Abstract
OBJECTIVE
The MB49 syngeneic, murine model of bladder cancer has been widely used for more than 35 years. In humans, bladder cancer is one third as prevalent in women as in men, with a trend toward lower prevalence in parous compared to nulliparous women. Our objective was to determine if the MB49 bladder cancer model reproduces the sex differences observed in humans, and to determine its sensitivity to testosterone and the pregnancy hormone, human chorionic gonadotropin (hCG).
METHODS
Male and female C57BL/6 mice were implanted with MB49 murine bladder cancer cells, and observed for tumor growth. MB49 dose responses to hCG and dihydrotestosterone were determined in vitro.
RESULTS
MB49 tumor growth was significantly greater in male mice than female mice. Pregnancy did not affect MB49 tumor growth in female mice. MB49 cells did not proliferate in response to hCG in vitro and the functional receptor for gonadotropins was absent. Dihydrotestosterone strongly stimulated growth of MB49 cells in vitro.
CONCLUSIONS
The MB49 murine model of bladder cancer reproduced some aspects of the sex differences observed in humans. Our results suggest that testosterone may stimulate MB49 cell proliferation, which may explain the more rapid MB49 tumor growth observed in male mice.
Keywords: urinary bladder neoplasms, chorionic gonadotropin, testosterone, MB49, sex differences
INTRODUCTION
Bladder cancer occurs in males approximately three times more frequently than in females [1]. This striking difference between the sexes cannot be accounted for by lifestyle factors [2]. The most reliable contributor to bladder cancer risk is smoking and this has been specifically excluded from the possible explanations for an increased rate of bladder cancer among men [3] Therefore, significant effort has gone into exploring possible mechanisms of protection against bladder cancer which may originate in some uniquely female attribute or biological process [4,5]. Mouse models of bladder cancer that reproduce the decreased susceptibility of females would be useful for these efforts. Therefore we investigated the extent to which the widely used MB49 model reproduces sex differences in bladder cancer.
MB49 cells are a urothelial carcinoma line derived from a C57BL/6 mouse by exposure of primary bladder epithelial cell explants to 7,12-dimethylbenz[a]anthracene (DMBA) for 24 hr followed by long-term culture [6]. The donor mouse was male, however, a 2012 karyotypic analysis found that the MB49 tumor cell line has lost the Y-chromosome [7], and therefore does not express male-specific antigens, which may trigger an immune response in syngeneic females. This would seem to make it an ideal model system with which to test the influence of sex and pregnancy on bladder cancer. Although the MB49 model has been in use since 1979, we were unable to find any studies directly comparing MB49 tumor growth in male and female mice. In this study, we directly compare growth of MB49 cells subcutaneously implanted in male and female mice, test the effect of concurrent pregnancy on MB49 tumor growth, report on the responsiveness of MB49 cells to human chorionic gonadotropin (hCG) and dihydrotestosterone in vitro, and their expression of the hCG receptor, LHCGR.
MATERIALS AND METHODS
Mice
Five week old male and female C57BL/6 mice were obtained from Harlan Laboratories (Indianapolis, IN). They were maintained in HEPA filtered cages on a 12 h light cycle with filtered water and food ad libitum. All procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee (IACUC).
Cells and reagents
MB49 murine bladder carcinoma cells were a gift from Dr. A. Böhle, Medical University of Lübeck, Borstel, Germany [8]. Human bladder cancer cell lines 5637, RT4, T24, TCC-Sup, and UMUC-3 were obtained from the American Type Culture Collection (Manassas, VA). All of these lines were maintained in DMEM containing 4.5 g glucose/ ml and antimicrobial-antimycotic (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Hyclone, Logan, UT). The human immortalized urothelial cell line, UROtsa, derived from normal human urothelial cells and immortalized with the SV40 T-antigen, was a gift from Dr. Donald Sens (University of North Dakota, Grand Forks, ND) [9]. UROtsa cells were maintained in DMEM containing 2.0 g glucose/ml, with 10% fetal calf serum and antimicrobial-antimycotic. All cell cultures were maintained at 37°C in a 5% CO2 atmosphere (Falcon, Bedford, MA). Human chorionic gonadotropin (hCG) (cat. # C0434) was purchased from Sigma (St. Louis, MO), dissolved in sterile saline and stored at −80°C. Dihydrotestosterone was obtained from Sigma-Aldrich, dissolved in 100% ethanol at a concentration of 10 mM, and stored at −20°C.
Tumor implantation and hCG treatment
All animal experiments were approved by the Medical University of South Carolina IACUC, and conducted under the supervision of the Medical University of South Carolina Department of Laboratory Animal Research. For implantation of MB49 cells, actively growing cells were trypsinized, counted and resuspended in sterile DMEM to a concentration of 2 million cells per ml. A volume of 0.1 ml (200,000 cells) was injected subcutaneously in the right flank of unanesthetized mice. For pregnancy experiments, female mice were housed with males either before or after MB49 cell implantation as indicated in results, and tumor growth was monitored by digital caliper measurement. Dates of birth of litters were used to calculate which day of gestation correlated with the day of cell implantation. For the comparison of tumor growth in non-pregnant male and female mice, on day 14 post-implantation, mice were euthanized and tumors were excised, weighed and photographed next to a ruler. Separate photographs were combined into one by matching the image sizes of the ruler to maintain proper scale. Tumor volumes were calculated using the ellipsoid volume formula (π/6 × L × W × H) [10].
MTS assay
Subconfluent, actively growing cells were trypsinized, counted, and plated in a 50 μl volume of complete medium at 5000 cells per well in a 96-well Corning Costar 3595 flat-bottom plate. Stock hCG was diluted in a series of 5-fold dilutions in complete medium and 50 μl added into triplicate wells per concentration for final concentrations of 50, 10, 2, 0.4, and 0.08 IU per ml. Plates were incubated for 48 hr at which time the medium was removed and replaced with warmed 100 μl HyClone DMEM without Phenol Red plus 10% MTS reagent (CellTiter 96® Aqueous One Solution Cell Proliferation Assay, Pierce Biotechnology, Rockford, IL) and read every 10 min in a Fluostar spectrophotometer according to kit instructions. MTS assays were performed 3 times in triplicate for each cell line tested.
Western blot detection of hCG receptor with LHCGR antibody
Two independent lysates were prepared on different days from separate passages of human bladder cancer cell lines 5637, RT4, T24, TCC-Sup, and UMUC-3, the human immortalized urothelial cell line, UROtsa, and murine MB49 cells. For each lysate, subconfluent, actively growing cells were scraped into phosphate buffered saline, pelleted, lysed with RIPA buffer (10 mM Tris-Cl, pH8.0, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS) plus mammalian protease inhibitor cocktail (P-8340 Sigma) and stored at −80°C. Testes from a C57BL/6 mouse were homogenized in RIPA buffer for a positive control. Lysates were centrifuged (20000 g) prior to performing protein assays on the supernatant (DC protein assay, BioRad, Hercules, MA). Protein was separated on 10% Bis/Tris NuPage gels in MES buffer (Invitrogen) and transferred to nitrocellulose (Bio-Rad) for 90 min at 30 V. Membranes were blocked in 5% milk in TBS-Tween prior to incubation with primary antibody (anti-LHR, H-50, sc-25828, Santa Cruz Biotechnology, Dalla TX) at 1:500 dilution. Following three washes with TBS-Tween, membranes were incubated with anti-rabbit HRP-conjugated secondary antibody (1:10 000) for 1 hr at room temperature, washed three times in TBS-Tween followed by chemiluminescent detection of the secondary conjugates with DuraWest Supersignal (Pierce Biotechnology).
Dihydrotestosterone dose curve growth assay
Subconfluent, actively growing MB49 cells were trypsinized, counted, and plated in a 50 μl volume of complete medium at 10,000 cells per well in a 96-well, black, Corning Costar (cat. # 3603) flat-bottom plate. Dihydrotestosterone was diluted in complete medium and 50 μl added into triplicate wells per concentration for final concentrations of 10, 7.5, 5, 2.5, 1.25 and 0.625 μM. Plates were incubated for 48 hr at which time CellTiter-Blue® Cell Viability Assay reagent (Pierce) was added and the plate read every 10 min in a Fluorstar spectrophotometer according to kit instructions. Assays were performed 3 times in triplicate.
Statistical analysis
Student’s two tailed t test was used to analyze all comparisons, with a Bonferroni adjustment for multiple comparisons when appropriate.
RESULTS
MB49 tumor growth is greater in male than female mice
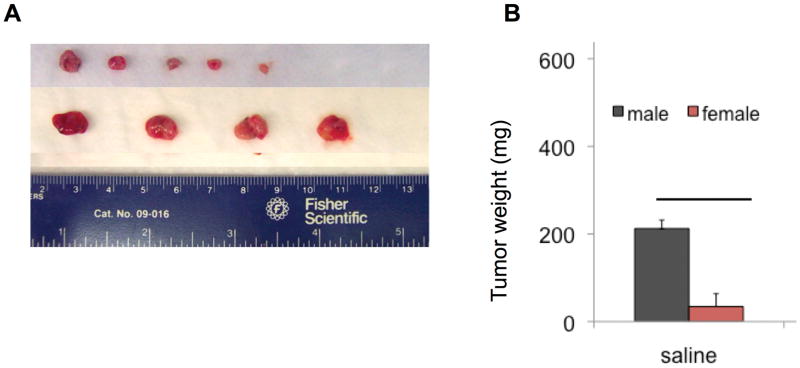
To investigate whether female mice have decreased susceptibility to MB49 tumor growth than male mice, equal numbers of MB49 cells were implanted in the right rear flanks of male and female syngeneic mice. On day 14 post-implantation, animals were sacrificed, and tumors were carefully dissected from normal tissues, photographed (Fig. 1A) and weighed (Fig. 1B) to obtain tumor mass. Comparison of tumor sizes showed a difference between male and female mice at P < 0.05, suggesting a more tumor permissive baseline environment in males. The experiment was repeated with similar results (not shown).
Figure 1. Growth of MB49 tumors is enhanced in male mice.
A. Image of excised tumors with size reference. B. Weight of excised tumors. Tumor take rate was 90% overall at sacrifice at 14 days. **P < 0.05.
Concurrent pregnancy did not inhibit MB49 tumor formation
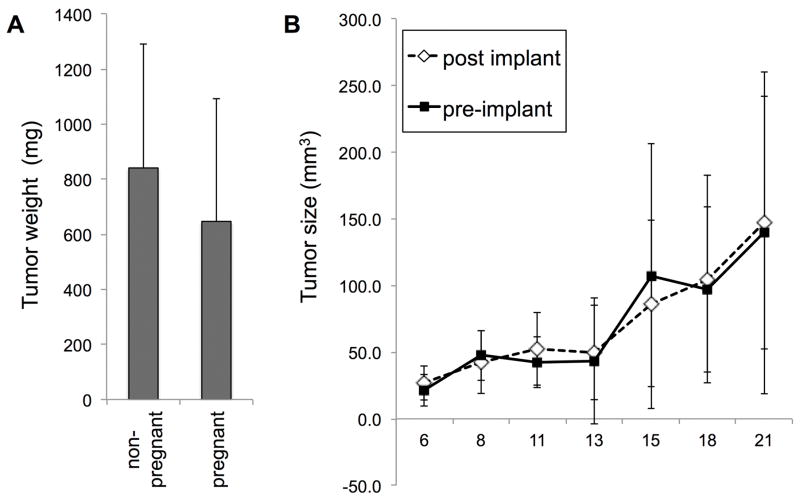
Sixteen female mice were housed with males for 3 weeks prior to subcutaneous MB49 cell implantation. Six produced litters, on dates corresponding to matings between 5 and 18 days prior to cell implantation, and 10 did not. At sacrifice, there was no significant difference in mean tumor weights in the females that gave birth and those that did not (Fig. 2A). Therefore, pregnancy at the time of MB49 cell implantation did not affect take rates, tumor size, or tumor growth variation. This first experiment could not exclude the possibility that the biological changes of very early pregnancy might be protective against tumor implantation since MB49 cells were introduced well after mating. We next compared females who were mated either one week prior (n = 6) or one week after (n = 7) MB49 cell implantation. These mice were tracked over the 3 week time course of the experiment by measuring length and width of tumors with digital calipers. No difference was seen between the two groups in regard to take rates, tumor sizes, or variation (Fig. 2B). Therefore, we concluded that all stages of pregnancy were non-protective against bladder cancer in the MB49 model of bladder cancer.
Figure 2. Pregnancy status does not predict tumor growth in MB49 model of bladder cancer.
A. Female mice were housed with males, underwent implantation with MB49 cells, and were grouped according to whether they gave birth during the course of the experiment. B. Female mice were mated before or after the subcutaneous implantation of MB49 cells, and tumor size monitored by caliper measurement.
hCG treatment does not stimulate MB49 bladder cancer cell proliferation in vitro
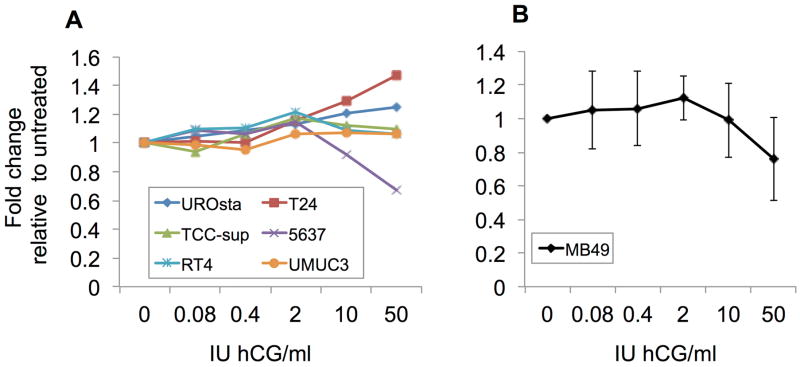
Experiments were next performed to determine whether MB49 cells could respond to hCG. The receptor for hCG is the Leutenizing Hormone Chorionic Gonadotropin Receptor (LHCGR) [11]. A recent paper by Zaravinos et al. reported that human bladder cancer cells undergo strong downregulation of the hCG receptor at the mRNA level [12]. The LHCGR is conserved across humans and mice, suggesting that MB49 mouse-derived bladder cancer cells could also be LHCGR deficient [11]. We tested this in vitro, using a dose curve of 0.08−50 IU hCG per ml on the MB49 cells as well as a panel of human bladder cancer lines, some with previously reported LHCGR expression and/ or hCG responsiveness as controls. An hCG dose of 4.5 IU per 20 g mouse is sufficient to induce ovulation in C57BL6 females [13,14]. The human bladder cancer line T24 has previously been shown to increase its proliferation rate when exposed to hCG [15,16]. Our results confirmed this finding (Fig. 3A). The cell line of immortalized normal bladder urothelial cells, UROtsa, was the only line other than T24 cells to show any positive response to hCG treatment (Fig. 3A). MB49 cells (Fig. 3B) and all other human bladder cancer cell lines tested had either no response or slightly slower proliferation when treated in vitro with hCG, consistent with previous reports of loss of the LHCGR in many bladder cancers.
Figure 3. Human chorionic gonadotropin (hCG) treatment does not stimulate MB49 cell proliferation in vitro.
A. A panel of human bladder lines, and B. murine MB49 cells were assessed for growth by MTS assay at various doses of hCG in vitro. Results represent three independent experiments for each cell line, with triplicate wells each time. Shown are fold differences in growth rates compared to the baseline for each cell line, in order to normalize rates. *P < .01 difference between treatment and control within the indicated cell line. Asterisks are color-coded to indicate which cell line to which they refer.
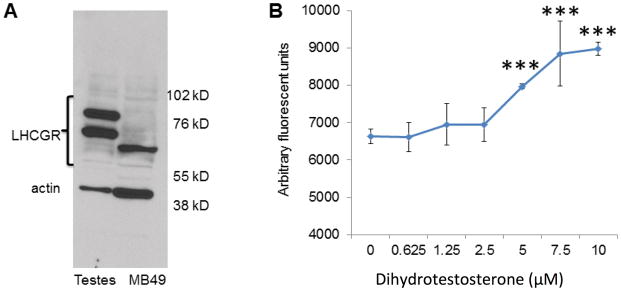
Lack of in vitro response to hCG by MB49 was further explained by Western blot analysis of LHCGR expression, which was found to be entirely absent from the MB49 cells (Fig. 4A). Interestingly, MB49 cells did manifest a low-molecular weight band which could represent an immature, alternatively spliced, or soluble version of the receptor, none of which would be expected to have signaling functionality [17,18]. This same band also appeared in the non-hCG-responsive human lines 5637, RT4, and UMUC, and was absent from the responsive T24 and UROtsa cells, further suggesting that the putative truncated protein was incompatible with hCG response (data not shown).
Figure 4. MB49 cells lack LHCGR, the functional receptor for hCG, but are stimulated in vitro by dihydrotestosterone.
A. Mouse testes lysate was used as a positive control for LHCGR expression, showing mature high molecular weight bands for the protein while MB49 cells lack these bands but express a single truncated short form at approximately 65 kD. B. MB49 cells were grown with increasing doses of dihydrotestosterone (0−10 μM), with a dose-dependent curve in growth, indicating cellular machinery responsive to this hormone. ***P < 0.0001
MB49 cells grow in response to dihydrotestosterone in vitro
MB49 cells were grown in the presence of dihydrotestosterone and were found to have enhanced growth in a dose-dependent manner indicating cellular machinery responsive to this hormone (Fig. 4B).
DISCUSSION
The striking difference between bladder cancer frequencies in males and females may offer an opportunity to study sex-specific factors that are either cancer protective or drivers of malignancy if suitable animal models are available. Here we have investigated the suitability of the well known, heterotopic, syngeneic MB49 model to play this role. We found that the MB49 model does reproduce a key aspect of the sex differences in bladder tumor growth seen in humans, in that implantation of identical numbers of cells results in significantly larger tumors in males than in females at 14 days. The mechanism of this difference has not been revealed by our work, and the approach to doing so may depend on whether this difference is viewed as enhancement of MB49 tumor growth in males or inhibition of MB49 tumor growth in females. It is our view that the additional results reported here and reports by many others make a strong case that females do not possess protective factors against bladder cancer, but instead represent a baseline frequency of bladder cancer disease while males produce factors that enhance bladder tumor progression. In this work, we showed that MB49 cells proliferate significantly, in a dose-dependent manner, in response to dihydrotestosterone in vitro. Testosterone has also been implicated in a spontaneous bladder tumor model in which castration reduced bladder tumor incidence [19], and Bertram, et al. observed that testosterone supplementation shortens the time needed to develop chemically induced bladder tumors in mice [20]. Similarly, Miyamoto, et al. showed an increase in incidence of N-butyl-N-(4-hydroxybutyl) nitrosamine-induced bladder tumors in mice with testosterone injections, and inhibition of bladder tumor induction in androgen receptor knock-out mice [21]. Finally, a recent retropective human study found that men with bladder cancer who were treated with androgen ablation therapy had reduced bladder cancer recurrences [22].
In contrast, the evidence that females produce factors protective against bladder cancer is weak. Studies designed to identify factors in females which are protective against bladder cancer have investigated parity and hormone replacement therapy in the human population. With regard to female hormone replacement therapy, most studies in the human population failed to find any link between rates of bladder cancer and its use, making estrogen an unlikely candidate for a protective mechanism in human females [23,24]. Since long-term estrogen replacement therapy in menopausal women is not associated with increased or decreased bladder cancer incidence in humans, we did not investigate estrogen in the MB49 model. Conflicting evidence has been presented in the literature regarding bladder cancer and parity in humans and mice [23–26]. One report from a group using a spontaneous mouse model of bladder cancer indicates that lower rates of cancer were seen in breeding females when compared to non-breeding females [19]. However, this observation was made retrospectively. In the work reported here, a prospective study design found no evidence of an effect of pregnancy on MB49 tumor growth, nor did hCG induce proliferation of MB49 cells in vitro. No difference was seen between groups of C57BL/6 female mice who were not mated and those that were, or between groups of female mice who were mated either before or after the implantation of MB49 bladder cancer cells. Consistent with this result, and as in 4 out of 5 human bladder cancer-derived cell lines tested in parallel, MB49 cells were found to be unresponsive in vitro to the pregnancy hormone, hCG, and express either no LHCGR or a defective version. While the tumor cells were implanted subcutaneously, not in the bladder itself, these results are strong evidence that pregnancy itself does not affect bladder cancer outcome, at least in this mouse model. In addition, and contrary to the hypothesis that pregnancy-associated hormones are protective against bladder cancer, is the robust finding that the beta subunit of hCG is expressed by a variety of cancers and its expression correlates with worse outcome [27–31]. This may be a result of its role in promoting angiogenesis, likely through increased expression of the vascular endothelial growth factor (VEGF), and to increased signaling by growth-promoting Wnt proteins [32–34]. Such alterations to the body’s environment would be generally supportive of cancer growth.
CONCLUSIONS
Our results indicate that the MB49 model may be a useful one for investigating sex-specific factors affecting human bladder cancer. If testosterone does promote human bladder tumor growth, as it does MB49 cell growth, this could have immediate clinical relevance. It may be prudent to screen for nascent bladder tumors prior to starting a course of testosterone boosting treatments, and testosterone lowering therapy, currently a foundation of prostate cancer treatment, may improve bladder cancer treatment.
Acknowledgments
This study was supported in part by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH Grant Number UL1 TR000062. The authors wish to thank Ben Gilbertson and Dr. Armand Glassman for helpful discussions during manuscript preparation. The authors declare that they have no conflicts of interest affecting the publication of this paper.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2012), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.
- 2.Hartge P, Harvey EB, Linehan WM, Silverman DT, Sullivan JW, et al. Unexplained excess risk of bladder cancer in men. J Natl Cancer Inst. 1990;82:1636–1640. doi: 10.1093/jnci/82.20.1636. [DOI] [PubMed] [Google Scholar]
- 3.Castelao JE, Yuan JM, Skipper PL, Tannenbaum SR, Gago-Dominguez M, et al. Gender- and smoking-related bladder cancer risk. J Natl Cancer Inst. 2001;93:538–545. doi: 10.1093/jnci/93.7.538. [DOI] [PubMed] [Google Scholar]
- 4.McGrath M, Michaud DS, De Vivo I. Hormonal and reproductive factors and the risk of bladder cancer in women. Am J Epidemiol. 2005;163:236–244. doi: 10.1093/aje/kwj028. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty SE, Lacey JV, Jr, Pfeiffer RM, Park Y, Hoover RN, et al. Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2013;133:462–472. doi: 10.1002/ijc.28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Summerhayes IC, Franks LM. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J Natl Cancer Inst. 1979;62:1017–1023. [PubMed] [Google Scholar]
- 7.Fabris VT, Lodillinsky C, Pampena MB, Belgorosky D, Lanari C, et al. Cytogenetic characterization of the murine bladder cancer model MB49 and the derived invasive line MB49-I. Cancer Genet. 2012;205:168–176. doi: 10.1016/j.cancergen.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Böhle A, Jurczok A, Ardelt P, Wulf T, Ulmer AJ, et al. Inhibition of bladder carcinoma cell adhesion by oligopeptide combinations in vitro and in vivo. J Urol. 2002;167:357–363. [PubMed] [Google Scholar]
- 9.Rossi MR, Masters JR, Park S, Todd JH, Garrett SH, et al. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ Health Perspect. 2001;109:801–808. doi: 10.1289/ehp.01109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice,” Cancer chemotherapy and pharmacology. Cancer Chemother Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 11.Maston GA, Ruvolo M. Chorionic gonadotropin has a recent origin within primates and an evolutionary history of selection. Mol Biol Evol. 2002;19:320–335. doi: 10.1093/oxfordjournals.molbev.a004085. [DOI] [PubMed] [Google Scholar]
- 12.Zaravinos A, Lambrou GI, Boulalas I, Delakas D, Spandidos DA. Identification of common differentially expressed genes in urinary bladder cancer. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vergara GJ, Irwin MH, Moffatt RJ, Pinkert CA. In vitro fertilization in mice: Strain differences in response to superovulation protocols and effect of cumulus cell removal. Theriogenology. 1997;47:1245–1252. doi: 10.1016/s0093-691x(97)00104-0. [DOI] [PubMed] [Google Scholar]
- 14.Wang XN, Greenwald GS. Human chorionic gonadotropin or human recombinant follicle-stimulating hormone (fsh)-induced ovulation and subsequent fertilization and early embryo development in hypophysectomized fsh-primed mice. Endocrinology. 1993;132:2009–2016. doi: 10.1210/endo.132.5.8477652. [DOI] [PubMed] [Google Scholar]
- 15.Gillott DJ, Iles RK, Chard T. The effects of beta-human chorionic gonadotrophin on the in vitro growth of bladder cancer cell lines. Br J Cancer. 1996;73:323–326. doi: 10.1038/bjc.1996.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole LA, Butler S. Hyperglycosylated hCG, hCGβ and Hyperglycosylated hCGβ: interchangeable cancer promoters. Mol Cell Endocrinol. 2011;349:232–238. doi: 10.1016/j.mce.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Apaja PM, Tuusa JT, Pietilä EM, Rajaniemi HJ, Petäjä-Repo UE. Luteinizing hormone receptor ectodomain splice variant misroutes the full-length receptor into a subcompartment of the endoplasmic reticulum. Mol Biol Cell. 2006;17:2243–2255. doi: 10.1091/mbc.E05-09-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers AE, Stanley PF, Randeva H, Banerjee S. Microvesicle-mediated release of soluble LH/hCG receptor (LHCGR) from transfected cells and placenta explants. Reprod Biol Endocrinol. 2011;9:64. doi: 10.1186/1477-7827-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson AM, O’Connell MJ, Messing EM, Reeder JE. Decreased bladder cancer growth in parous mice. Urology. 2008;72:470–473. doi: 10.1016/j.urology.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Bertram JS, Craig AW. Specific induction of bladder cancer in mice by butyl-(4-hydroxybutyl)-nitrosamine and the effects of hormonal modifications on the sex difference in response. Eur J Cancer. 1972;8:587–594. doi: 10.1016/0014-2964(72)90137-5. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto H, Yang Z, Chen Y, Ishiguro H, Uemura H, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 22.Izumi K, Taguri M, Miyamoto H, Hara Y, Kishida T, et al. Androgen deprivation therapy prevents bladder cancer recurrence. Oncotarget. 2014;5:12665–12674. doi: 10.18632/oncotarget.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich K, Demidenko E, Schned A, Zens MS, Heaney J, et al. Parity, early menopause and the incidence of bladder cancer in women: a case-control study and meta-analysis. Eur J Cancer. 2010;47:592–599. doi: 10.1016/j.ejca.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantwell MM, Lacey JV, Jr, Schairer C, Schatzkin A, Michaud DS. Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int J Cancer. 2006;119:2398–2401. doi: 10.1002/ijc.22175. [DOI] [PubMed] [Google Scholar]
- 25.Weibull CE, Eloranta S, Altman D, Johansson ALV, Lambe M. Childbearing and the risk of bladder cancer: a nationwide population-based cohort study. Eur Urol. 2013;63:733–738. doi: 10.1016/j.eururo.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Davis-Dao CA, Henderson KD, Sullivan-Halley J. Lower risk in parous women suggests that hormonal factors are important in bladder cancer etiology. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:1156–1170. doi: 10.1158/1055-9965.EPI-11-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, Wen X, Ghali L, Al-Shalabi FM, Docherty SM, et al. hCG beta expression by cervical squamous carcinoma--in vivo histological association with tumour invasion and apoptosis. Histopathology. 2008;53:147–155. doi: 10.1111/j.1365-2559.2008.03082.x. [DOI] [PubMed] [Google Scholar]
- 28.Shah T, Srirajaskanthan R, Bhogal M, Toubanakis C, Meyer T, et al. Alpha-fetoprotein and human chorionic gonadotrophin-beta as prognostic markers in neuroendocrine tumour patients. Br J Cancer. 2008;99:72–77. doi: 10.1038/sj.bjc.6604428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheaff MT, Martin JE, Badenoch DF, Baithun SI. beta hCG as a prognostic marker in adenocarcinoma of the prostate. J Clin Pathol. 1996;49:329–332. doi: 10.1136/jcp.49.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotakainen K, Lintula S, Jarvinen R, Paju A, Stenman J, et al. Overexpression of human chorionic gonadotropin beta genes 3, 5 and 8 in tumor tissue and urinary cells of bladder cancer patients. Tumour Biol. 2006;28:52–56. doi: 10.1159/000097703. [DOI] [PubMed] [Google Scholar]
- 31.Gori S, Porrozzi S, Roila F, Gatta G, De Giorgi U, et al. Germ cell tumours of the testis. Crit Rev Oncol Hematol. 2005;53:141–164. doi: 10.1016/j.critrevonc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Kuorelahti A, Rulli S, Huhtaniemi I, Poutanen M. Human chorionic gonadotropin (hCG) up-regulates wnt5b and wnt7b in the mammary gland, and hCGbeta transgenic female mice present with mammary Gland tumors exhibiting characteristics of the Wnt/beta-catenin pathway activation. Endocrinology. 2007;148:3694–3703. doi: 10.1210/en.2007-0249. [DOI] [PubMed] [Google Scholar]
- 33.Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Münstedt K, et al. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–5296. doi: 10.1210/jc.2002-020642. [DOI] [PubMed] [Google Scholar]
- 34.Michel RM, Aguilar JL, Arrieta O. Human chorionic gonadotropin as an angiogenic factor in breast cancer during pregnancy. Med Hypotheses. 2006;68:1035–1040. doi: 10.1016/j.mehy.2006.05.072. [DOI] [PubMed] [Google Scholar]