Abstract
Lack of access to human tissues with untreated tuberculosis (TB) has forced generations of researchers to use animal models and to adopt a paradigm that granulomas are the characteristic lesion of both primary and post primary TB. An extended search of studies of human lung tissues failed to find any reports that support this paradigm. We found scores of publications from gross pathology in 1804 through high resolution CT scans in 2015 that identify obstructive lobular pneumonia, not granulomas, as the characteristic lesion of developing post-primary TB. This paper reviews this literature together with other relevant observations to formulate a new paradigm of TB with three distinct stages: a three-act play. First, primary TB, a war of attrition, begins with infection that spreads via lymphatics and blood stream before inducing systemic immunity that contains and controls the organisms within granulomas. Second, post-primary TB, a sneak attack, develops during latent TB as an asymptomatic obstructive lobular pneumonia in persons with effective systemic immunity. It is a paucibacillary process with no granulomas that spreads via bronchi and accumulates mycobacterial antigens and host lipids for 1–2 years before suddenly undergoing caseous necrosis. Third, the fallout, is responsible for nearly all clinical post primary disease. It begins with caseous necrotic pneumonia that is either retained to become the focus of fibrocaseous disease or is coughed out to leave a cavity. This three-stage paradigm suggests testable hypotheses and plausible answers to long standing questions of immunity to TB.
Keywords: Post primary, Lung, Pathology, Pathogenesis, Cavity, Human
Introduction
More research papers were written about TB than any other disease in the 19th and early 20th centuries when the disease was common and autopsies were cutting edge science. This came to an abrupt end in the 1950s with the introduction of antibiotics and decline in autopsies. Research interest shifted away from the morphologic pathology to new areas of immunology, molecular microbiology, and genetics that could be addressed with isolated cells and animal models. While new technologies facilitated previously unimaginable progress in understanding the early stages of tuberculosis (TB), very little progress has been made in understanding the late stages that produce most disease and transmission of infection 1. This is increasingly recognized as a major obstacle to development of new vaccines and host directed therapies 2, 3.
Post-primary TB is exceedingly difficult to study because there are no recognized animal models and an extreme paucity of informative human specimens. Many attempts have been made to extrapolate findings of animal models to the human disease. However, most animal models mimic stages of primary, not post-primary TB. Consequently, it has not been possible to validate these models and errors have been perpetuated 4, 5. As a result, there has been little effective study of post primary TB and key questions remain unresolved: What protects most adults from disease following infection? Why doesn’t immunity to infection enable susceptible humans to resolve lung infection and thereby stop the development of disease? Why are immunocompetent young adults especially susceptible to disease and death? Why does recovery from disease fail to produce immunity, but actually produces increased susceptibility to recurrent disease 6? Why have vaccines that prevent disseminated TB in children, failed to protect adults from pulmonary TB? Why does post-primary TB localize in the upper lobes of the lungs?
The basic paradigm guiding research on the pathogenesis of TB is that the caseating granuloma is the characteristic lesion of all TB and that immunity is mediated by macrophages and T cells that enhance granuloma function 2. Surprisingly, this paradigm dates only from studies with animals in the late 20th century 7. We have not been able to find any articles written by persons who personally studied the pathology of human post-primary TB that support this paradigm. In contrast, studies of human tissues published from the 1804 through the 1960s report that pulmonary TB has not just one, but two characteristic lesions: the caseating granuloma (nodular tubercle or productive reaction) and tuberculous pneumonia (infiltration or exudative reaction) 8–23. The early stages of the pneumonia are typically asymptomatic and thus are classified as part of latent TB 24. However, the pneumonia undergoes necrosis to initiate clinical TB. The necrotic tissue either fragments and is expelled to produce cavities that are responsible for nearly all transmission of infection or it remains to become the focus of fibrocaseous disease and consumption. The existence of a second major pathology of pulmonary TB is not a hypothesis or speculation, but is an established historical fact supported by hundreds of papers by dozens of investigators over a period exceeding two centuries 8–23.
In the 1950’s, the introduction of antibiotics led many to believe that TB would soon be eliminated. Research on TB was continued by only a few investigators using animals until the rise of drug resistance and TB-HIV coinfection reignited interest in the 1980s. In particular, rabbits infected with M. bovis were the only commonly used animal model that produced fibrocaseous disease and cavities 7. Cavities in rabbits infected with M. bovis develop by erosion of caseating granulomas into bronchi and other structures. In the absence of any dissenting views or human tissue for study, this became the universally accepted paradigm of the pathogenesis of TB. Unfortunately, neither the rabbit nor M. bovis develop bronchogenic post-primary TB as it exists in humans 25. With lack of human tissues for study, this fallacious paradigm took root and has dominated TB research for decades. The few studies that mention tuberculous pneumonia usually dismiss it as the result of overwhelming infection rather than as the essential progenitor lesion of post primary TB 7, 26.
Descriptions of the pathology of TB from the pre antibiotic era
Our search for a new paradigm of TB began with review of the work of great scientists of the pre antibiotic era. It was exceedingly difficult until we obtained microscope slides of untreated pulmonary TB to study because the concepts and nomenclature were unfamiliar and there were very few pictures. In time most concepts became clear. In 1804, Rene Laennec reported that TB has two distinct pathologies, tubercular infiltration and tubercular granulation 9. Decades later in 1854, when microscopes came into general use, Bennett reported that terminal bronchioles are among the first structures affected and they obstruct three to twenty air-vesicles 10. Virchow vehemently challenged Laennec’s assertions that the two lesions were parts of the same disease 11. The infiltration and granulation were completely different when viewed with a microscope. The infiltration began with fatty degeneration of cells in alveoli to produce caseous pneumonia that softened to produce cavities. The tubercle was a nodule of cells within connective tissue. Virchow declared that the tubercle was a kind of tumor, while the infiltration was an inflammatory process 11. This battle raged with intensity until 1881 when Koch’s discovery of the tubercle bacillus proved that the two types of lesion were manifestations of one disease. Virchow was not pleased.
Douglas Powell wrote a particularly informative book in 1876 12. He reported that the lungs of people who succumbed to TB show “a great variety of pathologies, which may, nevertheless, be recognized as the results of two morbid processes that may occur separately or admixed.” These were a ‘ new growth’ or tubercle (granuloma) in the interstitial connective tissue of the lung and “inflammation” (pneumonia). The pneumonia was the most constant element of pulmonary TB, and the main factor in the more chronic and cachectic forms of lung disease. Importantly, “It begins in the alveoli and minute bronchi that become blocked with the large granular cells that stuff the alveoli. The lobules begin to undergo fatty degeneration and caseation.”
In the 1880s, Cornil & Ranvier, and Hamilton published drawings of the microscopic appearance of various lesions of pulmonary TB, Figure 1 14, 15. These are shown together with modern photomicrographs of the same processes. Each of the writers depicted pulmonary cavities arising from dissolution of the center of caseous pneumonic masses, not from granulomas, Figure 2.
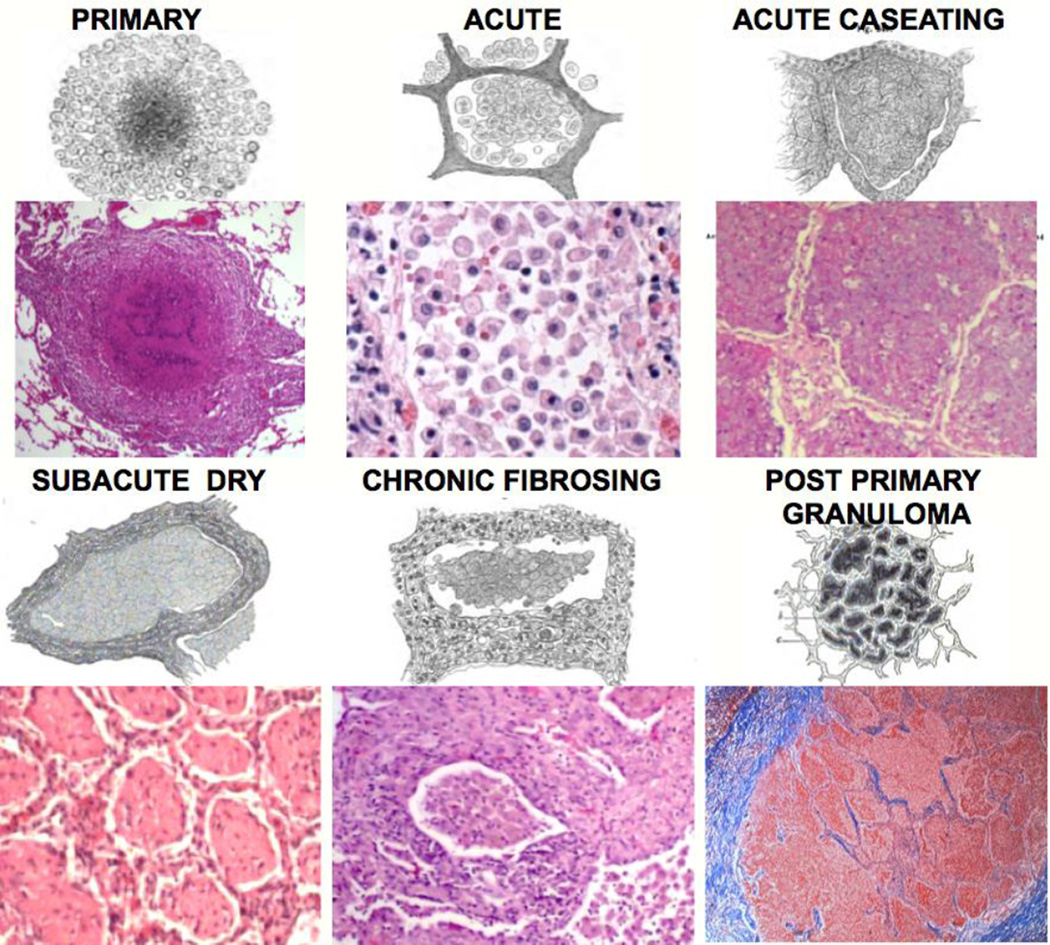
Figure 1. Characteristic pathologies of human post-primary TB from the 19th and 21st centuries.
Each pair of figures shows a drawing from the 19th century paired with a 21st century photomicrograph. The caseating granuloma is the characteristic lesion of primary TB. Post-primary TB begins a paucibacillary lobular pneumonia (Acute) that may undergo caseous necrosis (Acute Caseating), regress (Subacute Dry) or fibrose (Chronic Fibrosing). Finally, caseating granulomas in post-primary TB form only in response to caseous necrosis and are never the cause of it 22 (Post-primary Granuloma). The first 5 drawings are by Cornil and Ranvier (1881) 14, The last one is by Hamilton (1883) 15. The sections are H&E or trichrome stained and photographed at 40 to 400×.
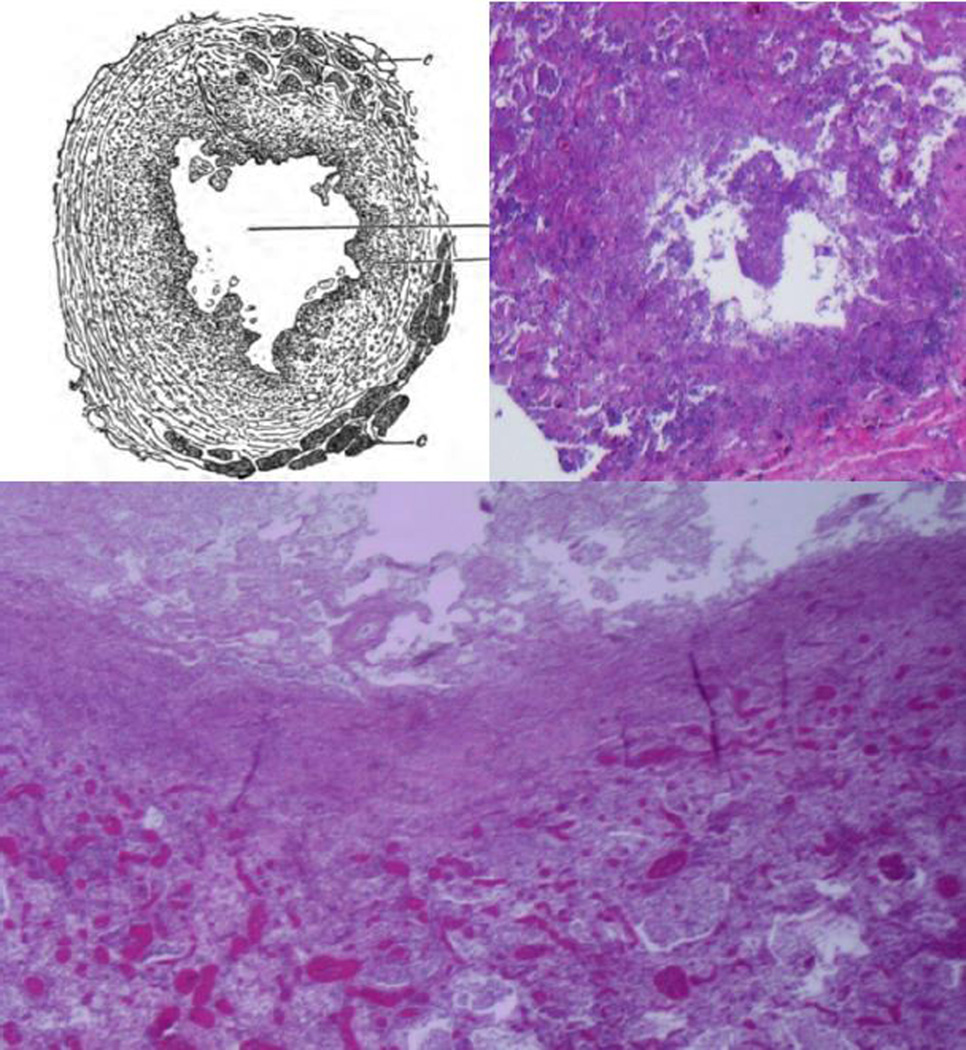
Figure 2. Histopathology of formation of cavities in the 19th and 21st centuries.
The caption of this drawing of a developing tuberculous cavity by Hamilton in 1883 stated: “The cavity formed by dissolution of the center of a caseous pneumonic mass” 15. The sections from persons who died recently of acute tuberculous pneumonia show the same pathology. Tuberculous pneumonia has undergone caseation necrosis, fragmentation and is being coughed out to form a cavity. The larger section shows necrotic lung above tuberculous pneumonia (H&E 40×).
Decades later, Osler in his ‘Principles and Practice of Medicine’ published lucid descriptions of TB and its pathology 17. “Caseous bronchopneumonia starts in the smaller tubes, which become blocked with a cheesy substance, while the air-cells in the lobule are filled with the products of a catarrhal pneumonia. By the fusion of contiguous masses an entire lobe may be render nearly solid. “ The disease could present in a wide spectrum of ways, some gradual and some sudden. Acute pulmonary TB “sets in abruptly with a chill, usually in in an individual who has enjoyed good health. … At this time, as a rule, no suspicion enters the mind of the practitioner that the case in anything but one of frank lobar pneumonia. Macroscopically the affection does not look tuberculous. The appearance of bronchopneumonia may be exceedingly deceptive.”
Several investigators at the end of the preantibiotic era in the 1950s confirmed and extended these findings. Rich reported that “It has been found by all who have studied early human pulmonary lesions that they represent areas of caseous pneumonia rather than nodular tubercles.” 20. Canetti, Pagel and Medlar all agreed that the pneumonic infection is a lipid pneumonia that spreads in the lung via bronchi, not via lymphatics or the blood stream and that it is frequently paucibacillary meaning that it contains few or no detectable acid fast bacilli 21–23. Pagel used fat stains on sections of multiple stages of pulmonary tuberculosis to demonstrate that stainable lipid increases progressively within foamy alveolar macrophages with development of the pneumonic lesions 27. Finally, clinical disease frequently had an acute onset resembling community acquired pneumonia and cavities formed by dissolution of caseous pneumonia.
Medlar examined thousands of human caseating granulomas looking for evidence that they had expanded in a way that might have produced cavities 28. He found none. Caseation necrosis develops in the center of granulomas of primary TB, but not in post-primary TB 5, 22. Granulomas in post-primary TB always follow production of caseation necrosis and never precede it 21, 22. They occur only as a reaction to pre existing caseous pneumonia 21–23. Granulomas in post-primary TB surround small areas of necrotic caseous pneumonia or produce fibrocaseous disease in reaction to large areas.
Recent and confirmatory studies
While a lack of specimens and changing scientific priorities resulted in loss of descriptions of the pathology of post-primary TB in the English language literature, it has been confirmed and extended in the Russian literature, the radiologic literature and our own observations of over 100 cases of untreated TB 4, 5, 25. In 2008, Erokhin published studies on a series of 250 patients with acute caseous pneumonia with pathologic changes similar to those described above 29. All of the cases were initially admitted to hospitals with the diagnosis of severe community-acquired pneumonia. The disease began abruptly and was paucibacillary in that acid fast bacilli (AFB) were found in the first 2 weeks in only 4% of the patients. AFB were identified in the first month in only half of the patients. Two findings, low CD4+ lymphocyte counts and profound coagulopathy, distinguished the patients with TB from those with ordinary bacterial pneumonia.
The development of x-rays enabled investigators to study the early stages of pulmonary lesions serially. Opie studied adolescent children of families with open tuberculosis 18. He found lesions unaccompanied by symptoms or physical signs that were progressive and manifest disease only after a period of latency. The lesions were soft shadows in the peripheral lung fields. It was often possible to predict clinical disease long before it made its appearance. “We have had opportunities of following the transitions from latent apical disease to advanced tuberculosis with loss of weight, cavity formulation, and appearance of tubercle bacilli in the sputum. It is noteworthy that adolescent children with latent apical lesions may be well nourished and in good health. Loss of weight does not usually make its appearance until the disease is moderately advanced. Opie concluded that “Examination by x-rays gives trustworthy evidence concerning the frequency of latent tuberculosis among children exposed to open tuberculosis and furnishes a means by which the severity of infection may be measured.” 18
High resolution CAT scans, developed in the 1990s, are able to visualize the time course of the same lesions with much greater detail. They identified a particular pattern known as the ‘tree-in-bud’ sign as characteristic of developing pulmonary TB, Figure 3 30. This sign results from alveoli filled with foamy macrophages and obstructed bronchioles as described by Powell in 1876 12. The infection quietly spreads via bronchi to adjacent lung frequently to occupy an entire lobe. The tree-in-bud sign was first thought to be diagnostic of TB, but has subsequently been found in other diseases. However, this sign was reported in 100% of cases of active pulmonary TB including drug sensitive, MDR and XDR infections, Figure 4. Tree-in bud is the first sign detectable in asymptomatic people, is characteristic of expanding lesions and disappears with effective therapy 31. With the literature on tree-in-bud, investigators since the 1850’s have identified obstructive lobular pneumonia as the characteristic lesion of developing post-primary TB.
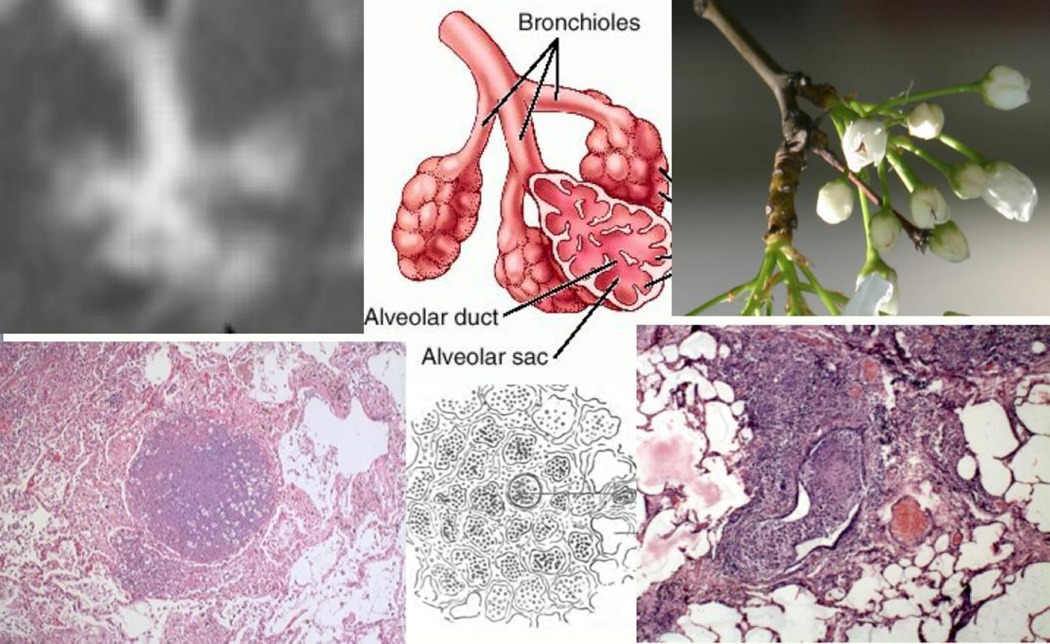
Figure 3. Bronchial obstruction in developing post-primary TB.
The tree-in-bud sign is a high definition CAT scan of the characteristic lesion of developing post-primary TB (upper left). It is an image of a pulmonary lobule with obstructed bronchioles and alveoli filled with cells and fluids (upper center). It was named for resemblance to a tree twig with buds (upper right). The identification of this lesion as the earliest stage of post-primary TB and its histology have been reported consistently since the 1880’s. Hamilton’s drawing and the flanking photomicrographs show the characteristic lobular obstruction and the associated lobular infiltration with mononuclear cells. Drawing reproduced from Hamilton 15 (H&E Stains 200×).
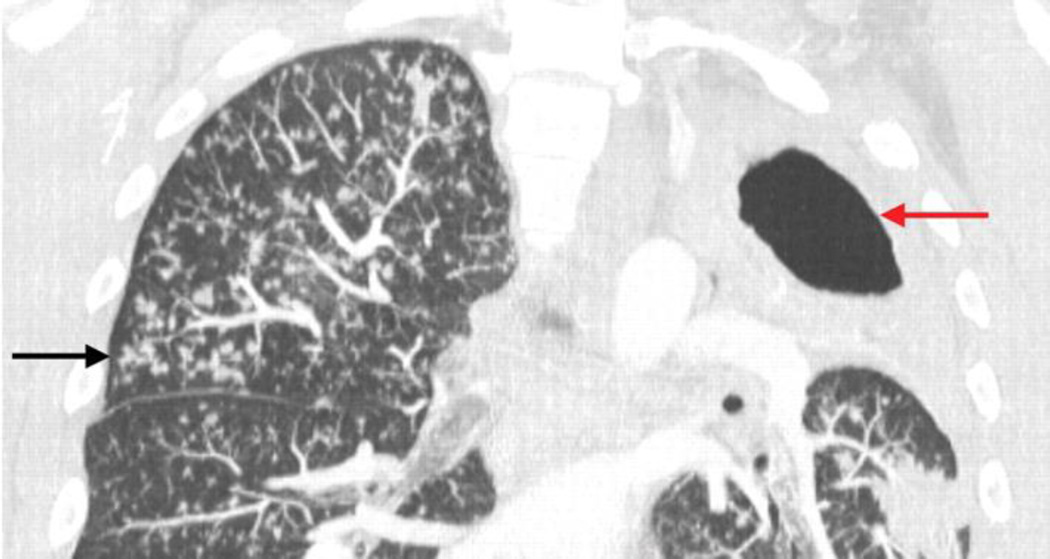
Figure 4. Tree-in-bud sign marking advancing cavitary TB.
A 4-mm coronal CT maximum intensity projection image. The black arrows point to nodules in the right upper and left lower lobes of the lungs, with a “tree-in-bud” appearance due to small airway spread of tuberculous infection. The large left upper lobe cavity (red arrow) is a hallmark of postprimary TB. The accompanying loculated left-sided tuberculous empyema (orange arrow) was subsequently drained percutaneously. Reproduced from Marshall et al 83.
Role of bronchial obstruction
As described above, bronchial obstruction has been found in 100% of cases of post-primary TB pneumonia 16, 32. Furthermore, relief of obstruction has been associated with spontaneous regression of disease 14, 20, 33. The obstruction may be due to external compression by a lymph node or by endobronchial tuberculosis 20. Chronic bronchial obstruction by any means causes post obstructive pneumonia. This is an endogenous lipid pneumonia similar to that produced by TB 34. Alveolar macrophages trapped behind an obstruction become foamy with lipids derived from continued synthesis of pulmonary surfactant 35, 36. Post obstructive pneumonia today, also known as golden pneumonia, is most commonly caused by cancers that obstruct bronchi. 13% of such lesions have been reported to undergo necrosis to produce cavities 37. We have seen two such cases both of which had cavities. The cavity of one occupied nearly the entire upper lobe of a lung 34. This cavity formed in one week following the initiation of cancer chemotherapy. While this lesion lacked the lymphocytes and inflammatory cells characteristic of TB, the cavity was limited to a single lobe and had formed by coughing out fragments of necrotic lung as is characteristic of post-primary TB. The remaining necrotic material in the lung was lipid rich resembling caseation necrosis. This demonstrates that post obstructive lipid pneumonia can be a predisposing factor for pulmonary necrosis and cavitation.
Antigen storage in alveolar cells
Immunohistochemistry has been used in multiple independent studies to demonstrate mycobacterial antigen accumulation in alveolar macrophages of developing tuberculous pneumonia in immunocompetent people, Figure 5 38–40. Such cells seldom have detectable AFB. Mustafa recently reported that these cells preferentially accumulate secreted, but not somatic, antigens of MTB 41. Typically, there is little or no inflammation around them until initiation of caseation necrosis when massive inflammation develops. In addition, the pathogenicity of MTB has recently been linked to its ability to release selected mycobacterial proteins 42. Collectively, these data indicate that developing post primary TB is characterized by prolonged asymptomatic accumulation of host lipids and secreted mycobacterial antigens in alveolar cells behind an obstructed bronchus. While the process can be much longer or shorter, there is typically a delay of 1–2 years between infection of people with MTB and onset of clinical tuberculosis 24. This suggest that the delay between infection and onset of clinical pulmonary TB is due to the time required for accumulation of sufficient host lipids and mycobacterial materials for a necrotizing reaction sufficient to produce a cavity from which MTB can escape to infect new hosts.
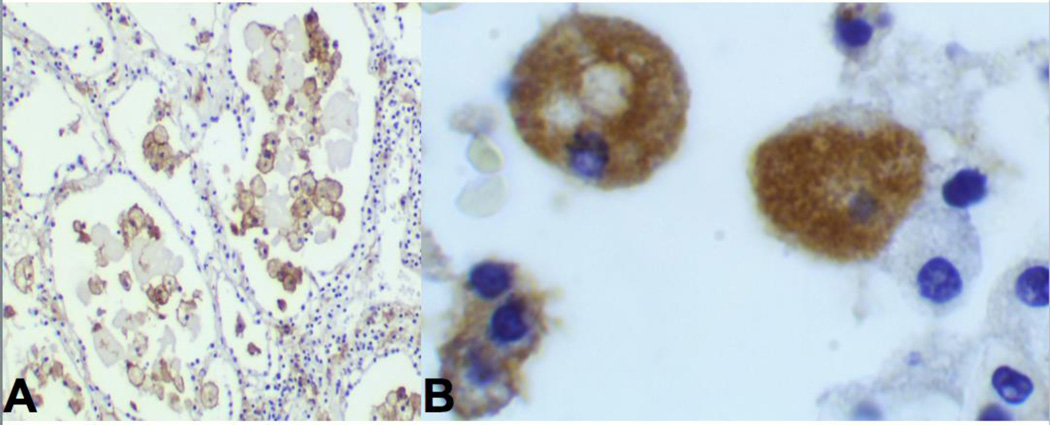
Figure 5. MTB antigen in alveolar cells of immunocompetent people.
A.
Immunohistochemical stain using polyclonal antibody against MTB in alveolar macrophages in lobular pneumonia of an immunocompetent person with developing post-primary TB (IHC Stain 200×).
B. Higher power of same section showing that the antigen is entirely intracellular. (IHC 1000×). AFB stain of these sections showed no organisms demonstrating a paucibacillary infection. The antigen staining is entirely intracellular until onset of necrosis. Reproduced from Hunter 5.
Apical Localization of Lesions
It is widely believed that MTB localize in the apices of lungs because of high oxygen tension. However, the situation is more complex. The disease may localize in the upper portions of the lower as well as the upper lobes. Smith et al reported several plausible theories including lack of blood supply, ventilation and movement of the apices 43. Goodwin in 1983, demonstrated that the apices have the lowest blood flow, ventilation and lymph flow of any part of the lung 44. Modern 4D cat scans demonstrate that they also have practically no movement 45. These observations, together with recognition of the physiologic effects of bronchial obstruction, suggest that TB develops in the apices of lungs because they provide an area that can be more easily isolated. This may be required for accumulation of sufficient mycobacterial products and host lipids to trigger necrosis sufficient to produce a cavity.
Conservation of mycobacterial T epitopes
Clinical disease begins when the developing lesions induce inflammation. It has long been known that hypersensitivity reactions to mycobacterial proteins are far more toxic to humans than any of the animals used for study 46–48. It has recently been observed that T epitopes of MTB are ‘hyper conserved’ 49, 50. This implies that the precise structures of these epitopes are required for survival and transmission of organism to new hosts. It has been proposed that they are conserved because they induce highly toxic hypersensitivity reactions 49,51. However, it seems unlikely that loss of toxicity of a single epitope of hundreds could doom the organism. We favor an alternative hypothesis that the precise epitopes are necessary for asymptomatically accumulating MTB antigens in alveolar macrophages in developing post-primary TB. Exposure of a single epitope at this stage could engage the systemic immune response and disrupt the process.
Perifocal inflammation
Perifocal inflammation is a type of inflammation that surrounds active foci of TB especially in the lung 20, 22. It can occur with either primary or post primary TB and is a major contributor to mortality and morbidity in miliary TB 52. Perifocal inflammation has generally been considered nonspecific inflammation with lipid rich edema, foamy macrophages and occasional red cells and other inflammatory cells. It contains few or frequently no AFB. However, it depends on live MTB since it may disappear from x-rays within 72 hours of the initiation of therapy 53. The significance of perifocal inflammation is that it frequently undergoes necrosis to contribute to caseous pneumonia and subsequent disease.
Perifocal lesions, indistinguishable from those that occur naturally, can be induced by injections of tuberculin. Koch discovered that injections of tuberculin caused stable lesions to progress to serious or lethal disease 46, 47. The inflammation was restricted to the acutely diseased parts and did not attack chronic lesions or healthy tissue 47 Tuberculin could induce intense focal reactions around existing tuberculous lesions even when there was only a mild reaction at the site of injection 46.
Perifocal inflammation in TB is morphologically similar to pulmonary alveolar proteinosis (PAP) in which alveoli are filed with lipid rich fluid and a variable number of macrophages. Publications in the literature suggest that PAP predisposes to TB 5, 54, 55. Most PAP is an autoimmune disease caused by neutralizing antibodies against GM-CSF. 56 This together with the observation that GM-CSF deficient mice develop intra-alveolar tuberculosis with proteinaceous fluid containing surfactant protein B (SP-B) suggests an important role for GM-CSF 57.
Cord factor
In considering the mechanisms of caseasion necrosis it seems noteworthy that cord factor (trehalose 6,6’ dimycolate or TDM) is the only chemically defined material known to induce caseating granulomas in sensitized animals 58. TDM is the most abundant lipid produced by virulent MTB 59. Its most unusual property is that it has multiple sets of biologic activities that depend on the mode of presentation 59. It is excreted by MTB, but is so insoluble that it remains free on the surface where it protects the organism from killing by macrophages. When it contacts a lipid droplet, TDM comes off the organism and spreads as a monolayer on the lipid surface where it becomes the most toxic lipid of MTB by orders of magnitude 60. It has recently been reported that the toxicity of TDM, measured by production of TNF, increases dramatically as the diameter of the particle exceeds 25 microns 5, 61. We propose that TDM encounters lipid droplets of increasing size during the course of post obstructive tuberculous pneumonia and that this is the trigger for initiating caseous necrosis.
Caseous pneumonia may soften and be coughed our to leave a cavity or remain to become surrounded by granulomatous inflammation of fibrocaseous TB. The softening phase has been associated with a profound coagulopathy and proteolytic enzymes 29,62. Caseous lesions may soften in either of two ways 22. First, the necrotic mass may became fissured and fragmented into large clumps and removed without any influx of inflammatory cells and few or frequently no AFB 22. The second pattern of softening involves massive influx of neutrophils at the periphery of the lesion and sometimes throughout 22. This may be associated with massive increase in AFB in the newly caseous tissue 22.
Discussion
Lack of progress in understanding host immunity to adult pulmonary TB is increasingly recognized as a major impediment to control and eventual elimination of the disease 2, 63. Modern science has produced masses of detail, without answering the central question as posed by North, “to explain why immunity to infection does not enable susceptible humans to resolve lung infection and thereby stop the development of disease” 64. Immunity effectively halts the increase in numbers of MTB in immunocompetent people and animals. However, it does not prevent the small number of remaining MTB in the lung from eventually causing disease 65. Disease is not caused by increasing numbers of MTB, but to hypersensitivity to MTB antigens. Accordingly, the onset of clinical post-primary TB in humans is typically paucibacillary 17, 29.
Multiple authors have called for a new paradigm to guide TB research 3, 66, 67. The paradigm that has directed research for decades envisions TB as a one-act play that repeats as one progresses from primary to post-primary disease. It is essentially a war of attrition in which MTB try to multiply while the host attempts to contain them within granulomas. Caseating granulomas driven by systemic immunity are considered the important lesion of all stages of TB. This conception of the pathogenesis of TB has failed to answer key questions 2, 3. It is also inconsistent with descriptions of the morphology of human TB from gross pathology published in 1804 through high resolution CT scans published in 2015. Several investigators have recognized the inconsistencies and proposed new mechanisms and models that include some of the concepts of this paper 26, 68–70.
The key departure of this paper was study of human tissues that led to the realization that post-primary TB develops for 1–2 years as part of latent TB (defined as persistent infection without symptoms) 24. MTB have evolved mechanisms to isolate pulmonary lobules within which they asymptomatically accumulate materials in preparation for a sudden necrotizing reaction to produce a cavity of sufficient size to mediate transmission of infection to new hosts. This process can be described using the metaphor of a three-act play.
TB as a three-act play
The diverse manifestations of the pathology of TB have long been a challenge for investigators attempting to discern its pathogenesis 21, 23,26. The genetics of both MTB and the host, the immune status of the host, nutrition and presence of other diseases all contribute to diversity of pathologies. In addition, multiple stages of disease may exist in single lungs. The present studies began with autopsies of immunocompetent young adults who died of acute pulmonary TB since they have only the early lesions of post primary TB. Their lungs had many stages of TB pneumonia, but no granulomas or fibrocaseous disease, much MTB antigen and very few AFB. With these results, the more complex pathologies of chronic TB and disease in immune compromised hosts began to form recognizable patterns. Lung tissue of some people with TB, especially immune compromised people and those with mature cavities, may have vast numbers of AFB that may or may not be cultivable 22. We have no explanation for these or many other phenomena. However, the present studies do demonstrate that in many fatal cases of tuberculous pneumonia, caseation necrosis associated with asymptomatic accumulation of mycobacterial antigens, not with large numbers of viable MTB. This together with knowledge of the pathology of developing post-primary TB, the effects of bronchial obstruction, the observation that perifocal inflammation can be induced by tuberculin and the observation that clinical disease typically begins after a latent period of 1–2 years after infection are key elements supporting the new paradigm. Accordingly, pulmonary TB progresses thorough distinct stages that suggest the metaphor of a three-act play, Figure 6.
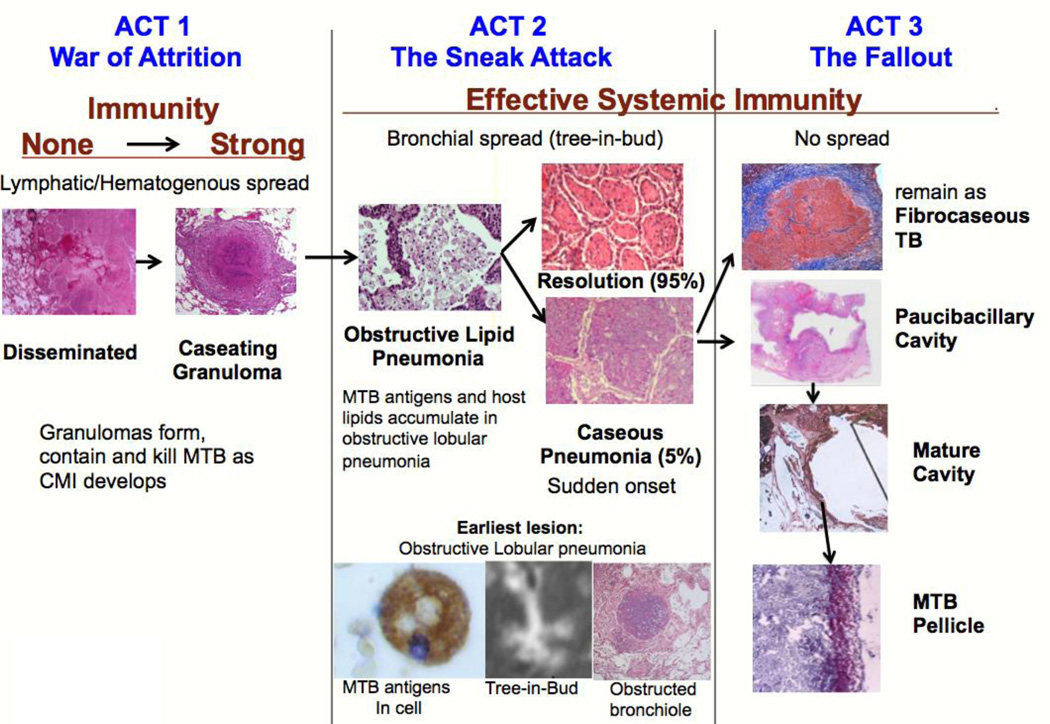
Figure 6. Tuberculosis as a Three Act Play.
The current paradigm of the pathogenesis of TB considers TB to a one act play in which the caseating granuloma modulated by CMI is the characteristic lesion of all TB. While this is an appropriate model for M. bovis and the early stages of post-primary TB, it fails to recognize the existence of obstructive lobular pneumonia that initiates and drives all of post-primary TB.
Act 1. War of Attrition; Caseating granulomas of primary TB
Macrophages ingest and attempt to destroy MTB while the MTB attempt to proliferate, disseminate and escape to infect new hosts. This type of infection occurs in most animal models and in people who have insufficient systemic immunity to control the organisms. The lesions enlarge until stopped by developing systemic cell mediated immunity (CMI). The organisms are then killed by activated macrophages or trapped within caseating granulomas. This war of attrition is won in weeks by most immunocompetent people, but progresses to death in immune compromised people and in most experimental animals. The immunocompetent person’s victory, however, is seldom complete. Small numbers of organisms adapt and persist asymptomatically as latent infection.
Act 2. The Sneak attack. Post-primary bronchogenic TB
begins asymptomatically in the apices of the lung, at some distance from the site initial infection that produced a Gohn complex. It is part of latent TB since there are no clinical symptoms 24. Small numbers of MTB in modified alveolar macrophages drive accumulation of host lipids and mycobacterial antigens in an isolated section of lung in preparation for a sudden necrotizing reaction sufficient to produce a cavity. Post-primary TB begins, as its name implies, after the host has established systemic immunity with primary TB. It begins with clandestine stockpiling of munitions. MTB infect alveolar macrophages, transform them into foam cells and obstruct bronchioles to physically isolate a lobule of lung in an otherwise immune host. This facilitates asymptomatic accumulation of mycobacterial antigens and host lipids within alveoli 71–73. Sensitized lymphocytes accumulate in the vicinity. The infection spreads via bronchi, not lymphatics, over a period of 1–2 years to adjacent lung. Most lesions regress spontaneously. A few suddenly undergo necrosis to produce either paucibacillary caseous pneumonia or caseous pneumonia with many neutrophils and AFB. The trigger for initiating necrosis may be changing conformation of TDM associated with lipid that causes expression of its toxicity 5, 59. This, in turn, releases stored MTB antigens that induce perifocal inflammation and acute caseous pneumonia. The nature and extent of the caseous pneumonia determines the future course of disease.
Act 3: The Fallout
encompasses the further evolution of necrotic caseous pneumonia. It is either coughed out to form a cavity or becomes surrounded by epitheloid cells and fibrosis that produce granulomatous inflammation and most clinical disease. Both usually occur in a single lung. Cavities form when caseous pneumonia softens, fragments and is coughed out of the body leaving a hole. The mechanisms of such softening have long been debated 20. Cavities may contain many neutrophils. Recent publications report that neutrophils and proteases contribute to the softening and fragmentation that leads to cavitation 7, 62, 74, 75. While this may be true, the data are not convincing because investigators have not been able to study developing post primary TB. Neutrophils and matrix metalloproteinases have been demonstrated in biopsies of the walls of mature cavities 76. This is a late stage of the fallout that differs in time and place from the formation of cavities.
MTB are seldom found in the sputum during the early stages of this process. They move in as the debris is cleared and induce a thin walled cavity to support pellicle growth of MTB with direct access to the airways. The pellicle appears to be a biofilm composed of masses of MTB and extracellular lipid, especially TDM 25, 77, 78. MTB are coughed out of such cavities for decades to infect new hosts as the person lives a fairly normal life.
Caseous pneumonia that is not coughed out remains to induce inflammation. It dries, hardens and becomes enveloped by epitheloid cells and fibrous tissue producing consumption and fibrocaseous TB. Granulomas in post-primary TB form only in response to caseation necrosis and are never the cause of it 21, 22. New lesions of obstructive lobular pneumonia, visible as tree-in-bud, may begin at any time and spread via bronchi in the lung. They may progress for 1–2 years to stockpile munitions before resolving or undergoing caseous necrosis. The initiation of such processes at different times explains the finding of multiple stages of TB in single lungs.
Addressing long-standing mysteries of the pathogenesis of TB
The value of a paradigm is often judged by how well it suggests testable hypotheses to important questions. The revised paradigm for the pathogenesis of post-primary TB suggests answers to several long-standing questions. The key new element is the sneak attack, an obstructive lobular pneumonia in which small numbers of MTB asymptomatically establish the conditions for a massive necrotizing reactions that leads to all subsequent manifestations of post-primary TB.
What is the nature of the immunity that protects most people from post-primary TB? Post-primary TB develops in people with sufficient immunity to prevent new infection in every part of their bodies except the vulnerable area of the lung. MTB selects the least active part of lung, isolates it further by bronchial obstruction and modulates alveolar macrophages to accumulate host and mycobacterial products quietly for 1–2 years in preparation for a necrotizing reaction that could be massive and sudden. However, progression to disease is a rare event (greater than 95% of lesions resolve spontaneously). Protection, therefore, could result from any factor that inhibits progression. Regression of lesions has been produced by relief of bronchial obstruction.20, 33. It could also be produced by failure to accumulate sufficient host lipids, mycobacterial products or sensitized cells. Alternatively, premature initiation of necrosis could abort the process resulting in an apical scar or small granuloma depending on the degree of caseation.
Why are immunocompetent young adults especially susceptible to disease and death? As its name implies, post-primary TB only occurs after primary TB has established a degree of immunity. Immunocompetent young adults have the strongest hypersensitivity and therefore develop the most severe reactions during a sneak attack.
How can multiple pulmonary lesions in a single lung act independently as if the others did not exist? Patients with chronic TB frequently have multiple stages lesions in a lung 7, 79. Both early and late stages may coexist in a single lung because bronchial obstruction starts the clock for storage of mycobacterial antigens and host lipids that drive the rest of the pathology. Obstruction is a local process that can begin in different parts of the lung at different times.
Why doesn’t recovery from post-primary TB produce immunity? “Recovery from active TB produces not immunity, but increased susceptibility to new clinical disease” 9. Such people are highly susceptible to new post primary bronchogenic infections 80, 81. Immunity typically remains sufficient to protect the rest of their bodies. Susceptibility to bronchogenic TB in such persons may be due to damage to bronchi that facilitates maintenance of bronchial obstruction by TB 32. Modified local immune responses such as T cell exhaustion likely contribute 82.
Why have vaccines that prevent disseminated TB in children, failed to protect adults from pulmonary TB? Totally different mechanisms of protection are required for primary and post-primary TB. These are systemic CMI for primary TB and failure to obstruct and accumulate mycobacterial antigens and appropriately sensitized cells for post-primary TB.
Why does post-primary TB localize in the upper lobes of the lungs? The apices have the least ventilation, perfusion, lymph flow and movement. This is necessary for maintaining obstructive lobular pneumonia that drives all subsequent manifestations of post-primary TB.
How can MTB be an obligate human parasite when people are more resistant than any of the animal models? MTB is an obligate human parasite because only humans develop post-primary TB that progresses to pulmonary cavities that facilitate transmission of infection to new hosts. MTB uses a special mechanism in humans, the sneak attack, to develop cavities in otherwise immune hosts. Most humans develop effective immunity against primary TB in weeks whereas most animals die of progressive disease. The systemic resistance benefits the organism because it maintains the health of the host who can then spread infection from the cavity for many years 17.
The differences between caseating granulomas in primary and post primary require discussion. It has long been recognized that caseation is caused by hypersensitivity reactions to mycobacterial antigens in lipid rich matrices 20, 58. In developing primary TB, an individual has insufficient immunity to prevent synthesis of mycobacterial antigens in developing granulomas. Consequently, the antigens may induce caseation within the granuloma as immunity develops. In developing post primary TB, MTB bacilli do not survive and produce antigens except in alveolar macrophages in sequestered sites in the lung. The antigens remain invisible inside of macrophages. When released, these antigens may interact with sensitized T cells to induce caseous pneumonia that, if not expelled, becomes surrounded with a granulomatous reaction. Histologically, the center of a caseating granuloma of primary TB is amorphous while that of post primary TB contains remnants of alveolar walls as illustrated in Figure 1.
This paper began with listing key unanswered questions for TB research. It is not surprising that these questions remain unanswered as research has been focused on caseating granulomas that have little to do with developing pulmonary disease, while the existence the true precursor lesion, obstructive lobular pneumonia, is seldom recognized. It is called post-primary TB because it occurs only after primary TB has established effective systemic immunity that protects the entire body except for particular lesions in the lungs. The early stages are a ‘sneak attack’ because MTB has evolved means to asymptomatically accumulate materials for a massive necrotizing reaction in a localized lesion in an otherwise immune host. Much evidence indicates that a strong systemic response is required for this lesion to produce cavities. Nevertheless, over 95% of sneak attacks fail because the lesions regress before undergoing caseous necrosis. This provides reason for optimism that research on spontaneous regression will lead to ways to make all lesions regress and thereby eradicate TB from the earth.
TB as a Three-Act Play: New Paradigm Based on 200 Years of Studies of Human Pulmonary TB.
-
Act 1. Building Defenses: Systemic CMI and caseating granulomas
-
-
A war of attrition between host and microbe.
Appropriate metaphor for primary TB and M. bovis
-
-
Intermission - latent MTB persists
-
Act 2. The Sneak Attack: Lobular obstructive pneumonia
-
-
Scene 1. Clandestine stockpiling of munitions
Over 95% regress asymptomatically & spontaneously
-
-
Scene 2. The explosion; sudden onset of caseous necrosis of post obstructive pneumonia
-
-
-
Act 3: The Fallout: Cavities and Consumption
-
-
Scene 1: Cavitation by expelling dead lung
Cavities mature to support massive growth of MTB with little inflammation in an otherwise immune host
-
-
Scene 2: Disease (Consumption/fibrocaseous disease) produced by host response to retained dead lung
Granulomas encase caseous necrotic lung that fails to soften and be coughed out producing most chronic disease.
-
-
Acknowledgments
Supported in part by USPHS grants AI078420 and HL068537
The author is indebted to many colleagues for advice, criticism and support in the development of this manuscript especially Drs. Chinnaswamy Jagannath, ShenAn Hwang, Jeffrey Actor and Elena Fedotova.
Footnotes
Ethical approval: This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Board of UT-Houston Medical School IRB protocol number HSC-MS-10-0109, Immunopathology of Tuberculosis. All materials were by products of regular autopsy practice. The specimens were treated as deidentified for the study.
Competing interests: None declared.
References
- 1.Russell DG, Barry CE, Flynn JL., 3rd Tuberculosis: what we don’t know can, and does, hurt us. Science. 2010;328:852–856. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufmann SH, Evans TG, Hanekom WA. Tuberculosis vaccines: time for a global strategy. Sci Transl Med. 2015;7:276fs8. doi: 10.1126/scitranslmed.aaa4730. [DOI] [PubMed] [Google Scholar]
- 3.Nunes-Alves C, et al. In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol. 2014;12:289–299. doi: 10.1038/nrmicro3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter RL, Jagannath C, Actor JK. Pathology of postprimary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis (Edinb) 2007;87:267–278. doi: 10.1016/j.tube.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Hunter RL, Actor JK, Hwang S, Karev V, Jagannath C. Pathogenesis of Post Primary Tuberculosis: Immunity and Hypersensitivity in the Development of Cavities. Ann Clin Lab Sci. 2014;44:365–387. [PubMed] [Google Scholar]
- 6.Verver S, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171:1430–1435. doi: 10.1164/rccm.200409-1200OC. [DOI] [PubMed] [Google Scholar]
- 7.Dannenberg AM., Jr . Insights from the rabbit model. Washington, DC: ASM press; 2006. Pathogenisis of human pulmonary tuberculosis. [Google Scholar]
- 8.Laennec R. A treatise on diseases of the chest in which they are described according to their anatomical characters, and their diagnosis established on a new principle by means of acoustick instruments. Birmingham AL: T&G Underwood, London reprinted 1979 by The Classics of Medicine Library; 1821. [Google Scholar]
- 9.Dubos R, Dubos G. Tuberculosis, Man, and Society. Brunswick, NJ: Rutgers Univesity Press; 1987. The White Plague. [Google Scholar]
- 10.Bennett JH. The pathology and treatment of pulmpnay tuberculosis. Philadelphia: Blanchard and Lea; 1854. [Google Scholar]
- 11.Virchow R. Cellular Pathology as based upon physiological and pathological histology. 511. Birmingham, AL., London: John Churchill; 1860. (reprinted by The Calssics of Medicine Library, 1978) [DOI] [PubMed] [Google Scholar]
- 12.Powell RD. On Consumption and on certin diseases of lungs and pleura 291. London: H.K. Lewis; 1876. [Google Scholar]
- 13.Tyndale J. The present status of the pathology of consumptoipn and tuberculosis. New York: Trow’s Printing and Bookbinding Co; 1878. [Google Scholar]
- 14.Cornil V, Ranvier L. A manual of pathological histology translated with notes and additions by EO Shakespeare and JHC Simms. Philadephia: Henry C Lea; 1880. pp. 394–445. [Google Scholar]
- 15.Hamilton JD. Available on Google books. London: Macmillan and Co; 1883. On the Pathology of Bronchitis, Catarrhal Pneumonia. Tubercle and allied lesions of the human lung. [Google Scholar]
- 16.Hektoen L, Reisman D. A Text-Book of Pathology for the use of students and practitoners of medicine and surgery. London and Philadelphia: W.B. Saunders & Company; 1901. [Google Scholar]
- 17.Osler W, McCrae T. The principles and practice of medicine. New York and London: D. Appleton and Company; 1921. pp. 184–255. [Google Scholar]
- 18.Opie EL. Pathology of the Tuberculosis of Childhood and Its Bearing on Clinical Work. Br Med J. 1927;2:1130–1135. doi: 10.1136/bmj.2.3493.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayne GG, Pagel W, O’Shaughenessy L. Pulmonary Tuberculosis, Pathology, Diagnosis and Management. London: Oxford University Press; 1939. [Google Scholar]
- 20.Rich A. The Pathogenesis of Tuberculosis. Second. Springfield, Illinois: Charles C Thomas; 1951. [Google Scholar]
- 21.Medlar EM. The behavior of pulmonary tuberculous lesions; a pathological study. Am Rev Tuberc. 1955;71:1–244. [PubMed] [Google Scholar]
- 22.Canetti G. Histobacteriology and its bearing on the therapy of pulmonary tuberculosis. New Yoyk: Springer Publishing Compani Inc; 1955. The tubercle bacillus in the pulmonary lesion of man. [Google Scholar]
- 23.Pagel W, Simmonds F, MacDonald N, Nassau E. In Pulmonary Tuberculosis, Bacteriology, Pathology, Management, Epidemiology and Prevention. London: Oxford University Press; 1964. pp. 36–63. [Google Scholar]
- 24.Salgame P, Geadas C, Collins L, Jones-Lopez E, Ellner JJ. Latent tuberculosis infection - Revisiting and revising concepts. Tuberculosis (Edinb) 2015 doi: 10.1016/j.tube.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Hunter RL. Pathology of post primary tuberculosis of the lung: an illustrated critical review. Tuberculosis (Edinb) 2011;91:497–509. doi: 10.1016/j.tube.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardona PJ. The key role of exudative lesions and their encapsulation: lessons learned from the pathology of human pulmonary tuberculosis. Front Microbiol. 2015;6:612. doi: 10.3389/fmicb.2015.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagel W, Zur WP. Histochemie der Lungentuberkulose, mit besonderer Berucksichtung der Fettsubstanzen und Lipoide(Fat, lipoid content to tuberculous tissue Histochemical investigation.) Virchows Arch Pathol Anat. 1925;256:629–640. [Google Scholar]
- 28.Medlar EM. The pathogenesis of minimal pulmonary tuberculosis; a study of 1,225 necropsies in cases of sudden and unexpected death. Am Rev Tuberc. 1948;58:583–611. doi: 10.1164/art.1948.58.6.583. [DOI] [PubMed] [Google Scholar]
- 29.Erokhin VV, Mishin VY, Chukanov VI, Guiller DV. Caseous pneumonia, Pathologic anatomy, pathogenesis, diagnosis, clinical course and treatment, A manual for practitioners. Moscow: Meditsina Publishers; 2008. [Google Scholar]
- 30.Im JG, Itoh H, Lee KS, Han MC. CT-pathology correlation of pulmonary tuberculosis. Crit Rev Diagn Imaging. 1995;36:227–285. [PubMed] [Google Scholar]
- 31.Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. Int J Infect Dis. 2015;32:87–93. doi: 10.1016/j.ijid.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Amaral AF, et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J. 2015 doi: 10.1183/13993003.02325-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchison JH. The pathogenesis of epituberculosis in children with a note on obstructive emphysema. Glasgow Med J. 1949;30:271–282. [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter RL. On the pathogenesis of post primary tuberculosis: the role of bronchial obstruction in the pathogenesis of cavities. Tuberculosis (Edinb) 2011;1(91 Suppl):S6–S10. doi: 10.1016/j.tube.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Betancourt SL, et al. Lipoid pneumonia: spectrum of clinical and radiologic manifestations. AJR Am J Roentgenol. 2010;194:103–109. doi: 10.2214/AJR.09.3040. [DOI] [PubMed] [Google Scholar]
- 36.Tamura A, Hebisawa A, Fukushima K, Yotsumoto H, Mori M. Lipoid pneumonia in lung cancer: radiographic and pathological features. Jpn J Clin Oncol. 1998;28:492–496. doi: 10.1093/jjco/28.8.492. [DOI] [PubMed] [Google Scholar]
- 37.Akinosoglou KS, Karkoulias K, Marangos M. Infectious complications in patients with lung cancer. Eur Rev Med Pharmacol Sci. 2013;17:8–18. [PubMed] [Google Scholar]
- 38.Humphrey DM, Weiner MH. Mycobacterial antigen detection by immunohistochemistry in pulmonary tuberculosis. Hum Pathol. 1987;18:701–708. doi: 10.1016/s0046-8177(87)80241-1. [DOI] [PubMed] [Google Scholar]
- 39.Barbolini G, et al. Immunohistologic analysis of mycobacterial antigens by monoclonal antibodies in tuberculosis and mycobacteriosis. Hum Pathol. 1989;20:1078–1083. doi: 10.1016/0046-8177(89)90226-8. [DOI] [PubMed] [Google Scholar]
- 40.Karimi S, et al. Histopathological findings in immunohistological staining of the granulomatous tissue reaction associated with tuberculosis. Tuberc Res Treat. 2014;2014:858396. doi: 10.1155/2014/858396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mustafa T, Leversen NA, Sviland L, Wiker HG. Differential in vivo expression of mycobacterial antigens in Mycobacterium tuberculosis infected lungs and lymph node tissues. BMC Infect Dis. 2014;14:535. doi: 10.1186/1471-2334-14-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majlessi L, Prados-Rosales R, Casadevall A, Brosch R. Release of mycobacterial antigens. Immunol Rev. 2015;264:25–45. doi: 10.1111/imr.12251. [DOI] [PubMed] [Google Scholar]
- 43.Smith DT, Abernathy RS. Selective Localization of Pulmonary Emboli: An Explanation for the Apical Localization of Reinfection Tuberculosis. Trans Am Clin Climatol Assoc. 1949;61:191–220. [PMC free article] [PubMed] [Google Scholar]
- 44.Goodwin RA, Des Prez Rm. Apical localization of pulmonary tuberculosis, chronic pulmonary histoplasmosis, and progressive massive fibrosis of the lung. Chest. 1983;83:801–805. doi: 10.1378/chest.83.5.801. [DOI] [PubMed] [Google Scholar]
- 45.Du K, et al. Reproducibility of registration-based measures of lung tissue expansion. Med Phys. 2012;39:1595–1608. doi: 10.1118/1.3685589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lincoln EM, Grethmann W. The potetial dangers of tuberculin tests. J Pediatrics. 1939;15:682–696. [Google Scholar]
- 47.Birnbaum M. Project Gutenberg Ebook method to cure tuberculosis. ( http://www.gutenberg.net EBook #27181, 2008)
- 48.Rook GA, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–284. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 49.Comas I, et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindestam Arlehamn CS, et al. Immunological consequences of intragenus conservation of Mycobacterium tuberculosis T-cell epitopes. Proc Natl Acad Sci U S A. 2015;112:E147–E155. doi: 10.1073/pnas.1416537112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orme IM. Development of new vaccines and drugs for TB: limitations and potential strategic errors. Future Microbiol. 2011;6:161–177. doi: 10.2217/fmb.10.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhalla A, Mahapatra M, Singh R, D’Cruz S. Acute lung injury in miliary tuberculosis. Indian Journal of Tuberculosis. 2002;49:125–128. [Google Scholar]
- 53.Massaro D, Katz S. Rapid Clearing in Hematogenous Pulmonary Tuberculosis. Arch Intern Med. 1964;113:573–577. doi: 10.1001/archinte.1964.00280100081013. [DOI] [PubMed] [Google Scholar]
- 54.Witty LA, Tapson VF, Piantadosi CA. Isolation of mycobacteria in patients with pulmonary alveolar proteinosis. Medicine (Baltimore) 1994;73:103–109. doi: 10.1097/00005792-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Pereira-Silva JL, Marinho MM, Veloso TV, Coelho JJ. Pulmonary alveolar proteinosis and tuberculosis in a diabetic patient: a rare or a seldom diagnosed association. Braz J Infect Dis. 2002;6:188–195. doi: 10.1590/s1413-86702002000400006. [DOI] [PubMed] [Google Scholar]
- 56.Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol. 2010;135:223–235. doi: 10.1016/j.clim.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chroneos ZC, Midde K, Sever-Chroneos Z, Jagannath C. Pulmonary surfactant and tuberculosis. Tuberculosis (Edinb) 2009;1(89 Suppl):S10–S14. doi: 10.1016/S1472-9792(09)70005-8. [DOI] [PubMed] [Google Scholar]
- 58.Hunter RL, Olsen M, Jagannath C, Actor JK. Trehalose 6,6’-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. Am J Pathol. 2006;168:1249–1261. doi: 10.2353/ajpath.2006.050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci. 2006;36:371–386. [PubMed] [Google Scholar]
- 60.Schabbing RW, Garcia A, Hunter RL. Characterization of the trehalose 6,6’-dimycolate surface monolayer by scanning tunneling microscopy. Infect Immun. 1994;62:754–756. doi: 10.1128/iai.62.2.754-756.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geisel RE, Sakamoto K, Russell DG, Rhoades ER. In Vivo Activity of Released Cell Wall Lipids of Mycobacterium bovis Bacillus Calmette-Guerin Is Due Principally to Trehalose Mycolates. J Immunol. 2005;174:5007–5015. doi: 10.4049/jimmunol.174.8.5007. [DOI] [PubMed] [Google Scholar]
- 62.Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med. 2014;190:9–18. doi: 10.1164/rccm.201311-2106PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karp CL, Wilson CB, Stuart LM. Tuberculosis vaccines: barriers and prospects on the quest for a transformative tool. Immunol Rev. 2015;264:363–381. doi: 10.1111/imr.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 65.Dannenberg AM, Jr, Collins FM. Progressive pulmonary tuberculosis is not due to increasing numbers of viable bacilli in rabbits, mice and guinea pigs, but is due to a continuous host response to mycobacterial products. Tuberculosis (Edinb) 2001;81:229–242. doi: 10.1054/tube.2001.0287. [DOI] [PubMed] [Google Scholar]
- 66.Andersen P, Woodworth JS. Tuberculosis vaccines--rethinking the current paradigm. Trends Immunol. 2014;35:387–395. doi: 10.1016/j.it.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Lalvani A, Sridhar S, von Reyn CF. Tuberculosis vaccines: time to reset the paradigm. Thorax. 2013;68:1092–1094. doi: 10.1136/thoraxjnl-2013-203456. [DOI] [PubMed] [Google Scholar]
- 68.Henao-Tamayo M, Ordway DJ, Orme IM. Memory T cell subsets in tuberculosis: what should we be targeting. Tuberculosis (Edinb) 2014;94:455–461. doi: 10.1016/j.tube.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Orme IM, Basaraba RJ. The formation of the granuloma in tuberculosis infection. Semin Immunol. 2014;26:601–609. doi: 10.1016/j.smim.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Elkington PT, Friedland JS. Permutations of time and place in tuberculosis. Lancet Infect Dis. 2015 doi: 10.1016/S1473-3099(15)00135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh V, et al. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe. 2012;12:669–681. doi: 10.1016/j.chom.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 72.Peyron P, et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M.tuberculosis persistence. PLoS Pathog. 2008;4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Korf JE, et al. Macrophage reprogramming by mycolic acid promotes a tolerogenic response in experimental asthma. Am J Respir Crit Care Med. 2006;174:152–160. doi: 10.1164/rccm.200507-1175OC. [DOI] [PubMed] [Google Scholar]
- 74.Huynh KK, Joshi SA, Brown EJ. A delicate dance: host response to mycobacteria. Curr Opin Immunol. 2011;23:464–472. doi: 10.1016/j.coi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Kubler A, et al. Mycobacterium tuberculosis dysregulates MMP/TIMP balance to drive rapid cavitation and unrestrained bacterial proliferation. J Pathol. 2015;235:431–444. doi: 10.1002/path.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ong CW, et al. Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis. PLoS Pathog. 2015;11:e1004917. doi: 10.1371/journal.ppat.1004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ojha AK, et al. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol. 2008;69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hunter RL, Venkataprasad N, Olsen MR. The role of trehalose dimycolate(cord factor) on morphology of virulent M.tuberculosis in vitro. Tuberculosis (Edinb) 2006;86:349–356. doi: 10.1016/j.tube.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 79.Lenaerts A, Barry CE, 3rd, Dartois V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev. 2015;264:288–307. doi: 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.den Boon S, et al. High prevalence of tuberculosis in previously treated patients, Cape Town, South Africa. Emerg Infect Dis. 2007;13:1189–1194. doi: 10.3201/eid1308.051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Rie A, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–1179. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 82.Henao-Tamayo M, Irwin SM, Shang S, Ordway D, Orme IM. T lymphocyte surface expression of exhaustion markers as biomarkers of the efficacy of chemotherapy for tuberculosis. Tuberculosis (Edinb) 2011;91:308–313. doi: 10.1016/j.tube.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marshall GB, Babar JL, Muller NL. Postprimary tuberculosis. CMAJ. 2007;177:148. doi: 10.1503/cmaj.070051. [DOI] [PMC free article] [PubMed] [Google Scholar]