Abstract
Background:
Na+/Ca2+ exchanger (NCX) plays a crucial role in pentylenetetrazol-induced convulsion. However, it is unclear whether NCX is critically involved in hyperthermia-induced convulsion. In this study, we examined the potential changes in NCX3 in the hippocampus and cerebrocortex of rats with hyperthermia-induced convulsion.
Methods:
Twenty-one Sprague Dawley rats were randomly assigned to control group, convulsion-prone group and convulsion-resistant group (n = 7 in each group). Whole-cell patch-clamp method was used to record NCX currents. Both the Western blotting analysis and immunofluorescence labeling techniques were used to examine the expression of NCX3.
Results:
NCX currents were decreased in rats after febrile convulsion. Compared to the control group, NCX3 expression was decreased by about 40% and 50% in the hippocampus and cerebrocortex of convulsion-prone rats, respectively. Furthermore, the extent of reduction in NCX3 expression seemed to correlate with the number of seizures.
Conclusions:
There is a significant reduction in NCX3 expression in rats with febrile convulsions. Our findings also indicate a potential link between NCX3 expression, febrile convulsion in early childhood, and adult onset of epilepsy.
Keywords: Cerebrocortex, Febrile Convulsion, Hippocampus, Hyperthermia, Na+/Ca2+ Exchanger 3
INTRODUCTION
Febrile convulsion usually occurs in infants and young children. Nearly 2–5% of individuals below the age of 5 years’ experience febrile convulsion.[1,2] Although most children do not develop any adverse neural sequelae after experiencing a febrile seizure, epidemiological studies indicate that 30–70% of people with temporal lobe epilepsy (TLE) have febrile convulsions in their early life.[3,4,5] Multiple studies have investigated hyperthermia-induced seizure in animal models. These studies have reported that cell loss, inflammation, and altered patterns of gene expression are responsible for the development of epileptogenesis.[6,7,8] However, researchers still do not know the mechanism through which epilepsy develops in patients who have experienced febrile seizures in their early life.
The Na+/Ca2+ exchanger (NCX) consists of three isoforms: NCX1, NCX2, and NCX3. NCX1 is ubiquitously expressed, whereas NCX2 and NCX3 are strongly expressed in the brain and skeletal muscle, respectively.[9] Previous studies have found that these NCX isoforms are involved in the development of a variety of diseases associated with the central nervous system, including brain ischemia, multiple sclerosis, Alzheimer's disease, and Parkinson's disease.[10,11,12] Calcium homeostasis is a critical factor that governs neuronal excitability and sensitivity of seizures.[13] Furthermore, NCX is a major mechanism that maintains a low level of Ca2+ inside neurons that have undergone intense electrical activity.[14]
NCX3 is the most abundant isoform in the oriens and radiatum layers of the hippocampal area, CA1.[15] Furthermore, NCX3 is involved in mesial temporal sclerosis, a condition that manifests in many TLE patients who have experienced febrile convulsions in their early childhood.[16] In this study, we determined NCX currents (INCX) and NCX3 expression after inducing febrile convulsion in young rats. We hypothesized that reduction in NCX3 protein and frequent occurrence of febrile convulsion play a pivotal role in the development of TLE.
METHODS
Febrile convulsion
Twenty-one timed-pregnant Sprague Dawley rats were obtained from the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology, China. They were exposed to 12 h light/dark cycle. Moreover, they had unrestricted access to standard rodent chow and water. The day of their birth was designated as postnatal day 0 (P0).
Febrile convulsion was elicited by immersion in warm water, as previously described.[17] Briefly, on postnatal day 20 (P20), pups were placed in a glass jar containing warm water (44.5°C). The water level in the jar was such that the animals could stand upright with only their heads above the water level for 5 min or until they suffered a seizure. This session was conducted twice a day for 5 consecutive days. Subjects that experienced seizures in their first session were defined as convulsion-prone rats (named as HT+FC group, n = 7), whereas subjects that did not develop seizures in their first session were termed as convulsion-resistant rats (named as HT–FC group, n = 7). The latency of seizure was 4.39 ± 0.08 min, a parameter that was measured from the moment the rats were placed in water till they first showed the signs of a seizure. The seizure duration was 5.38 ± 0.07 min; this parameter was measured from the moment the rat showed signs of seizure to the instant when the rat regained consciousness. At the onset of seizure, the core temperature of rats was 41.87 ± 0.06°C. The rats of the control group (named as NT group, n = 7) were exposed to water at a temperature of 37°C.
Cell preparation
Rats were sacrificed by decapitation after their last behavioral episode. Then, we rapidly dissected the hippocampus and cerebrocortex of sacrificed rats. The cells were treated with 0.05% trypsin and then they were fragmented with a fire-polished Pasteur pipette. The digestion was terminated by adding 5% bovine serum albumin. Cells were centrifuged at 1000 ×g for 5 min. The cell pellets were re-suspended in phosphate buffered saline.
Whole-cell patch-clamp recording
Whole-cell patch-clamp recording (INCX) was carried out at 20–22°C using a PC–2C patch–clamp amplifier (Yibo life science instrument limited corporation, China).[2] The external solution contained the following reagents: NaCl (140 mmol/L), CsCl (5 mmol/L), CaCl2 (2 mmol/L), MgCl2 (1 mmol/L), HEPES (15 mmol/L) (pH = 7.4), and glucose (10 mmol/L). The pipette solution contained the following reagents: CsCl (110 mmol/L), K-gluconate (20 mmol/L), NaCl (20 mmol/L), CaCl2 (0.1 mmol/L), MgCl2 (4 mmol/L), HEPES (10 mmol/L) (pH = 7.1), and glucose (5 mmol/L). The holding potential was −40 mV: This applied potential blocked the current induced by contaminating Ca2+ and Na+ ions. INCX was defined as the Ni2+ -sensitive current of slow pulses that were produced through an applied voltage of +100 mV (reverse mode) and −100 mV (forward mode) in 10-mV steps for 300 ms at an alternating frequency of 0.5 Hz.
Western blotting analysis
Protein was extracted from the tissue using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, China). The protein concentration was measured by bicinchoninic acid assay. Briefly, 60 μg of proteins were separated from their samples by performing 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then they were transferred to a membrane made from polyvinylidene fluoride. The blots were blocked with 5% nonfat milk in tris-buffered saline. Finally, they were incubated overnight with a primary anti-NCX3 antibody (1:200 dilution; Biorbyt, UK). The membrane was then incubated with a secondary antibody (1:2000−1:4000 dilution) and visualized using a standard ECL plus kit (Pierce Biotech Inc., USA). β-actin was used as the loading control for the total protein. A P20 sample was included in each gel as a control (100%).
Immunofluorescence labeling
Sections were processed by performing deparaffinization, rehydration, antigen retrieval, and nonspecific antigen site blocking. Then, these sections were incubated overnight at 4°C with anti-NCX3 antibody (1:40). Thereafter, they were fluorescently tagged with a secondary antibody for 1 h. We did not include a negative control by incubating a section with a primary antibody. Images were captured using a laser scanning confocal microscope (Nikon corporation, Japan).
Statistical analysis
Results are expressed as a mean ± standard error (SE). These results were statistical analyzed using unpaired Student's t-test. Statistical significance was set at P < 0.05. SPSS version 17.0 (SPSS Inc., USA) was used for statistical analysis. To statistically compare the forward and reverse modes of INCX, we chose currents at −100 mV and +100 mV, respectively.
RESULTS
Decreased INCX in hippocampal and cortical neurons in convulsion-prone rats
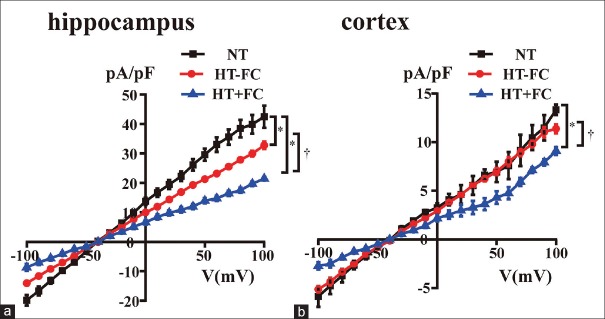
To understand the biophysical properties of neurons in the hippocampus and cortex, INCX was recorded in the pyramidal neurons of median size, which were observed under the electron microscope. We observed the neurons of rats included in the NT, HT−FC, and HT + FC groups. Furthermore, I–V relationship curves of normal pyramidal neurons were recorded at a holding potential of −40 mV, following an extracellular application of 5 mmol/L NiCl2. A dramatic reduction in current amplitudes was observed after 5 min owing to the INCX existing in pyramidal neurons of the hippocampus and cortex. Compared with the INCX density in convulsion-resistant rats and control rats, the current density in the neurons of convulsion-prone rats was significantly lower. We also noticed a significant change in the current density of hippocampal neurons; however, there was no significant change in the current density of cortical neurons of convulsion-resistant rats and control rats [Figure 1a and 1b].
Figure 1.
INCX in hippocampal and cortical neurons recorded by patch-clamp technique (a) hippocampus, (b) cerebrocortex. n = 7. *P < 0.05 versus NT, †P < 0.05 versus HT−FC.
Reduction of NCX3 expression in the hippocampus and cerebrocortex of convulsion-prone rats
Western blotting analysis revealed that NCX3 expression was lower in the hippocampus and cerebrocortex of convulsion-prone rats than that exhibited by either convulsion-resistant rats or control rats [Figure 2]. Compared to control rats, NCX3 expression was lower by about 40% and 50% in the hippocampus and cerebrocortex of convulsion-prone rats, respectively. There was no significant difference between convulsion-resistant rats and control rats. Immunofluorescence labeling revealed that NCX3 isoform was mainly present in the membrane of neurons located in both the hippocampus and cerebrocortex area of the brain [Figure 3a]. As the number of seizures increased, the decline in the expression of NCX3 seemed to accelerate in convulsion-prone rats [Figure 3b-e].
Figure 2.
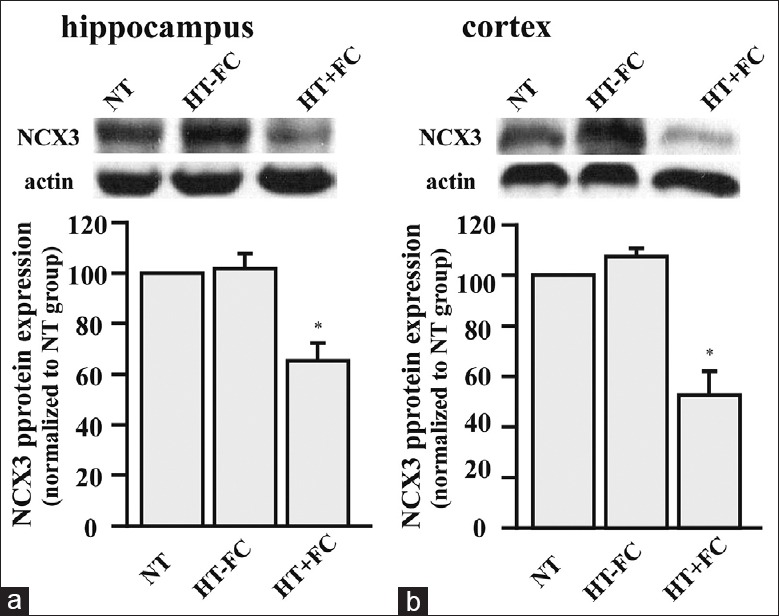
Na+/Ca2+ exchanger 3 expression detected by Western blotting analysis. (a) Hippocampus. (b) Cerebrocortex. β-actin was used as a loading control. n = 7. *P < 0.05 versus NT.
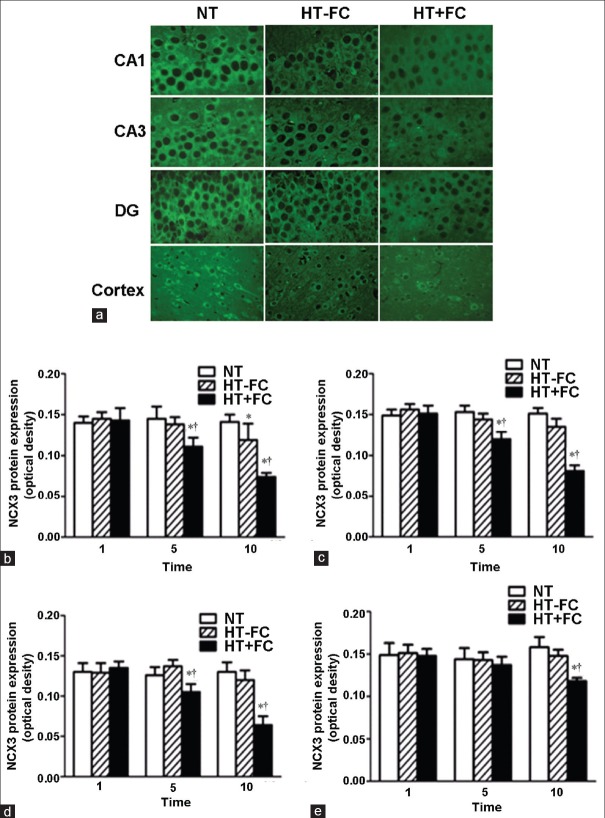
Figure 3.
Immunofluorescence examination of Na+/Ca2+ exchanger 3 expression in rats with varying number of hyperthermia episodes (1, 5, or 10). Na+/Ca2+ exchanger 3 protein appeared as green signals in the neuronal membrane. (a) Representative immunofluorescence images after 10 hyperthermia episodes. (b) Na+/Ca2+ exchanger 3 expression in the CA1 region. (c) Na+/Ca2+ exchanger 3 expression in the CA3 region. (d) Na+/Ca2+ exchanger 3 expression in the dentate gyrus region. (e) Na+/Ca2+ exchanger 3 expression in the cerebrocortex. n = 7. *P < 0.05 versus NT group, †P < 0.05 versus HT−FC group.
In CA1, CA3, and dentate gyrus areas, we found that the expression of NCX3 decreased in patients who had experienced more than five seizures [Figure 3b-d]. In the cerebrocortex, the reduction of NCX3 was more pronounced in patients who had suffered more than 10 seizures [Figure 3e]. NCX3 expression in hippocampus and cerebrocortex areas of the brain was detected by Western blotting analysis. NCX3 expression was not found to be different between convulsion-resistant rats and control rats [Figure 2]. However, immunofluorescense labeling detected a significant decline in NCX3 expression in neurons of the hippocampal CA1 region [Figure 3a].
DISCUSSION
NCX3 is a critical protein that is involved in neural degeneration. The NCX3 protein levels were reduced in patients with Alzheimer's disease,[13] and a calcium overload was caused by the reversal of NCX currents in patients with multiple sclerosis.[18] In this study, we reported that recurring febrile convulsion reduced the expression of NCX3 in the neurons of cerebrocortex and hippocampus area in the brain. Moreover, the downregulation of NCX3 can be correlated with the number of febrile convulsions. Our findings suggest that NCX3 participates in brain damage in patients who experience febrile convulsion.
Cell death may occur due to intracellular calcium overload that is caused by pathological conditions.[19] There is an excessive calcium influx in neurons of patients suffering from various diseases of the central nervous system, including epilepsy. For example, an over-stimulation of NMDA receptor triggers calcium influx, which causes neuronal injury.[20] NCXs maintain calcium homeostasis by removing excessive calcium released during neuronal discharge. In mice subjected to global cerebral ischemia, the NCX3 gene gets silenced in neurons. As a result, there is an increase in intracellular calcium.[21] Our results have shown that hyperthermia can reduce the expression of NCX3 gene [Figures 2 and 3], leading to a decrease in INCX [Figure 1a]. It will induce the overload of intracellular calcium.
Nearly, 40% adult patients with TLE have experienced febrile convulsion in their early childhood.[22] Moreover, there is a correlation between complex febrile convulsion that occur in early childhood and TLE that manifests in the adult stage of life. However, the link between childhood febrile convulsion and TLE is still controversial.[23]
In a recent report, researchers have proved that in rats with hyperthermia-induced seizures, γ-aminobutyric acid A receptors can be up-regulated, which induce ectopic granule cells to cause epilepsy in adulthood.[24] Hyperthermia-induced seizures can significantly modify neuronal excitability in limbic circuits.[25] A significant increase in neuronal excitability can ultimately lead to the development of epilepsy.
A reduction in the expression of NCX3 leads to an increase in intracellular calcium, stimulating neuronal excitability and lowering seizure threshold. In addition, calcium overload can activate calcium-dependent apoptosis signal. Due to apoptosis, there is a neuronal loss that adversely affects the structure and function of the brain. This may ultimately lead to hippocampus atrophy and sclerosis in TLE patients.
Calpain cleaves NCX when it is activated by neuronal injury.[26] In previous studies conducted on other seizure models, researchers have proved that NCX3 is more sensitive to calpain cleavage.[27] This indicates that NCX3 has a close relationship with neuronal degeneration, which is caused by excitotoxicity.
In conclusion, our results have proved that there is a significant reduction in NCX3 expression and INCX in neurons of rats with febrile convulsion. This finding provides a potential mechanistic link between complex febrile convulsion and TLE. The results also indicate that NCX3 can be used as a target in the treatment of febrile convulsions in order to prevent the subsequent emergence of TLE.
Financial support and sponsorship
This study was supported by grants from the Natural Science Foundation Project of Hubei Province (No. 2004ABA234), Wuhan Morning Program Project (No. 200850731390), and Project of Wuhan Clinical Medical Research Center in Children's Neural Disease (No. 2014-160).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Huang CC, Wang ST, Chang YC, Huang MC, Chi YC, Tsai JJ. Risk factors for a first febrile convulsion in children: A population study in southern Taiwan. Epilepsia. 1999;40:719–25. doi: 10.1111/j.1528-1157.1999.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 2.Østergaard JR. Febrile seizures. Acta Paediatr. 2009;98:771–3. doi: 10.1111/j.1651-2227.2009.01200.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuks JB, Cook MJ, Fish DR, Stevens JM, Shorvon SD. Hippocampal sclerosis in epilepsy and childhood febrile seizures. Lancet. 1993;342:1391–4. doi: 10.1016/0140-6736(93)92754-h. [DOI] [PubMed] [Google Scholar]
- 4.Blair RD. Temporal lobe epilepsy semiology. Epilepsy Res Treat 2012. 2012 doi: 10.1155/2012/751510. 751510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cendes F, Andermann F, Dubeau F, Gloor P, Evans A, Jones-Gotman M, et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: An MRI volumetric study. Neurology. 1993;43:1083–7. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- 6.de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–95. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 7.Reid AY, Galic MA, Teskey GC, Pittman QJ. Febrile seizures: Current views and investigations. Can J Neurol Sci. 2009;36:679–86. doi: 10.1017/s0317167100008246. [DOI] [PubMed] [Google Scholar]
- 8.Brewster A, Bender RA, Chen Y, Dube C, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591–9. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quednau BD, Nicoll DA, Philipson KD. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am J Physiol. 1997;272(4 Pt 1):C1250–61. doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]
- 10.Pignataro G, Esposito E, Cuomo O, Sirabella R, Boscia F, Guida N, et al. The NCX3 isoform of the Na+/Ca2+ exchanger contributes to neuroprotection elicited by ischemic postconditioning. J Cereb Blood Flow Metab. 2011;31:362–70. doi: 10.1038/jcbfm.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokolow S, Luu SH, Headley AJ, Hanson AY, Kim T, Miller CA, et al. High levels of synaptosomal Na(+)-Ca(2+) exchangers (NCX1, NCX2, NCX3) co-localized with amyloid-beta in human cerebral cortex affected by Alzheimer's disease. Cell Calcium. 2011;49:208–16. doi: 10.1016/j.ceca.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pignataro G, Cuomo O, Vinciguerra A, Sirabella R, Esposito E, Boscia F, et al. NCX as a key player in the neuroprotection exerted by ischemic preconditioning and postconditioning. Adv Exp Med Biol. 2013;961:223–40. doi: 10.1007/978-1-4614-4756-6_19. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto EM, Vivar C, Camandola S. Physiology and pathology of calcium signaling in the brain. Front Pharmacol. 2012;3:61. doi: 10.3389/fphar.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annunziato L, Pignataro G, Di Renzo GF. Pharmacology of brain Na+/Ca2+ exchanger: From molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–54. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- 15.Molinaro P, Viggiano D, Nisticò R, Sirabella R, Secondo A, Boscia F, et al. Na+-Ca2+ exchanger (NCX3) knock-out mice display an impairment in hippocampal long-term potentiation and spatial learning and memory. J Neurosci. 2011;31:7312–21. doi: 10.1523/JNEUROSCI.6296-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marucci G, Giulioni M, Rubboli G, Paradisi M, Fernández M, Del Vecchio G, et al. Neurogenesis in temporal lobe epilepsy: Relationship between histological findings and changes in dentate gyrus proliferative properties. Clin Neurol Neurosurg. 2013;115:187–91. doi: 10.1016/j.clineuro.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W, Duong TM, de Lanerolle NC. The neuropathology of hyperthermic seizures in the rat. Epilepsia. 1999;40:5–19. doi: 10.1111/j.1528-1157.1999.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 18.Kurnellas MP, Donahue KC, Elkabes S. Mechanisms of neuronal damage in multiple sclerosis and its animal models: Role of calcium pumps and exchangers. Biochem Soc Trans. 2007;35(Pt 5):923–6. doi: 10.1042/BST0350923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316:493–8. doi: 10.1056/NEJM198702263160901. [DOI] [PubMed] [Google Scholar]
- 20.Cross JH. Fever and fever-related epilepsies. Epilepsia. 2012;53(Suppl 4):3–8. doi: 10.1111/j.1528-1167.2012.03608.x. [DOI] [PubMed] [Google Scholar]
- 21.Koyama R, Tao K, Sasaki T, Ichikawa J, Miyamoto D, Muramatsu R, et al. GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy. Nat Med. 2012;18:1271–8. doi: 10.1038/nm.2850. [DOI] [PubMed] [Google Scholar]
- 22.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 23.Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–10. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- 24.Jeffs GJ, Meloni BP, Sokolow S, Herchuelz A, Schurmans S, Knuckey NW. NCX3 knockout mice exhibit increased hippocampal CA1 and CA2 neuronal damage compared to wild-type mice following global cerebral ischemia. Exp Neurol. 2008;210:268–73. doi: 10.1016/j.expneurol.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–94. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–85. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 27.Brustovetsky T, Bolshakov A, Brustovetsky N. Calpain activation and Na+/Ca2+ exchanger degradation occur downstream of calcium deregulation in hippocampal neurons exposed to excitotoxic glutamate. J Neurosci Res. 2010;88:1317–28. doi: 10.1002/jnr.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]