Abstract
Background:
Majority of the research on cardiac arrest (CA) have focused on post-CA brain injury and myocardial dysfunction, the renal dysfunction and acute kidney injury (AKI) in other critical illnesses after CA have not been well described. This study was designed to assess AKI with renal Doppler and novel AKI biomarkers in a swine model of ventricular fibrillation cardiac arrest (VFCA).
Methods:
Thirty healthy piglets were divided into VFCA group (n = 22) and Sham group (n = 8) in a blinded manner. Mean arterial pressure, heart rate, and cardiac output were recorded continuously. Cardiac arrest (CA) was induced by programmed electric stimulation in the VFCA group, and then cardiopulmonary resuscitation was performed. Twenty piglets returned of spontaneous circulation (ROSC) and received intensive care. Blood and urine samples were collected for AKI biomarkers testing, and Color Doppler flow imaging was performed at baseline, 6 h, 12 h, and 24 h, respectively after ROSC. At ROSC 24 h, the animals were sacrificed and a semi-quantitative evaluation of pathologic kidney injury was performed.
Results:
In the VFCA group, corrected resistive index (cRI) increased from 0.47 ± 0.03 to 0.64 ± 0.06, and pulsatility index (PI) decreased from 0.82 ± 0.03 to 0.68 ± 0.04 after ROSC. Cystatin C (CysC) in both serum and urine samples increased at ROSC 6 h, but neutrophil gelatinase-associated lipocalin (NGAL) in serum increased to 5.34 ± 1.68 ng/ml at ROSC 6 h, and then decreased to 3.16 ± 0.69 ng/ml at ROSC 24 h while CysC increasing constantly. According to the renal histopathology, 18 of 20 animals suffered from kidney injury. The grade of renal injury was highly correlated with RI, cRI, NGAL, and CysC. Linear regression equation was established: Grade of renal injury = 0.002 × serum CysC + 6.489 × PI + 4.544 × cRI – 8.358 (r2 = 0.698, F = 18.506, P < 0.001).
Conclusions:
AKI is common in post-CA syndrome. Renal Doppler and novel AKI biomarkers in serum and urine are of significant importance as early predictors of post-CA AKI.
Keywords: Acute Kidney Injury, Cardiac Arrest, Cystatin C, Doppler Ultrasonography, Neutrophil Gelatinase-associated Lipocalin
INTRODUCTION
Cardiac arrest (CA) leads to more than 300,000 deaths in North America annually, but cardiopulmonary resuscitation (CPR) yields a functional survival rate of only 1.4–5%.[1,2] Postresuscitation morbidity has been termed as “post-CA syndrome”: A multi-system dysfunction resulting from cerebral, myocardial, and prolonged, complete, and whole body ischemia-reperfusion injury.
Over the past decade, while majority of the research on CA have focused on post-CA brain injury and myocardial dysfunction, the renal dysfunction and acute kidney injury (AKI) in other critical illnesses after CA have not been well described. In actual, renal dysfunction is common following resuscitation from CA,[3] and it is strongly associated with increased early and long-term patient mortality, as well as the subsequent development of chronic kidney disease (CKD). Serum creatinine (sCr) and urine output are the standard diagnostic indexes of the RIFLE (risk, injury, failure, loss, and end-stage) criteria, the AKIN (the Acute Kidney Injury Network criteria), the KDIGO (Kidney Disease: Improving Global Outcomes), and clinical practice guidelines for AKI.[4] These criteria have several limitations since oliguria is a nonspecific marker of AKI and even mild sCr elevation occurs only after several hours of a profound decrease in the glomerular filtration rate.[5] Therefore, the identification of markers allowing the early detection of renal dysfunction is a research priority.[6] Color Doppler flow imaging (CDFI) is a rapid, noninvasive, and repeatable method for detecting AKI. Recently, Doppler-based renal resistive index (RI) has been suggested for assessing changes in renal perfusion in severely ill patients.[7,8] Neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C (CysC), which are the clinical utility of certain novel AKI biomarkers, have been already evaluated in various clinical conditions and deemed as good as or better than Cr in detecting AKI, particularly in the early stage.[9,10] The main objective of this study was to assess AKI with renal Doppler as novel AKI biomarkers in a swine model of ventricular fibrillation cardiac arrest (VFCA).
METHODS
The procedures have been approved by the Capital Medical University Institutional Animal Care Committee and the Beijing Chao-Yang Hospital, Capital Medical University Animal Care and Use Committee. All animals received treatments in compliance with the National Research Council's 1996 Guide for the Care and Use of Laboratory Animals. Anesthesia was titrated in all surgical interventions to avoid unnecessary suffering. The study was performed according to Utstein-style guidelines[11] on 30 healthy inbred Wuzhishan miniature piglets of either sex, aged 6–8 months, whose average weight was 20.12 ± 2.01 kg. Before any procedure, animals were divided into two groups by envelope method in a blinded manner as VFCA group (n = 22) and Sham group (n = 8).
Animal preparation
Briefly, initial sedation in each animal was achieved with intramuscular injection of 10 mg/kg ketamine, followed by ear vein injection of 1 mg/kg propofol and 4 μg/kg fentanyl to reach the desired depth of anesthesia and analgesia. The anesthetized animals were intubated with a 6.5 mm cuffed endotracheal tube and were mechanically ventilated by a volume-controlled ventilator (Servo 900c; Siemens, Berlin, Germany) with a tidal volume of 10 ml/kg and a respiratory frequency of 12/min with room air (FiO2 = 21%). Aortic blood pressure (ABP) was measured using a fluid-filled catheter advanced from the left femoral artery into the thoracic aorta. A 7F Swan-Ganz catheter (Edwards Life Sciences, Irvine, CA, USA) was advanced from the left femoral vein and flow-directed into the pulmonary artery to measure cardiac output (CO) by the thermodilution method. A 5F pacing catheter was advanced from the right internal jugular vein into the right ventricle to induce ventricular fibrillation (VF) for the VFCA group. An 18F urine catheter connected to a urine bag was inserted into the bladder by cystostomy to collect urine. Electrocardiographic lead II was continuously recorded with a multichannel physiological recorder (BL-420F Data Acquisition and Analysis System, Chengdu TME Technology Co. Ltd., Chengdu, Sichuan, China). Hemodynamics was monitored by an HP monitor (M1165; Hewlett-Packard Co., CA, USA). All animals received intraoperative normal saline solution (10 ml·kg−1·h−1) to replenish fluid losses.
Experimental protocol
After surgery, the piglets were allowed to equilibrate for 1 h to achieve a stable resting level and the baseline data were collected. In the Sham group, animals received intensive care for 24 h. In the VFCA group, VF was induced by a programmed electrical stimulation instrument (GY-600A; Kaifeng Huanan Instrument Co., Henan, China) with mode S1S2 (300/200 ms), 40 V, 8:1 proportion, and −10 ms step length. VF was verified by the presence of a characteristic electrocardiographic waveform and an immediate drop in ABP. After 8 min of untreated CA,[12] mechanical ventilation was resumed with 100% oxygen, and CPR was performed manually. Manual chest compressions were rapidly initiated at a rate of 100/min with equal compression-relaxation duration. After 2 min of CPR, epinephrine (0.02 mg/kg) was injected into the right atrium and then CPR was resumed for 2 min. After a total of 4 min of CPR, defibrillation was attempted using 4 J/kg (biphasic waveforms shock) for the first attempt. CPR was resumed for another 2 min after defibrillation attempt. The sequence continued until the restoration of spontaneous circulation (ROSC) or death. The duration from CA attempt to ROSC was recorded as ROSC time. ROSC was defined as the maintenance of a systolic blood pressure of at least 50 mmHg (1 mmHg = 0.133 KPa) for at least 10 consecutive min. The animal was announced dead when the resuscitation maintained 30 min, but the ROSC was still not achieved. The animals with ROSC received intensive care for 24 h, and mechanical ventilation with supplemental oxygen continued throughout the immediate postresuscitation period. No vasoactive agent was used to avoid bias for the hemodynamic monitor. Twenty-four hours after ROSC, all catheters were removed by a surgical procedure.[13] Then, the animals were sacrificed with intravenous injection of 60 mg of propofol and 20 ml of 2 mol/L potassium chloride solution. If the animals cannot survive to the prospective endpoint, the living time was recorded.
Hemodynamics
4°C saline was injected into the right atrium through the Swan-Ganz catheter per hour to determine CO by the transpulmonary thermodilution method. Heart rate (HR) and mean arterial pressure (MAP) were recorded continuously by the HP monitor.
Acute kidney injury biomarkers measurements
Blood and urine samples were collected for the laboratory test at baseline, 6 h, 12 h, and 24 h (In the VFCA group, the ROSC time was recorded as 0 h). The blood samples were used to measure serum Cr (sCr), CysC (sCysC) and NGAL (sNGAL) while the urine samples were used to determine urine CysC and NGAL. Briefly, the blood and urine samples were centrifuged (3000 r/min) for 20 min, and the supernatants were stored at −80°C for the analyses that were performed by an investigator blinded to the study design. Commercially available enzyme-linked immunosorbent assays were used for both serum and urine AKI biomarkers measurements, according to the manufacturer's instructions (Swine Cr/CysC/NGAL, Beijing Xinfangcheng Biotechnology Company, Ltd., China), in a duplicated manner. All intra-assay coefficients of variation were <10%.
Color Doppler flow imaging
CDFI was performed on right kidney at baseline, 6 h, 12 h, and 24 h. All CDFI studies were performed by an ultrasound physician with more than 10 years of experience, using an iU22 probe (Philips Ultrasound, USA). The probe was put on the right side of the animal's abdomen to obtain a clear image of the right kidney. The Doppler gain was set to obtain a clear outline of flow waves with minimal background noise. The spectrum was considered optimal when at least three similar consecutive waveforms were visualized. An interlobar or arcuate artery was selected, and the measurements were obtained. The main indexes included pulsatility index (PI), RI, and corrected RI (cRI), which were calculated using the following equations.[7]
PI = (peak systolic velocity [PSV] – minimum diastolic velocity [MDV])/mean velocity.
RI = (PSV – MDV)/PSV.
cRI = (observed RI – 0.0026 × [80 – observed HR]).
Three to five measurements were performed and averaged to obtain the mean RI value.
Pathologic examination and terminal deoxynucleotidyl transferase-mediated 2-deoxyuridine 5-triphosphate nick end labeling assay
After ROSC 24 h, animals were sacrificed. The upper pole of right renal cortex was surgically removed and preserved in 10% formaldehyde or 4% paraformaldehyde to observe pathologic changes under a light microscope (LM). Then, a grade of renal injury of semi-quantitative evaluation was done in each animal [Table 1]. Terminal deoxynucleotidyl transferase-mediated 2-deoxyuridine 5-triphosphate nick end labeling (TUNEL) assay was used to label cells that suffered severe DNA damage/fragmentation induced by apoptotic signaling cascades. The TUNEL positive cells were counted to determine the apoptotic index (AI). AI = Apoptotic cells stained brown/total TUNEL-positive cells. The pathologic evaluations were performed by an independent pathologist who has more than 10 years of experience and was blinded to this study.
Table 1.
Pathologic change for a semi-quantitative evaluation of kidney injury
| Grade | Pathologic changes under light microscope |
|---|---|
| 0 - Normal | Renal histopathology |
| 1 - Mild | Mild interstitial edema, renal tubular necrosis, slight glomerular capillaries angiectasis, and inflammatory cells infiltration |
| 2 - Moderate | Pathologic changes of renal between mild and severe |
| 3 - Severe | Severe interstitial edema, renal tubular necrosis, severe glomerular capillaries angiectasis, mesangial proliferation, and severe inflammatory cells infiltration |
Statistical analysis
Statistical analysis was performed by IBM SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Discrete variables were compared with Fisher's exact test. All continuous variables in this study were normal distribution, which were detected by the Kolmogorov-Smirnov test and reported as mean ± standard deviation (SD). The time and treatment effects were detected by the repeated measure analysis of variance (ANOVA). The difference between the two groups was compared by an independent sample t-test (AI) or the multivariate ANOVA (hemodynamics, AKI biomarkers, and CDFI parameters). Pearson correlation coefficient and linear regression were performed to determine the correlations of parameters. A value of P < 0.05 was considered statistically significant.
RESULTS
Characteristics of animals
The sham group and the VFCA group did not show significant difference in the animal profile (sex [10/12 vs. 3/5, P = 0.682] and weight [20.2 ± 0.9 kg vs. 20.3 ± 0.7 kg, t = −0.210, P = 0.835]), time of preparatory phase (61.2 ± 2.3 min vs. 60.6 ± 2.7 min, t = −0.618, P = 0.541), the extra doses of propofol (115.7 ± 2.1 mg vs. 116.6 ± 2.5 mg, t = 0.994, P = 0.329), and fentanyl (72.1 ± 1.7 μg vs. 71.5 ± 2.2 μg, t = −0.853, P = 0.401) administered during the preparation phase.
In the VFCA group, 20 of 22 (90.91%) animals got ROSC and survived to 24 h. The mean ROSC time was 16.23 ± 2.10 min. Eighteen of 20 (90.00%) animals showed some degree of kidney injury according to renal histopathology: Two animals were Grade 0; seven animals were Grade 1; six animals were Grade 2; and five animals were Grade 3.
Hemodynamics
HR, MAP, and CO at baseline did not differ significantly between the two groups. Compared with the Sham group, HR increased significantly, while MAP and CO decreased significantly after ROSC in the VFCA group. HR reached a peak at ROSC 2 h while MAP and CO decreased to the lowest point at ROSC 4 h. Significant difference of MAP was found between the two groups until ROSC 6h, whereas of HR and CO in the whole protocol (P < 0.05) [Table 2].
Table 2.
Hemodynamics of HR, MAP and CO of the VFCA group and Sham group (Sham group, n = 8; VFCA group, n = 22) (mean ± SD)
| Items | HR (bpm/min) | F | P | MAP (mmHg) | F | P | CO (L/min) | F | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | VFCA | Sham | VFCA | Sham | VFCA | |||||||
| Baseline | 95.0 ± 9.2 | 93.0 ± 5.4 | 0.470 | 0.499 | 92.3 ± 4.1 | 91.9 ± 5.0 | 0.914 | 0.843 | 2.80 ± 0.08 | 2.84 ± 0.09 | 1.435 | 0.242 |
| ROSC 2 h | 90.1 ± 6.2 | 143.7 ± 10.0* | 194.983 | <0.001 | 92.0 ± 4.8 | 102.3 ± 10.0* | 606.229 | 0.010 | 2.79 ± 0.10 | 2.14 ± 0.08* | 323.575 | <0.001 |
| ROSC 4 h | 90.4 ± 6.2 | 123.2 ± 5.9* | 171.565 | <0.001 | 91.9 ± 5.1 | 84.9 ± 5.8* | 282.004 | 0.006 | 2.84 ± 0.10 | 2.06 ± 0.08* | 447.207 | <0.001 |
| ROSC 6 h | 91.9 ± 6.3 | 114.5 ± 9.3* | 29.398 | <0.001 | 91.3 ± 3.0 | 86.1 ± 4.9† | 151.557 | 0.011 | 2.84 ± 0.08 | 2.15 ± 0.06* | 606.029 | <0.001 |
| ROSC 12 h | 92.4 ± 5.4 | 111.6 ± 8.3* | 36.349 | <0.001 | 92.9 ± 3.9 | 90.4 ± 5.2 | 36.432 | 0.227 | 2.83 ± 0.08 | 2.18 ± 0.05* | 599.364 | <0.001 |
| ROSC 24 h | 94.3 ± 5.5 | 103.0 ± 9.6† | 5.781 | 0.024 | 91.1 ± 3.6 | 93.6 ± 5.4 | 33.604 | 0.255 | 2.82 ± 0.11 | 2.23 ± 0.06* | 337.949 | <0.001 |
*P < 0.01, †P < 0.05 versus baseline, which were detected by the repeated measure ANOVA. P value in the table: Sham group versus VFCA group, which was detected by the multivariate ANOVA. SD: Standard deviation; ROSC: Return of spontaneous circulation; HR: Heart rate; MAP: Mean arterial pressure; CO: Cardiac output; VFCA: Ventricular fibrillation cardiac arrest.
Serum and urine acute kidney injury biomarkers
NGAL and CysC in serum and urine increased from baseline to ROSC 6 h, but NGAL decreased from ROSC 6 h while CysC continued to increase. However, sCr did not increase until ROSC 12 h (P > 0.05) [Table 3].
Table 3.
AKI biomarkers and Color Doppler parameters of the VFCA group and Sham group (Sham group, n = 8; VFCA group, n = 22) (mean ± SD)
| Items | sCr (umol/L) | F | P | sNGAL (ng/ml) | F | P | uNGAL (ng/ml) | F | P | sCysC (ng/ml) | F | P | uCysC (ng/ml) | F | P | PI | F | P | RI | F | P | cRI | F | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | VFCA | Sham | VFCA | Sham | VFCA | Sham | VFCA | Sham | VFCA | Sham | VFCA | Sham | VFCA | Sham | VFCA | |||||||||||||||||
| Baseline | 63.2 ± 1.3 | 62.9 ± 3.3 | 0.104 | 0.749 | 2.21 ± 0.10 | 2.23 ± 0.08 | 0.387 | 0.540 | 0.62 ± 0.04 | 0.63 ± 0.04 | 0.203 | 0.656 | 513.5 ± 3.6 | 515.4 ± 9.8 | 0.296 | 0.591 | 212.6 ± 4.4 | 214.0 ± 5.6 | 0.420 | 0.523 | 0.82 ± 0.03 | 0.82 ± 0.03 | 0.021 | 0.887 | 0.44 ± 0.03 | 0.43 ± 0.03 | 0.030 | 0.846 | 0.48 ± 0.03 | 0.47 ± 0.03 | 0.286 | 0.597 |
| ROSC 6 h | 62.3 ± 2.5 | 63.1 ± 2.8 | 0.530 | 0.473 | 2.22 ± 0.06 | 5.34 ± 1.68* | 27.060 | <0.001 | 0.62 ± 0.03 | 3.33 ± 1.52* | 25.076 | <0.001 | 511.2 ± 4.4 | 777.5 ± 169.7* | 19.251 | <0.001 | 215.2 ± 8.9 | 359.8 ± 86.8* | 25.076 | <0.001 | 0.82 ± 0.03 | 0.73 ± 0.03* | 67.969 | <0.001 | 0.44 ± 0.03 | 0.53 ± 0.04* | 33.666 | <0.001 | 0.48 ± 0.02 | 0.62 ± 0.04* | 83.167 | <0.001 |
| ROSC 12 h | 63.5 ± 3.0 | 65.8 ± 2.5 | 4.578 | 0.042 | 2.23 ± 0.11 | 4.05 ± 0.95* | 28.591 | <0.001 | 0.62 ± 0.03 | 2.29 ± 0.91* | 26.096 | <0.001 | 514.9 ± 6.8 | 864.2 ± 202.3* | 23.298 | <0.001 | 215.1 ± 7.4 | 426.1 ± 125.0* | 22.264 | <0.001 | 0.82 ± 0.03 | 0.68 ± 0.05* | 59.336 | <0.001 | 0.43 ± 0.02 | 0.54 ± 0.06* | 24.425 | <0.001 | 0.46 ± 0.03 | 0.62 ± 0.07* | 42.244 | <0.001 |
| ROSC 24 h | 63.2 ± 3.4 | 91.8 ± 20.5* | 15.036 | 0.001 | 2.20 ± 1.12 | 3.16 ± 0.69* | 15.072 | 0.001 | 0.62 ± 0.03 | 1.41 ± 0.31* | 51.03 | <0.001 | 519.7 ± 3.5 | 1304.4 ± 407.8* | 28.949 | <0.001 | 213.6 ± 3.1 | 475.5 ± 115.7* | 40.058 | <0.001 | 0.82 ± 0.03 | 0.68 ± 0.04* | 70.000 | <0.001 | 0.44 ± 0.03 | 0.59 ± 0.06* | 51.136 | <0.001 | 0.47 ± 0.04 | 0.64 ± 0.06* | 53.771 | <0.001 |
*P<0.01 versus baseline, which were detected by the repeated measure ANOVA. P value in the tabel: Sham group versus VFCA group, which was detected by an independent sample t-test or the multivariate ANOVA. SD: Standard deviation; AKI: Acute kidney injury; ROSC: Return of spontaneous circulation; sCr: Serum creatinine; sNGAL: Serum neutrophil gelatinase-associated lipocalin; uNGAL: Urine neutrophil gelatinase-associated lipocalin; sCysC: Serum cystatin C; uCysC: Urine cystatin C; PI: Pulsatility index; RI: Resistive index; cRI: Corrected resistive index; VFCA: Ventricular fibrillation cardiac arrest.
Color Doppler flow imaging
No significant difference in PI, RI, and cRI was found at the baselines in both groups. In the VFCA group, RI and cRI increased, and PI decreased significantly after ROSC. Significant differences were found between the two groups by multivariate ANOVA (P < 0.001) [Table 3 and Figure 1].
Figure 1.
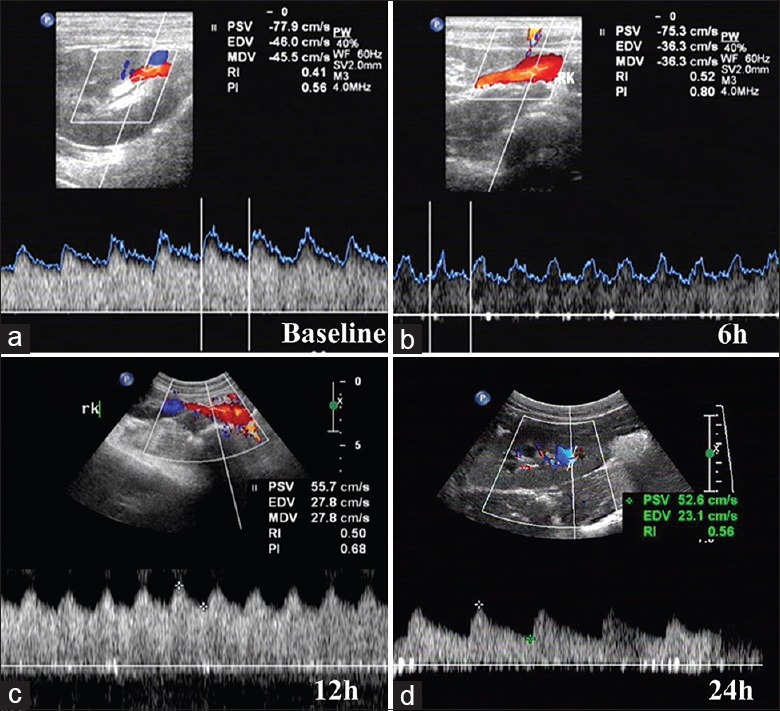
Renal Color Doppler flow image. (a) Color Doppler flow image at baseline. (b) Color Doppler flow image at ROSC 6 h. (c) Color Doppler flow image at ROSC 12 h. (d) Color Doppler flow image at ROSC 24 h. ROSC: Restoration of spontaneous circulation; PSV: Peak systolic velocity; EDV: End diastolic velocity; MDV: Minimum diastolic velocity; RI: Resistive index; PI: Pulsatility index.
Renal histopathology
Compared with the Sham group [Figure 2a], the renal injury was prominent in the VFCA group [Figure 2b-2d]. Interstitial edema, renal tubular necrosis, glomerular capillaries angiectasis, mesangial proliferation, and inflammatory cells infiltration were observed. Under the LM, the grade of renal injury was divided into 0 to 3 [Table 1]. All animals in the sham group were normal, whereas the mean grade of renal injury in the VFCA group was 1.70 ± 0.98; significant difference was found between the two groups (t = −7.768, P < 0.001). TUNEL assay revealed that there were greater numbers of apoptotic cells in the VFCA group than the sham group [Figure 2e and 2f]. AI of the sham group and the VFCA group was 7.68 ± 2.29% and 51.04 ± 6.44%, respectively, and significant difference was found between the two groups (t = −26.238, P < 0.001).
Figure 2.
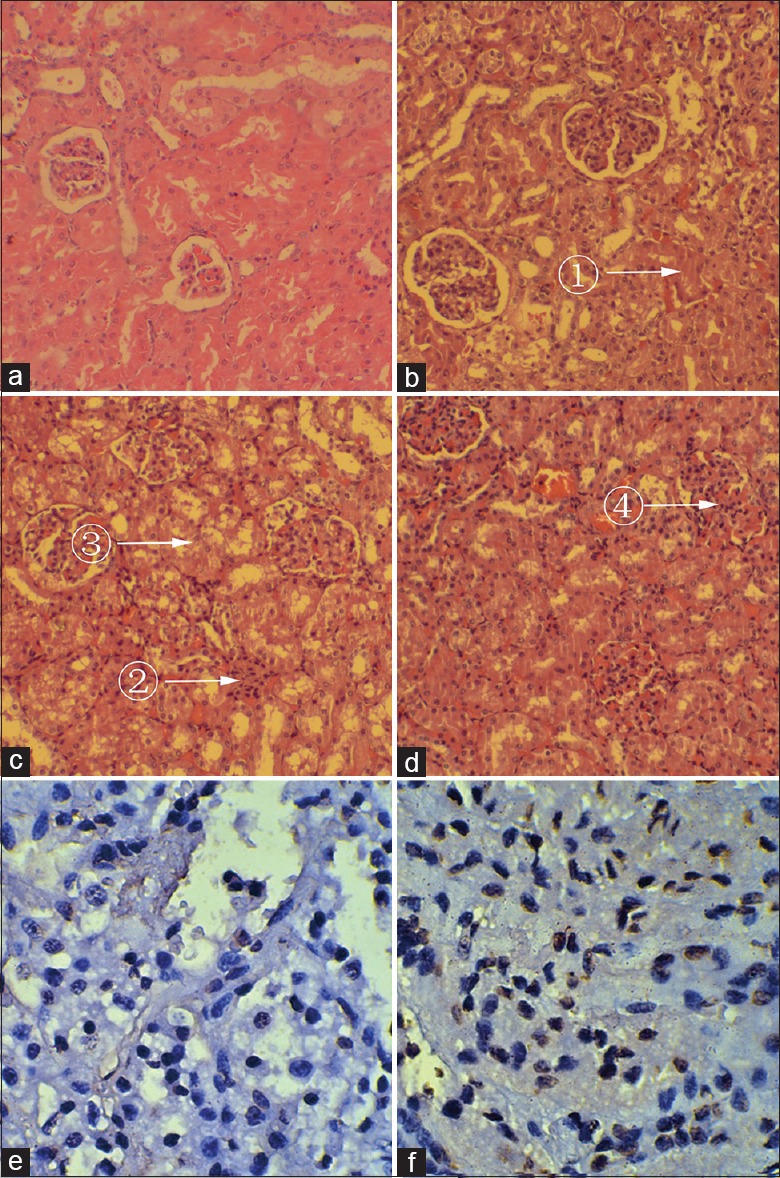
Renal ultra-microstructure and TUNEL staining images of renal apoptotic cells 24 h after ROSC of the VFCA (b-d, f), Sham (a, e) group (a-d, H and E, original magnification ×400; e, f, TUNEL, original magnification ×400). (a) Normal, Grade 0; (b) Grade 1, Mild injury: ① Interstitial edema. (c) Grade 2, Moderate injury: ② Mesangial inflammatory cells infiltration. ③ Renal tubular necrosis. (d) Grade 3, Severe injury: ④ Glomerular capillaries angiectasis. TUNEL: Terminal deoxynucleotidyl transferase-mediated 2-deoxyuridine 5-triphosphate nick end labeling; H and E: Hematoxylin and erosin; VFCA: Ventricular fibrillation cardiac arrest.
Correlations and regression
High correlations between serum and urine were found in NGAL and CysC. There were significant inverse correlations between RI and CO or between cRI and CO. There were positive correlations between grade of renal injury and ROSC time, sNGAL, sCysC, RI, or cRI [Table 4]. Regression analysis was performed among grade of renal injury and sNGAL, sCysC, PI, RI, or cRI. Linear regression equation can be established using the following equation: Grade of renal injury = 0.002 × sCysC + 6.489 × PI + 4.544 × cRI − 8.358 (r2 = 0.698, F = 18.506, P < 0.001).
Table 4.
Correlations of sNGAL, sCysC, CO and grade of renal injury
| Index 1 | Index 2 | r | P |
|---|---|---|---|
| sNGAL | uNGAL | 0.946 | <0.001 |
| sCysC | uCysC | 0.891 | <0.001 |
| CO | RI | −0.653 | <0.001 |
| CO | cRI | −0.723 | <0.001 |
| Grade of renal injury | ROSC time | 0.701 | <0.001 |
| Grade of renal injury | sNGAL | 0.459 | <0.001 |
| Grade of renal injury | sCysC | 0.809 | <0.001 |
| Grade of renal injury | RI | 0.402 | 0.034 |
| Grade of renal injury | cRI | 0.439 | 0.019 |
NGAL: Neutrophil gelatinase-associated lipocalin; sNGAL: Serum neutrophil gelatinase-associated lipocalin; uNGAL: Urine neutrophil gelatinase-associated lipocalin; CysC: cystatin C; sCysC: Serum cystatin C; uCysC: Urine cystatin C; CO: Cardiac output; ROSC: Return of spontaneous circulation; RI: Resistive index; cRI: Corrected resistive index.
DISCUSSION
Patients of CA often suffer from multiple organ dysfunction syndromes after ROSC and kidney is one of the most common injured organs. The incidence of renal dysfunction in patients worldwide is about 23.2%, and in patients with CA is 12–28%.[14] Despite increasing ability to support vital organs and resuscitate patients, the morbidity and mortality of AKI remain dismally high. AKI can gradually exacerbate to acute kidney failure and then ultimately to CKD. It may have a significant influence on the long-term survival. Therefore, the ability to predict the occurrence of AKI is crucial for the development of preventive strategies. In current clinical practice, the international gold standard for identification and classification of AKI is dependent on serial Cr and urine output,[15] which are unreliable during acute changes in kidney function.[16] Cr and urine output are not ideal for screening the early stages of AKI, because they could be normal until several days after renal injury occurs, when 25–50% of kidney function has already been lost.[17] Early detection of AKI requires markers that are sensitive, noninvasive, repeatable, and easily applicable in clinical practice. In this study, we used renal Doppler ultrasonography and two novel renal biomarkers combining with Cr to assess the renal function after CA. We found both of the renal novel biomarkers and the renal Doppler ultrasonography markers were abnormal in the early stage after ROSC in the VFCA group while sCr did not increase until ROSC 24 h. We also found that 18 animals in the VFCA group suffered various degree of kidney injury according to the histopathology result. These results demonstrate that the AKI in the CA is common and renal Doppler ultrasonography, NGAL, and CysC are useful in detecting AKI.
NGAL and CysC are two promising biomarkers in detecting early stage of AKI. In our previous study, it has been confirmed that NGAL and CysC have a higher sensitivity in the diagnosis of AKI and increase as early as ROSC 2 h.[18] In the present study, we found that NGAL decreased after ROSC 6 h, but CysC continued to increase, suggesting the increase of CysC may sustain longer than NGAL. Furthermore, the changes of NGAL and CysC in the serum were correlated with the changes in urine. The grade of renal injury was highly correlated with the levels of the serum biomarkers, which indicate that both NGAL and CysC are prognostic factors of renal injury after VFCA.
Renal Doppler ultrasonography is a noninvasive method to evaluate renal vascular condition. RI and PI are useful indexes to assess renal perfusion. More and more evidence suggests that Doppler-based renal RI and PI hold promise in monitoring renal function and in predicting AKI of critically ill patients.[19] Since RI depends on the minimum diastolic shift of renal arteriola, it could be influenced by the HR. To avoid the influence of HR change, we calculated cRI and found a decrease in PI and an increase in RI and cRI after ROSC, which indicating deterioration of renal perfusion. As same as the novel AKI biomarkers, high correlations were also found between the grade of renal injury and PI or cRI. Since cRI is a useful index to assess renal perfusion, it was not surprising that there is a high correlation between cRI and CO while linear regression equation was established between grade of renal injury and cRI in our investigation. Those results indicate that Doppler ultrasonography increases the diagnostic value of AKI.
Renal ischemia is the most common reason of AKI after CA, which causes functional impairment by multiple factors, including renal vasoconstriction, tubular obstruction, tubular back-leakage of glomerular filtrate, reduced glomerular permeability, and apoptosis.[20,21,22] In our study, the perfusion of renal stopped during 8 min of untreated CA, and a hypoperfusion may persist because of the deterioration of hemodynamic after ROSC, which may induce apoptosis. This result implied that hypoperfusion was one of the main risk factors of AKI.
The cause of AKI is complicated. In our study, even under the same experiment conditions, significant variation of AKI grade was observed among the animals, which may be due to different ROSC time. The longer ROSC time the worse AKI grade, which was confirmed by the positive correlation between the grade of AKI and ROSC time. Early detection of AKI will help to prevent or attenuate persistent AKI in patients with transient AKI. Our current study indicate that the combination of the novel AKI biomarkers and the Doppler ultrasonography can predict the risk of AKI in the early stage at the micro (molecular level) and macro (systemic level) level.
The current experiments were conducted on healthy animals, but the changes during pathophysiologic condition may be different. Because of the sample limitation, we did not analyze the differences of PI, RI and cRI at the same time point between the different AKI grades in the current study.
In conclusion, post-CA AKI, which occurs at an early stage of postresuscitation, is common in CA. Both renal Doppler ultrasonography and novel AKI biomarkers in serum and urine, such as NGAL and CysC, are of significant importance as early predictors of post-CA AKI.
Financial support and sponsorship
This research was supported by a grant from the Natural Science Foundation of China (No. 81372025).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Ning-Ning Wang and Ya-Lin Bao
REFERENCES
- 1.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics-2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.Yanta J, Guyette FX, Doshi AA, Callaway CW, Rittenberger JC. Post Cardiac Arrest Service. Renal dysfunction is common following resuscitation from out-of-hospital cardiac arrest. Resuscitation. 2013;84:1371–4. doi: 10.1016/j.resuscitation.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 4.KDIGO. Clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:8–12. [Google Scholar]
- 5.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–9. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Society of Nephrology. American Society of Nephrology Renal Research Report. J Am Soc Nephrol. 2005;16:1886–903. doi: 10.1681/ASN.2005030285. [DOI] [PubMed] [Google Scholar]
- 7.Schnell D, Darmon M. Renal Doppler to assess renal perfusion in the critically ill: A reappraisal. Intensive Care Med. 2012;38:1751–60. doi: 10.1007/s00134-012-2692-z. [DOI] [PubMed] [Google Scholar]
- 8.Schnell D, Camous L, Guyomarc’h S, Duranteau J, Canet E, Gery P, et al. Renal perfusion assessment by renal Doppler during fluid challenge in sepsis. Crit Care Med. 2013;41:1214–20. doi: 10.1097/CCM.0b013e31827c0a36. [DOI] [PubMed] [Google Scholar]
- 9.Sharma RK. Biomarkers of acute kidney injury. Clin Queries Nephrol. 2012;1:13–7. [Google Scholar]
- 10.Adiyanti SS, Loho T. Acute kidney injury (AKI) biomarker. Acta Med Indones. 2012;44:246–55. [PubMed] [Google Scholar]
- 11.Idris AH, Becker LB, Ornato JP, Hedges JR, Bircher NG, Chandra NC, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a task force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Institute of Critical Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine. Writing Group. Circulation. 1996;94:2324–36. doi: 10.1161/01.cir.94.9.2324. [DOI] [PubMed] [Google Scholar]
- 12.van Alem AP, Vrenken RH, de Vos R, Tijssen JG, Koster RW. Use of automated external defibrillator by first responders in out of hospital cardiac arrest: Prospective controlled trial. BMJ. 2003;327:1312–7. doi: 10.1136/bmj.327.7427.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xanthos T, Bassiakou E, Koudouna E, Tsirikos-Karapanos N, Lelovas P, Papadimitriou D, et al. Baseline hemodynamics in anesthetized landrace-large white swine: Reference values for research in cardiac arrest and cardiopulmonary resuscitation models. J Am Assoc Lab Anim Sci. 2007;46:21–5. [PubMed] [Google Scholar]
- 14.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–93. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 17.Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: What do we know? What do we need to learn. Pediatr Nephrol. 2009;24:265–74. doi: 10.1007/s00467-008-1060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hang CC, Li CS, Wu CJ, Yang J. Acute kidney injury after cardiac arrest of ventricular fibrillation and asphyxiation swine model. Am J Emerg Med. 2014;32:208–15. doi: 10.1016/j.ajem.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Lerolle N, Guérot E, Faisy C, Bornstain C, Diehl JL, Fagon JY. Renal failure in septic shock: Predictive value of Doppler-based renal arterial resistive index. Intensive Care Med. 2006;32:1553–9. doi: 10.1007/s00134-006-0360-x. [DOI] [PubMed] [Google Scholar]
- 20.Burne-Taney MJ, Kofler J, Yokota N, Weisfeldt M, Traystman RJ, Rabb H. Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am J Physiol Renal Physiol. 2003;285:F87–94. doi: 10.1152/ajprenal.00026.2003. [DOI] [PubMed] [Google Scholar]
- 21.Cerchiari EL, Safar P, Klein E, Diven W. Visceral, hematologic and bacteriologic changes and neurologic outcome after cardiac arrest in dogs. The visceral post-resuscitation syndrome. Resuscitation. 1993;25:119–36. doi: 10.1016/0300-9572(93)90090-d. [DOI] [PubMed] [Google Scholar]
- 22.Hutchens MP, Nakano T, Kosaka Y, Dunlap J, Zhang W, Herson PS, et al. Estrogen is renoprotective via a nonreceptor-dependent mechanism after cardiac arrest in vivo. Anesthesiology. 2010;112:395–405. doi: 10.1097/ALN.0b013e3181c98da9. [DOI] [PMC free article] [PubMed] [Google Scholar]


