransferase; PpA, inorganic pyrophosphatase; aldolase, sialic acid aldolase, CSS, CMP-sialic acid synthetase; SiaT, sialyltransferase.
One-pot multienzyme (OPME) strategy for the synthesis of carbohydrates with PGMs
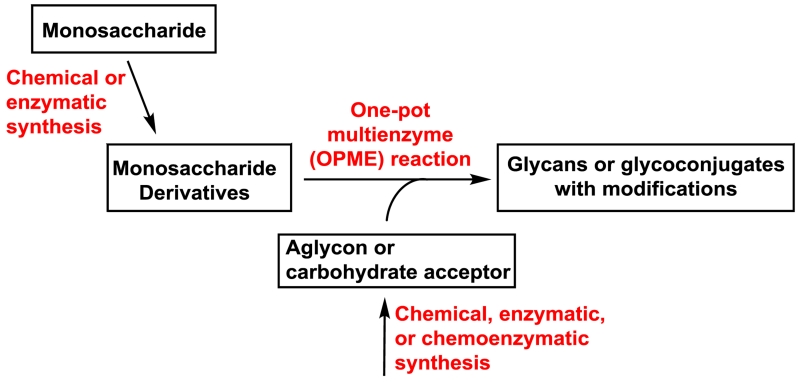
In order to synthesize carbohydrates with PGMs in a controllable manner, highly efficient one-pot multienzyme (OPME) chemoenzymatic methods (Figure 4)33 have been developed. In this system, instead of nature’s way of introducing PGMs after the formation of glycosidic bonds, modifications can be installed at the monosaccharide level by chemical or enzymatic processes to allow tailored synthesis of desired structures. Glycosyltransferase acceptors can be obtained chemically, enzymatically, or chemoenzymatically.
Fig. 4.
One-pot multienzyme (OPME) chemoenzymatic synthetic strategy for producing complex carbohydrates with PGMs.
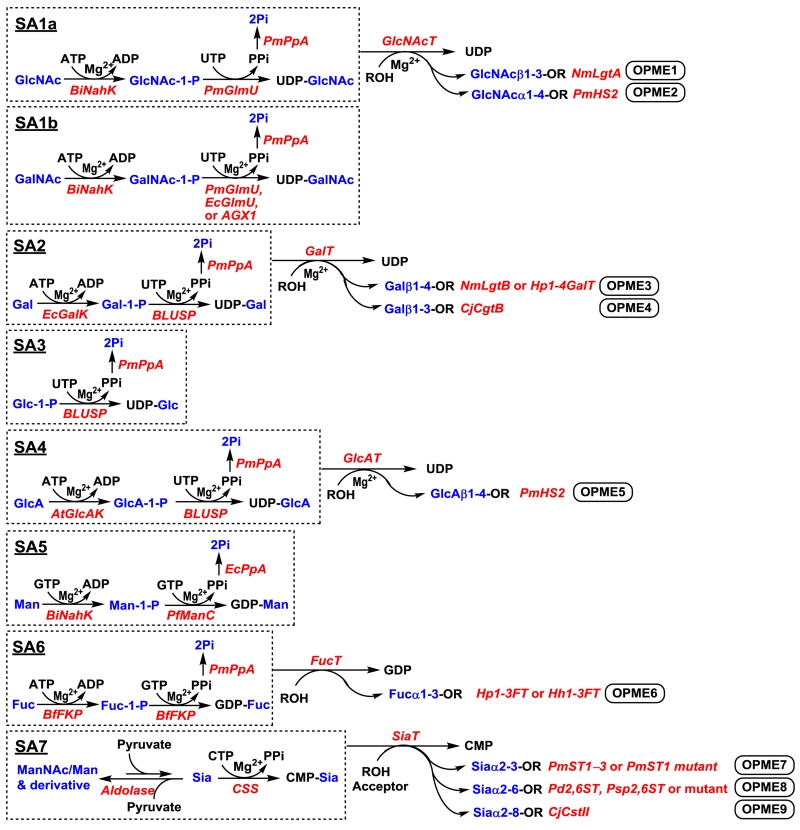
The key to the success of economic OPME chemoenzymatic synthesis of diverse carbohydrates with PGMs is to obtain suitable enzymes that have high activity, can be easily expressed in large amounts in simple expression systems (e.g. Escherichia coli system). More importantly, they need to be able to tolerate modified donor and/or acceptor substrates. Bacterial enzymes from different species have been found to be suitable candidates. To this end, we and our collaborator Peng G. Wang’s group have developed various OPME systems for the synthesis of sugar nucleotides (SA1a, SA1b, and SA2–SA7)34-38 and oligosaccharides (OPME1–OPME9)30,33,36,37,39-44 containing one of the eight common mammalian monosaccharide building blocks and/or their derivatives (Figure 5). The identification of enzymes involved in the salvage pathway of the sugar nucleotide formation allows the use of a smaller number of enzymes in the OPME system compared to using the enzymes from the de novo pathway,28 thus simplifies the OPME processes.
Fig. 5.
One-pot multienzyme (OPME) systems that we and our collaborator Peng G. Wang’s group have established for the synthesis of sugar nucleotides (SA1a, SA1b, and SA2–SA7) and oligosaccharides (OPME1–OPME9). A standard system contains 1) 2–3 enzymes for generation of valuable sugar nucleotide from NTP and monosaccharides and 2) one glycosyltransferase for glycosylation reaction. An exception is SA3 where low-cost glucose-1-phosphate (Glc-1-P) is directly used as a starting material for the production of UDP-Glc. The same set of enzymes are involved for the production of UDP-GlcNAc (SA1a) and UDP-GalNAc (SA1b) from GlcNAc and GalNAc, respectively. Enzyme abbreviations: BiNahK, Bifidobacterium infantis N-acetylhexosamine-1-kinase;46 PmGlmU, Pasteurella multocida N-acetylglucosamine 1-phosphate uridylyltransferase;34 EcGlmU, Escherichia coli N-acetylglucosamine 1-phosphate uridylyltransferase;50 AGX1, human UDP-GalNAc pyrophosphorylase;51 PmPpA, Pasteurella multocida inorganic pyrophosphatase;45 NmLgtA, Neisseria meningitidis β1–3-N-acetylglucosaminyltransferase;39 PmHS2, Pasteurella multocida heparosan synthase 2;40 EcGalK, Escherichia coli K-12 galactose kinase;35,54 BLUSP: Bifidobacterium longum UDP-sugar synthase;35 NmLgtB, Neisseria meningitidis β1–4-galactosyltransferase;45 Hp1–4GalT, H. pylori β1–4-galactosyltransferase;45 CjCgtB, Campylobacter jejuni β1–3-galactosyltransfearse;37 AtGlcAK, Arabidopsis thaliana glucuronokinase;36 PfManC, Pyrococcus furiosus DSM3638 GDP-Man pyrophosphorylase;38 EcPpA, Escherichia coli inorganic pyrophosphatase;38 BfFKP, a bifunctional enzyme from Bacteroides fragilis that has both L-fucokinase and GDP-fucose pyrophosphorylase activities;55 Hp1–3FT, Helicobacter pylori α1–3FucT;33,41,42 Hh1–3FT, Helicobacter hepaticus α1–3FucT;43 Aldolase, Escherichia coli56,57 or Pasteurella multocida58 sialic acid aldolase or lyase; CSS, CMP-sialic acid synthetase;56 PmST1–3, Pasteurella multocida α2–3-sialyltransferase 1–3;57,59,60 PmST1 mutant, PmST1 E271F/R313Y61 or PmST1 M144D42 mutant; Pd2,6ST, Photobacterium damselae α2–6-sialyltransferase;62,63 Psp2,6ST or mutant, Photobacterium species α2–6-sialyltransferase64 or Psp2,6ST A366G mutant;65 CjCstII, Campylobacter jejuni α2–3/8-sialyltransferase.44,66
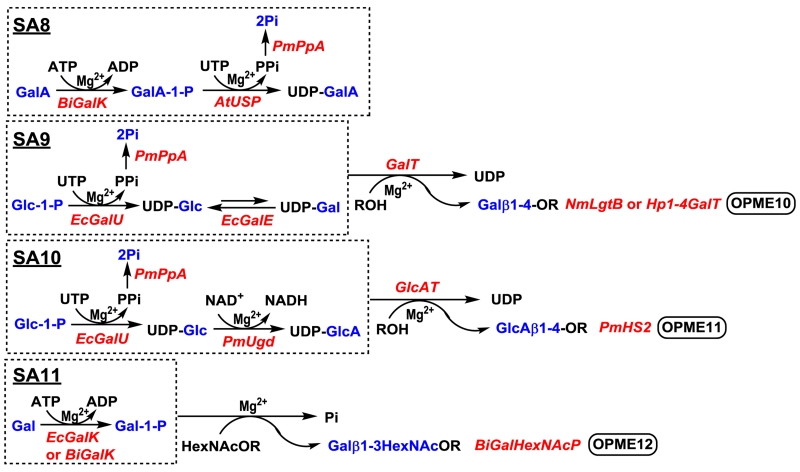
A one-pot three-enzyme (OP3E) system (SA8) (Figure 6) similar to UDP-GlcA in situ generation system (SA4) (Figure 5) has also been developed for generating uridine 5′-diphosphate galacturonic acid (UDP-GalA).36 In addition, it should be noted that the OPME3 system with directly activation of Gal (SA2) (Figure 5)37,39 is an improved system compared to a previously developed OPME1045 (Figure 6) relying on a UDP-Gal epimerase-catalyzed reaction for the formation of UDP-Gal from UDP-Glc obtained by UDP-Glc uridylyltransferase-catalyzed process (SA9) which requires an extra enzymatic process. Similarly, OPME5 system with direct activation of GlcA (SA4) (Figure 5)36 is a more efficient approach compared to a previous system (OPME11) (Figure 6) which requires the conversion of UDP-Glc to UDP-GlcA (SA10) by a nicotinamide adenine dinucleotide (NAD+)-dependent oxidation process catalyzed by a UDP-Glc dehydrogenase (Ugd).40
Fig. 6.
Additional one-pot multienzyme (OPME) systems that have been developed in our and Peng G. Wang’s groups. A. Enzyme abbreviations: BiGalK, Bifidobacterium infantis galactokinase;36,47,48 AtUSP, Arabidopsis thaliana UDP-sugar pyrophosphorylase;36 EcGalU, Escherichia coli UDP-glucose pyrophosphorylase;45 EcGalE, Escherichia coli UDP-galactose C4-epimerase;45 PmUgd, Pasteurella multocida UDP-glucose dehydrogenase;40 BiGalHexNAcP, Bifidobacterium infantis Galβ1–3HexNAc phosphorylase.52
It is important to point out that many enzymes used in the OPME systems are promiscuous towards substrates and modifications. For example, Bifidobacterium infantis N-acetylhexosamine-1-kinase (BiNahK)46 can use GlcNAc, GalNAc, mannose, and their C2- or C6-modified derivatives as substrates. 4-Deoxymannose was also a tolerable substrate for BiNahK. This property has been used in SA1a, SA1b, and SA5 for the OPME synthesis of UDP-GlcNAc, UDP-GalNAc, and GDP-Man respectively (Figure 5). Quite interestingly, Bifidobacterium infantis galactokinase (BiGalK)36,47,48 does not only recognize Gal but also GalA as substrates. It has been used in SA8 (Figure 6) for synthesizing UDP-GalA.36 Galactokinase mutants with broad substrate promiscuity49 can also be used for OPME synthetic purposes. Other than the substrate promiscuity noticed for glycokinases (GlyK), several nucleotidyltransferases (NucTs) are quite promiscuous towards substrates and modifications. For example, Pasteurella multocida N-acetylglucosamine 1-phosphate uridylyltransferase (PmGlmU),34 Escherichia coli N-acetylglucosamine 1-phosphate uridylyltransferase (EcGlmU),50 and human UDP-GalNAc pyrophosphorylase (AGX1)51 can tolerate GlcNAc-1-P, GalNAc-1-P, and their derivatives as substrates for the production of corresponding UDP-sugars. In fact, the same set of enzymes can be used for one-pot synthesis of UDP-GlcNAc (SA1a) and UDP-GalNAc (SA1b) (Figure 5). However, for a given UDP-HexNAc derivative, detailed comparison of the substrate specificities of NucTs are needed as they have some overlapping but complementary promiscuities. Bifidobacterium longum UDP-sugar pyrophosphorylase (BLUSP)35 is another promiscuous NucT. It can use Gal-1-P, Glc-1-P, GlcA-1-P, Man-1-P, and the derivatives of Glc-1-P and Man-1-P as substrates for the synthesis of UDP-Gal (SA2), UDP-Glc (SA3), UDP-GlcA (SA4), and the derivatives of UDP-Glc and UDP-Man.35,36
Other than the reactions catalyzed by sugar nucleotide-dependent glycosyltransferases, a more simplified one-pot two-enzyme (OP2E) system containing a galactokinase (GalK) and a Galβ1–3HexNAc phosphorylase (GalHexNAcP) (OPME12) has been developed for the synthesis of β1–3-linked galactosides (Figure 6).52 Although the products are limited to relatively small molecules, this two-enzyme OPME system can be considered as the simplest glycosylation process for the synthesis of glycans from simple monosaccharides. The development of glycoside phosphorylase-based53 OPME can be further explored for the synthesis of other glycans.
Although illustrated (Figures 5–6) for glycans containing non-modified monosaccharide building blocks for simplicity, the OPME systems will be the most powerful tools for synthesizing glycans containing modified monosaccharide units as the corresponding sugar nucleotides are not readily available and have to be generated. OPME systems can skip the tedious sugar nucleotide purification processes and avoid the instability issues of some sugar nucleotides and their derivatives. For this purpose, it is beneficial to test the substrate specificities of various enzymes from different species as they have different coverage on the scope of modifications that they can tolerate. Many of these enzymes were cloned and characterized previously by other groups but their substrate promiscuities were not fully explored and their synthetic applications were not maximized, especially in the content of producing carbohydrates with PGMs. By protein sequence homology search based on enzymes with known functions, we were able to identify new enzymes. In some cases, when wild-type enzymes could not satisfy the need for effective synthesis of target glycans, crystal structure-based mutagenesis42,61,65 was applied to obtain mutants with desired properties. For example, while a double site mutant of Pasteurella multocida α2–3-sialyltransferase 1 (PmST1)57,67,68 E271F/R313Y led to more than 6000-fold decrease of its α2–3-sialidase activity61 to allow easier control of sialoside synthetic process, PmST1 M144D42 single site mutant allowed the use of fucosylated glycans such as Lewis x and its 6′- and/or 6-O-sulfated derivatives as effective acceptors for the synthesis of sialyl Lewis x antigens containing different sialic acid forms with or without O-sulfation in a systematic manner.42,69 More recently, a single site mutation A366G65 of Photobacterium species α2–6-sialyltransferase (Psp2,6ST)64 was shown to improve its activity and expression level in Escherichia coli which provides larger amount of a more powerful catalyst to facilitate the synthetic effort.
OPME systems containing promiscuities sugar nucleotide biosynthetic enzymes and glycosyltransferases have been used for the synthesis of diverse arrays of glycans with PGMs or with non-natural modifications, especially for sialosides containing different sialic acid forms and various internal glycans.42,44,57,59,60,62,64-66,69
Sequential OPME processes for the synthesis of extended chain carbohydrates
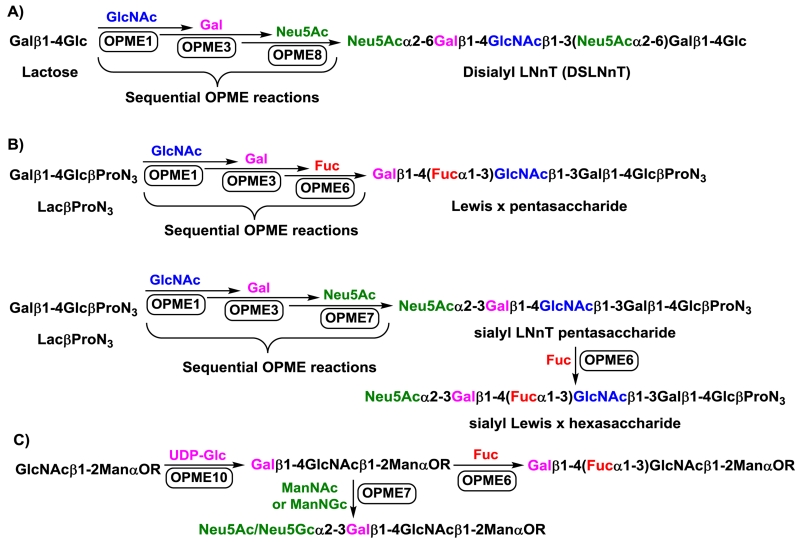
The OPME systems shown in Figures 5–6 can be carried out in sequential to build up complex mammalian glycomes in vitro. To avoid the complication of reactions in the downstream processes, product purification after each OPME reaction is commonly applied. Such an example has been successfully shown for the synthesis of a hexasaccharide disialyl lacto-N-neotetraose (DSLNnT) mimicking human milk disialyl lacto-N-tetraose (DSLNT) (Figure 7A).39 In this sequential OPME process, lactose was used as a starting material to go through Neisseria meningitidis β1–3-N-acetylglucosaminyltransferase (NmLgtA)-containing OPME1, Neisseria meningitidis β1–4-galactosyltransferase (NmLgtB)-containing OPME3, and Photobacterium damselae α2–6-sialyltransferase (Pd2,6ST)-containing OPME8 (Figure 5) reactions for sequential addition of a β1–3-linked GlcNAc, a β1–4-linked Gal, and two α2–6-linked Neu5Ac. The resulting DSLNnT was shown to have the beneficial effects of resembling human milk oligosaccharides (HMOSs)70 in protecting neonatal rats from necrotizing enterocolitis (NEC) in an animal model.39 Other examples have been shown for sequential OPME syntheses of Lewis x and sialyl LNnT pentasaccharides,71 sialyl Lewis x hexasaccharide (Figure 7B),71 and sialylated or fucosylated α-dystroglycan core M1 O-mannose glycans (Figure 7C).72
Fig. 7.
Sequential one-pot multienzyme (OPME) systems for the synthesis of a human milk hexasaccharide analog (A), Lewis x and sialyl LNnT pentasaccharides, sialyl Lewis x hexasaccharide (B), and sialylated or fucosylated α-dystroglycan core M1 O-mannose glycans (C).
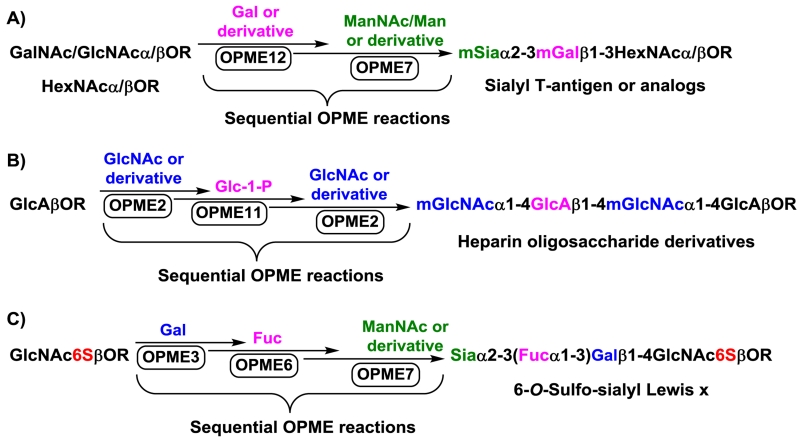
If modified monosaccharide units are introduced by the sequential OPME processes (Figure 8), both donor and acceptor substrate promiscuities of the glycosyltransferases used in the processes need to be considered. Such examples have been shown for the synthesis of sialyl Thomsen-Friedenreich (ST)-antigens containing various sialic acid forms and with different GalNAc/GlcNAc73 or Gal derivatives,48,74 heparin oligosaccharide derivatives,40 and more recently for the synthesis of O-sulfated sialyl Lewis x glycans containing different sialic acid forms.69
Fig. 8.
Sequential one-pot multienzyme (OPME) system for the synthesis of oligosaccharides and analogs containing modified monosaccharide building blocks including sialyl T-antigen or analogs (A), heparin oligosaccharide derivatives (B), and 6-O-sulfo-sialyl Lewis x (C). Abbreviations: mSia, modified sialic acid; mGal, modified galactose; mGlcNAc, modified N-acetylglucosamine
Chemoenzymatic synthesis of complex carbohydrates using building blocks produced by OPME processes for chemical glycosylation
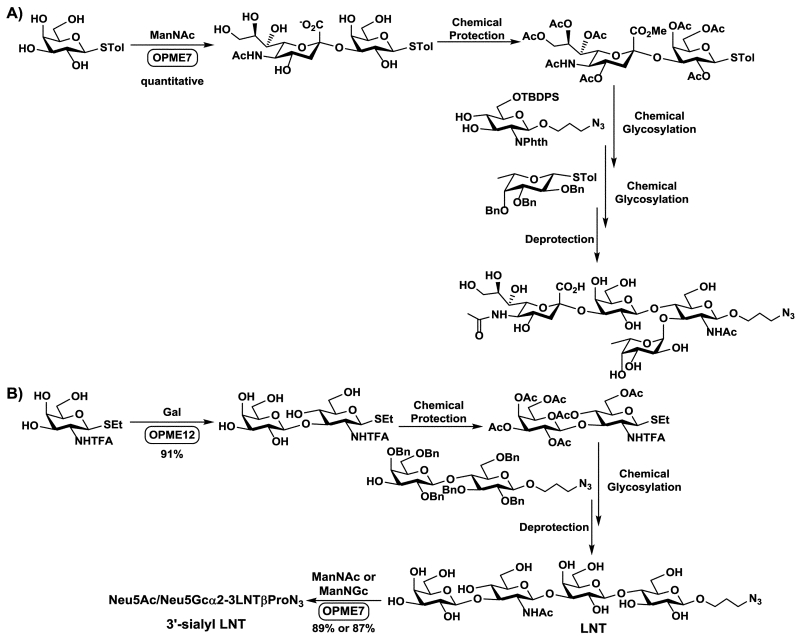
OPME process can also be used to efficiently produce oligosaccharide building blocks or synthons for chemical glycosylation reactions. This strategy is particularly useful for enzymatic construction of synthetically challenging sialylated glycosyl synthons for chemical synthesis. This has been demonstrated for the chemoenzymatic synthesis of sLex tetrasaccharides (Figure 9A),75 and in the Hongzhi Cao’s group for the synthesis of lacto-N-tetraose (LNT) and 3′-sialyl LNT (Figure 9B).76
Fig. 9.
Chemoenzymatic synthesis of sLex tetrasaccharide (A) and LNT (B) by chemical glycosylation of oligosaccharide building blocks/synthons obtained from OPME glycosylation process.
Additional strategies for OPME chemoenzymatic synthesis of desired complex carbohydrates
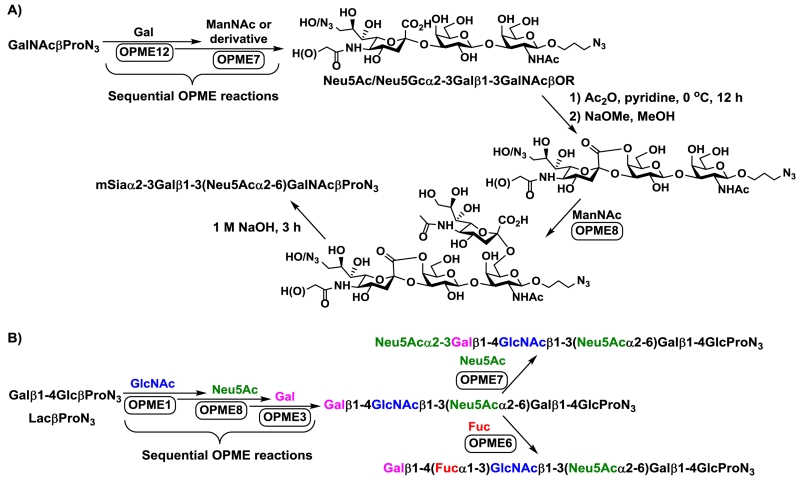
Additional strategies have also been developed in the Cao group for OPME chemoenzymatic synthesis of desired sequence of complex carbohydrates. For example, chemical manipulation of an α2–3-sialyltrisaccharide synthesized by sequential OPME reactions provided desired regioselectivity for additional OPME α2–6-sialylation for the synthesis of terminal disialyl tetrasaccharide sequence of gangliosides GD1α, GT1aα, and GQ1bα (Figure 10A).77 Furthermore, altering the sequence of sequential OPME processes provided alternative synthetic targets with different regioselective sialyl linkages (Figure 10B)71 compared to the previously reported one (Figure 7A).39
Fig. 10.
Chemical manipulation of trisaccharide synthesized by sequential OPME reactions for regioselective OPME α2–6-sialylation (A) and altering the sequence of sequential OPME processes for desired residue selective OPME sialylation (B).
Conclusions and outlook
In conclusion, OPME and sequential OPME are powerful tools for accessing mammalian glycomes and for developing carbohydrate-based diagnostics and therapeutics. Among 10 common building blocks for mammalian glycomes, 9 have accessible sugar nucleotides. Effective OPME systems have been developed by us and our collaborators for 7 of them for direct production of sugar nucleotides (UDP-GlcNAc, UDP-GalNAc, UDP-Gal, UDP-GlcA, GDP-Man, GDP-Fuc, CMP-Sia) from the corresponding simple monosaccharides. UDP-Glc can also be readily obtained from inexpensive Glc-1-P. For UDP-Xyl production, enzymatic or engineered yeast systems have been developed but they are achieved from UDP-Glc via an oxidation process for the production of UDP-GlcA with or without in situ regeneration of NAD+ cofactor followed by a decarboxylation process.19,78,79 UDP-Xyl has also been synthesized from xylose-1-phosphate and UDP-Glc via a UDP-Gal uridylyltransferase (GalU)-catalyzed reaction.80 Nevertheless, combining a suitable kinase, PpA, and a promiscuous UDP-sugar pyrophosphorylase such as that from pea sprouts81 or Thermus caldophilus82 should form a more efficient OPME system for the synthesis of UDP-Xyl directly from xylose, ATP, and UTP.
The OPME strategy can be extended to constructing structures in the glycomes of other species such as plants, fungi, and bacteria which produce many medicinally useful carbohydrate-containing structures.83,84 However, these are more challenging targets as a much higher diversity is presented in these glycomes. In addition to exploring the substrate promiscuity of the enzymes involved in the current OPME systems using new building blocks that are not presented in mammalian glycomes, new OPME systems will also be needed. Glycosyltransferases and sugar nucleotide biosynthetic enzymes with high substrate promiscuities will allow the access of many of these structures and will continue to be key components for making the OPME chemoenzymatic synthetic strategy successful. Wild-type enzymes from different species continue to be rich source for exploring their substrate promiscuity. Nevertheless, these enzymes may not be able to satisfy all chemoenzymatic synthetic needs. Protein engineering11,85-89 by crystal structure-based rational design and directed evolution will allow to obtain enzymes with desired properties including substrate promiscuities, stability, high expression level, high activity, etc. Both functional genomics and protein engineering studies will allow the access to more effect OPME systems that have broader applications in accessing large libraries of glycan structures to satisfy the increasing demand of carbohydrate research.
Supplementary Material
Acknowledgements
The authors are grateful for financial supports from NIH (grant numbers: R01GM094523 and R01HD065122), NSF (grant numbers: CHE-1012511 and CHE-1300449), European Union FP7 TransLink, and HDTRA GRANT11631742.
Footnotes
Footnotes relating to the title and/or authors should appear here. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Varki A. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanson S, Best M, Bryan MC, Wong CH. Trends Biochem. Sci. 2004;29:656–663. doi: 10.1016/j.tibs.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Ernst B, Magnani JL. Nat. Rev. Drug Discov. 2009;8:661–677. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulati K, Poluri KM. Glycoconj. J. 2016;33:1–17. doi: 10.1007/s10719-015-9642-2. [DOI] [PubMed] [Google Scholar]
- 5.Bauer J, Osborn HM. Future Med. Chem. 2015;7:2285–2299. doi: 10.4155/fmc.15.135. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Varki A. ACS Chem. Biol. 2010;5:163–176. doi: 10.1021/cb900266r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Linhardt RJ. Nat. Prod. Rep. 2014;31:1676–1685. doi: 10.1039/c4np00076e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X, Schmidt RR. Angew. Chem. Int. Ed. 2009;48:1900–1934. doi: 10.1002/anie.200802036. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CH, Hung SC, Wu CY, Wong CH. Angew. Chem. Int. Ed. 2011;50:11872–11923. doi: 10.1002/anie.201100125. [DOI] [PubMed] [Google Scholar]
- 10.Lepenies B, Yin J, Seeberger PH. Curr. Opin. Chem. Biol. 2010;14:404–411. doi: 10.1016/j.cbpa.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Shaikh FA, Withers SG. Biochem. Cell Biol. 2008;86:169–177. doi: 10.1139/O07-149. [DOI] [PubMed] [Google Scholar]
- 12.Kittl R, Withers SG. Carbohydr. Res. 2010;345:1272–1279. doi: 10.1016/j.carres.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Chen X. ACS Chem. Biol. 2011;6:14–17. doi: 10.1021/cb100375y. [DOI] [PubMed] [Google Scholar]
- 14.Muthana S, Cao H, Chen X. Curr. Opin. Chem. Biol. 2009;13:573–581. doi: 10.1016/j.cbpa.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luzhetskyy A, Bechthold A. Appl. Microbiol. Biotechnol. 2008;80:945–952. doi: 10.1007/s00253-008-1672-2. [DOI] [PubMed] [Google Scholar]
- 16.Langenhan JM, Griffith BR, Thorson JS. J. Nat. Prod. 2005;68:1696–1711. doi: 10.1021/np0502084. [DOI] [PubMed] [Google Scholar]
- 17.Chokhawala HA, Chen X. Enzymatic approaches to O-glycoside introduction: Glycosyltransferases. In: Kamerling JP, Boons Geert-Jan, Lee YC, Suzuki Akemi, Taniguchi Naoyuki, Voragen Alphons G. J., editors. Comprehensive Glycoscience, from Chemistry to Systems Biology. Vol. 1. Elsevier; Oxford, UK: 2007. pp. 415–451. Subject Editors: [Google Scholar]
- 18.Yu H, Chen X. Org. Biomol. Chem. 2007;5:865–872. doi: 10.1039/b700034k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai L. J. Carbohydr. Chem. 2012;31:535–552. [Google Scholar]
- 20.Jungblut PR, Holzhutter HG, Apweiler R, Schluter H. Chem. Cent. J. 2008;2:16. doi: 10.1186/1752-153X-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith LM, Kelleher NL, P. Consortium for Top Down Nat. Methods. 2013;10:186–187. doi: 10.1038/nmeth.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA, 3rd, Nishi A, Hsieh-Wilson LC. Nat. Chem. Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 23.Sjoberg ER, Powell LD, Klein A, Varki A. J. Cell Biol. 1994;126:549–562. doi: 10.1083/jcb.126.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crick F. Nature. 1970;227:561–563. doi: 10.1038/227561a0. [DOI] [PubMed] [Google Scholar]
- 25.Prendergast JG, Chambers EV, Semple CA. Genome Biol. Evol. 2014;6:1758–1771. doi: 10.1093/gbe/evu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roundtree IA, He C. Curr. Opin. Chem. Biol. 2015;30:46–51. doi: 10.1016/j.cbpa.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson KF. Glycoconj. J. 1999;16:141–146. doi: 10.1023/a:1026440509859. [DOI] [PubMed] [Google Scholar]
- 28.Bulter T, Elling L. Glycoconj. J. 1999;16:147–159. doi: 10.1023/a:1026444726698. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Hoffmeister D, Liu L, Fu X, Thorson JS. Bioorg. Med. Chem. 2004;12:1577–1584. doi: 10.1016/j.bmc.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 30.Yu H, Chokhawala HA, Huang S, Chen X. Nat. Protoc. 2006;1:2485–2492. doi: 10.1038/nprot.2006.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichikawa Y, Look GC, Wong CH. Anal. Biochem. 1992;202:215–238. doi: 10.1016/0003-2697(92)90099-s. [DOI] [PubMed] [Google Scholar]
- 32.Koeller KM, Wong CH. Glycobiology. 2000;10:1157–1169. doi: 10.1093/glycob/10.11.1157. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, Lau K, Li Y, Sugiarto G, Chen X. Curr. Protoc. Chem. Biol. 2012;4:233–247. doi: 10.1002/9780470559277.ch110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Thon V, Li Y, Yu H, Ding L, Lau K, Qu J, Hie L, Chen X. Chem. Commun. 2011;47:10815–10817. doi: 10.1039/c1cc14034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthana MM, Qu J, Li Y, Zhang L, Yu H, Ding L, Malekan H, Chen X. Chem. Commun. 2012;48:2728–2730. doi: 10.1039/c2cc17577k. [DOI] [PubMed] [Google Scholar]
- 36.Muthana MM, Qu J, Xue M, Klyuchnik T, Siu A, Li Y, Zhang L, Yu H, Li L, Wang PG, Chen X. Chem. Commun. 2015;51:4595–4598. doi: 10.1039/c4cc10306h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malekan H, Fung G, Thon V, Khedri Z, Yu H, Qu J, Li Y, Ding L, Lam KS, Chen X. Bioorg. Med. Chem. 2013;21:4778–4785. doi: 10.1016/j.bmc.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Liu Y, Wan Y, Li Y, Chen X, Zhao W, Wang PG. Org. Lett. 2013;15:5528–5530. doi: 10.1021/ol402585c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H, Lau K, Thon V, Autran CA, Jantscher-Krenn E, Xue M, Li Y, Sugiarto G, Qu J, Mu S, Ding L, Bode L, Chen X. Angew. Chem. Int. Ed. 2014;53:6687–6691. doi: 10.1002/anie.201403588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Li Y, Yu H, Sugiarto G, Thon V, Hwang J, Ding L, Hie L, Chen X. Angew. Chem. Int. Ed. 2013;52:11852–11856. doi: 10.1002/anie.201305667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugiarto G, Lau K, Yu H, Vuong S, Thon V, Li Y, Huang S, Chen X. Glycobiology. 2011;21:387–396. doi: 10.1093/glycob/cwq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugiarto G, Lau K, Qu J, Li Y, Lim S, Mu S, Ames JB, Fisher AJ, Chen X. ACS Chem. Biol. 2012;7:1232–1240. doi: 10.1021/cb300125k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Lau K, Cheng J, Yu H, Li Y, Sugiarto G, Huang S, Ding L, Thon V, Wang PG, Chen X. Glycobiology. 2010;20:1077–1088. doi: 10.1093/glycob/cwq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu H, Cheng J, Ding L, Khedri Z, Chen Y, Chin S, Lau K, Tiwari VK, Chen X. J. Am. Chem. Soc. 2009;131:18467–18477. doi: 10.1021/ja907750r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lau K, Thon V, Yu H, Ding L, Chen Y, Muthana MM, Wong D, Huang R, Chen X. Chem. Commun. 2010;46:6066–6068. doi: 10.1039/c0cc01381a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Yu H, Chen Y, Lau K, Cai L, Cao H, Tiwari VK, Qu J, Thon V, Wang PG, Chen X. Molecules. 2011;16:6396–6407. doi: 10.3390/molecules16086396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Liu Y, Wang W, Cheng J, Zhao W, Wang P. Carbohydr. Res. 2012;355:35–39. doi: 10.1016/j.carres.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Liu Y, Li T, Wang W, Yu Z, Ma C, Qu J, Zhao W, Chen X, Wang PG. Chem. Commun. 2015;51:10310–10313. doi: 10.1039/c5cc03746h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Fu X, Liao J, Liu L, Thorson JS. Chem. Biol. 2005;12:657–664. doi: 10.1016/j.chembiol.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Guan W, Cai L, Fang J, Wu B, George Wang P. Chem. Commun. 2009:6976–6978. doi: 10.1039/b917573c. [DOI] [PubMed] [Google Scholar]
- 51.Guan W, Cai L, Wang PG. Chemistry. 2010;16:13343–13345. doi: 10.1002/chem.201002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu H, Thon V, Lau K, Cai L, Chen Y, Mu S, Li Y, Wang PG, Chen X. Chem. Commun. 2010;46:7507–7509. doi: 10.1039/c0cc02850a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Neill EC, Field RA. Carbohydr. Res. 2015;403:23–37. doi: 10.1016/j.carres.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Fang J, Zhang J, Liu Z, Shao J, Kowal P, Andreana P, Wang PG. J. Am. Chem. Soc. 2001;123:2081–2082. doi: 10.1021/ja005738v. [DOI] [PubMed] [Google Scholar]
- 55.Yi W, Liu X, Li Y, Li J, Xia C, Zhou G, Zhang W, Zhao W, Chen X, Wang PG. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4207–4212. doi: 10.1073/pnas.0812432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu H, Yu H, Karpel R, Chen X. Bioorg. Med. Chem. 2004;12:6427–6435. doi: 10.1016/j.bmc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 57.Yu H, Chokhawala H, Karpel R, Yu H, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. J. Am. Chem. Soc. 2005;127:17618–17619. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Yu H, Cao H, Lau K, Muthana S, Tiwari VK, Son B, Chen X. Appl. Microbiol. Biotechnol. 2008;79:963–970. doi: 10.1007/s00253-008-1506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thon V, Lau K, Yu H, Tran BK, Chen X. Glycobiology. 2011;21:1206–1216. doi: 10.1093/glycob/cwr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thon V, Li Y, Yu H, Lau K, Chen X. Appl. Microbiol. Biotechnol. 2012;94:977–985. doi: 10.1007/s00253-011-3676-6. [DOI] [PubMed] [Google Scholar]
- 61.Sugiarto G, Lau K, Li Y, Khedri Z, Yu H, Le DT, Chen X. Molecular BioSyst. 2011;7:3021–3027. doi: 10.1039/c1mb05182b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu H, Huang S, Chokhawala H, Sun M, Zheng H, Chen X. Angew. Chem. Int. Ed. 2006;45:3938–3944. doi: 10.1002/anie.200600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun M, Li Y, Chokhawala HA, Henning R, Chen X. Biotechnol. Lett. 2008;30:671–676. doi: 10.1007/s10529-007-9588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding L, Yu H, Lau K, Li Y, Muthana S, Wang J, Chen X. Chem. Commun. 2011;47:8691–8693. doi: 10.1039/c1cc12732b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding L, Zhao C, Qu J, Li Y, Sugiarto G, Yu H, Wang J, Chen X. Carbohydr. Res. 2015;408:127–133. doi: 10.1016/j.carres.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng J, Yu H, Lau K, Huang S, Chokhawala HA, Li Y, Tiwari VK, Chen X. Glycobiology. 2008;18:686–697. doi: 10.1093/glycob/cwn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ni L, Sun M, Yu H, Chokhawala H, Chen X, Fisher AJ. Biochemistry. 2006;45:2139–2148. doi: 10.1021/bi0524013. [DOI] [PubMed] [Google Scholar]
- 68.Ni L, Chokhawala HA, Cao H, Henning R, Ng L, Huang S, Yu H, Chen X, Fisher AJ. Biochemistry. 2007;46:6288–6298. doi: 10.1021/bi700346w. [DOI] [PubMed] [Google Scholar]
- 69.Santra A, Yu H, Tasnima N, Muthana MM, Li Y, Zeng J, Kenyon NJ, Louie AY, Chen X. Chem. Sci. 2016 doi: 10.1039/c5sc04104j. DOI: 10.1039/C5SC04104J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X. In: Advances in Carbohydrate Chemistry and Biochemistry. Baker DC, Horton D, editors. Vol. 72. Academic Press, ACCB; UK: 2015. pp. 113–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen C, Zhang Y, Xue M, Liu XW, Li Y, Chen X, Wang PG, Wang F, Cao H. Chem. Commun. 2015;51:7689–7692. doi: 10.1039/c5cc01330e. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Meng C, Jin L, Chen X, Wang F, Cao H. Chem. Commun. 2015;51:11654–11657. doi: 10.1039/c5cc02913a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lau K, Yu H, Thon V, Khedri Z, Leon ME, Tran BK, Chen X. Org. Biomol. Chem. 2011;9:2784–2789. doi: 10.1039/c0ob01269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan J, Chen X, Wang F, Cao H. Org. Biomol. Chem. 2013;11:842–848. doi: 10.1039/c2ob26989a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao H, Huang S, Cheng J, Li Y, Muthana S, Son B, Chen X. Carbohydr. Res. 2008;343:2863–2869. doi: 10.1016/j.carres.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao W, Yan J, Chen X, Wang F, Cao H. Carbohydr. Res. 2015;401:5–10. doi: 10.1016/j.carres.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 77.Meng X, Yao W, Cheng J, Zhang X, Jin L, Yu H, Chen X, Wang F, Cao H. J. Am. Chem. Soc. 2014;136:5205–5208. doi: 10.1021/ja5000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oka T, Jigami Y. The FEBS journal. 2006;273:2645–2657. doi: 10.1111/j.1742-4658.2006.05281.x. [DOI] [PubMed] [Google Scholar]
- 79.Eixelsberger T, Nidetzky B. Adv. Synth. Catal. 2014;356:3575–3584. doi: 10.1002/adsc.201400766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Errey JC, Mukhopadhyay B, Kartha KP, Field RA. Chem. Commun. 2004:2706–2707. doi: 10.1039/b410184g. [DOI] [PubMed] [Google Scholar]
- 81.Kotake T, Yamaguchi D, Ohzono H, Hojo S, Kaneko S, Ishida HK, Tsumuraya Y. J. Biol. Chem. 2004;279:45728–45736. doi: 10.1074/jbc.M408716200. [DOI] [PubMed] [Google Scholar]
- 82.Kim JS, Koh S, Shin HJ, Lee DS, Lee SY. Biotechnol. Appl. Biochem. 1999;29(Pt 1):11–17. [PubMed] [Google Scholar]
- 83.Elshahawi SI, Shaaban KA, Kharel MK, Thorson JS. Chem. Soc. Rev. 2015;44:7591–7697. doi: 10.1039/c4cs00426d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goff RD, Thorson JS. MedChemComm. 2014;5:1036–1047. doi: 10.1039/C4MD00117F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang A, Singh S, Phillips GN, Jr., Thorson JS. Curr. Opin. Biotechnol. 2011;22:800–808. doi: 10.1016/j.copbio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams GJ, Gantt RW, Thorson JS. Curr. Opin. Chem. Biol. 2008;12:556–564. doi: 10.1016/j.cbpa.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gantt RW, Peltier-Pain P, Thorson JS. Nat. Prod. Rep. 2011;28:1811–1853. doi: 10.1039/c1np00045d. [DOI] [PubMed] [Google Scholar]
- 88.Hancock SM, Vaughan MD, Withers SG. Curr. Opin. Chem. Biol. 2006;10:509–519. doi: 10.1016/j.cbpa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 89.McArthur JB, Chen X. Biochem. Soc. Trans. 2016;44:129–142. doi: 10.1042/BST20150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.