Abstract
Background:
Hyperbaric oxygen (HBO) and Ginkgo biloba extract (e.g., EGB 761) were shown to ameliorate cognitive and memory impairment in Alzheimer's disease (AD). However, the exact mechanism remains elusive. The aim of the present study was to investigate the possible mechanisms of HBO and EGB 761 via the function of nuclear factor kappa-B (NF-κB) pathway.
Methods:
AD rats were induced by injecting β-amyloid 25–35 into the hippocampus. All animals were divided into six groups: Normal, sham, AD model, HBO (2 atmosphere absolute; 60 min/d), EGB 761 (20 mg·kg−1·d−1), and HBO/EGB 761 groups. Morris water maze tests were used to assess cognitive, and memory capacities of rats; TdT-mediated dUTP Nick-End Labeling staining and Western blotting were used to analyze apoptosis and NF-κB pathway-related proteins in hippocampus tissues.
Results:
Morris water maze tests revealed that EGB 761 and HBO significantly improved the cognitive and memory ability of AD rats. In addition, the protective effect of combinational therapy (HBO/EGB 761) was superior to either HBO or EGB 761 alone. In line, reduced apoptosis with NF-κB pathway activation was observed in hippocampus neurons treated by HBO and EGB 761.
Conclusions:
Our results suggested that HBO and EGB 761 improve cognitive and memory capacity in a rat model of AD. The protective effects are associated with the reduced apoptosis with NF-κB pathway activation in hippocampus neurons.
Keywords: Ginkgo Biloba Extract 761, Hyperbaric Oxygen, Nuclear Factor Kappa-B, Rats, Alzheimer's Disease
INTRODUCTION
Alzheimer's disease (AD) is a devastating neurodegenerative disease characterized by progressive cognitive impairment and psychobehavioral disturbances or language impairment. Accumulating evidence indicated that excess production of β-amyloid (Aβ) occurs early in the disease progression, it may represent a crucial step in AD pathogenesis.[1] Recently, it has been demonstrated that there is a close link between nuclear factor kappa-B (NF-κB) and nerve degeneration diseases such as AD, Parkinson's disease (PD), and Huntington's disease (HD).[2] In the early stage, the activation of NF-κB pathway with deposition of amyloid was observed in neurons and astrocytes in human ADs.[3] However, the exact function of NF-κB activation in such context remains unclear.
The concentrated extracts of Ginkgo biloba leaves (EGB 761) have been found to protect against various neural and vascular damages.[4,5] EGB 761 is a well-known anti-oxidant which inhibits Aβ-derived fibril formation and apoptosis. In addition, the herb can modulate brain cholinergic transmission, increase brain cholinergic activity, and normalize the acetylcholine receptors in the hippocampus area.[6] Hyperbaric oxygen (HBO) therapy ameliorates the disease course of a number of human diseases such as hypoxia, ischemia, and reperfusion. Further, recent studies have shown that the HBO therapy reduces spinal cord injury (SCI) and facilitates recovery of neurological functions.[7,8] In this regard, it has been reported that HBO exerts its protective effect through TLR2/NF-κB pathway inhibition and consequent suppression of pro-inflammatory cytokine release.[9] In this study, we analyzed the biological effects of Aβ25–35 on neurons, which is an active fragment of toxic Aβ (Aβ1–40 and Aβ1–42).[10] Further, we investigated the potential role of NF-κB in mediating protective effects of HBO/EGB 761 against cognitive and memory impairment using a rat model of AD.
METHODS
Animals
Seventy-two male Sprague-Dawley rats (age: 5–6 months and weight: 250–350 g) were obtained from Shanghai Laboratory Animal Center. The animals were acclimatized for 7 days at 23°C with a 12-h light-dark cycle and allowed free access to drinking water and pellet diet. All animal experiments were evaluated and approved by the Animal and Ethics Review Committee of the Southeast University (Nanjing, Jiangsu, China), and all efforts were made to minimize the number of used animals as well as their suffering.
Preparation of β-amyloid 25–35 and Ginkgo biloba extract 761
Aβ25–35 (A4559, Sigma-Aldrich, USA) was resolved in sterile saline solution at 37°C for 7 days for the preparation of aggregated Aβ25–35 (1 g/L). EGB 761 (40 mg/tablet, German pharmaceutical company, Dr. Weimashupei, Germany) was prepared in drinking water (20 mg/ml).
Injection of aggregated β-amyloid 25–35
In brief, all animals were anesthetized by intraperitoneal injection of chloral hydrate (300 mg/kg; Tianjin Chemical Reagent Development Center, China). Surgery and injection of Aβ25–35 was carried out as previously described.[11] The aggregated Aβ25–35 (10 μl; 1 μl/min) was injected into bilateral hippocampus. The sham control was injected with the same amount of saline. Postoperatively, animals were treated with prophylactic antibiotics (penicillin; 80,000 unit/0.25 ml) for 3 days. After 3 weeks, animals were randomly assigned into six groups (n = 12/each group): Normal, sham, AD model, HBO, EGB 761, and HBO/EGB 761 groups according to different treatment regiments (see below).
Hyperbaric oxygen and Ginkgo biloba extract 761 administrations
The gavage of EGB 761 was performed in the EGB 761 group as previously described.[12] For HBO treatment in the HBO group, 100% oxygen was administered at a pressure of 2.0 atmosphere absolute in a hyperbaric chamber (SHC3200–8500, Shanghai 701 Yang garden hyperdaric oxygen chamber Co Ltd., China) for 1 h including 15 min of compression and decompression. Depending on experimental design, EGB 761 and HBO was administrated in the HBO/EGB 761 group. The treatment groups (HBO, EGB 761 and HBO/EGB 761) consisted of two courses (10 days per course) with an interval of 3 days between two courses. There was no treatment with the other groups (normal, sham, AD model). Moreover, all of the animals were bred with food pellets and water throughout the experiment.
Morris water maze
The Morris water maze was performed in a circular water tank (160 cm × 50 cm) filled with water to a depth of 25 cm. The tank was divided into four quadrants (I, II, III, and IV). A platform was placed inside the quadrant I. The hidden platform (from day 1 to day 6) and probe trial (on day 7) was performed. For the hidden platform trial, rats were tested twice per day. Specifically, rats were placed in the water at the starting location (quadrants II and III). The trial was considered to be successful when they managed to escape to the platform within 90 s. If a rat failed to escape within 90 s, the escape latency was recorded as 90 s. All rats were allowed to spend 20 s on the platform and returned to their cages. The escape latency, swimming speed, and trajectory were recorded for each rat. For the probe trial, the platform was removed and the starting point was located in quadrant III. Within 90 s, the number of crossings time spent in the quadrant I were record.
TdT-mediated dUTP Nick-End Labeling staining
After trials, rats (n = 6/each group) were immediately anesthetized and perfused with 0.01 mol/L phosphate buffer (pH 7.4), followed by ice-cold 4% paraformaldehyde through the left ventricle. Brains were removed and embedded in paraffin. Sections were taken from each brain for TdT-mediated dUTP Nick-End Labeling (TUNEL) test. The process was conducted according to the manufacturer's instructions of Tunel kit (Nanjing KeyGEN Biotech. Co., Ltd., Jiangsu, China). The data were represented as the apoptotic index (AI = apoptosis/total cellular score × 100%) in the CA1 of hippocampus.
Western blotting analysis
The protein of hippocampus tissue was prepared using tissue isolation kit and measured using BCA kit (Beyotime Biotechnology, Jiangsu, China). Samples of protein (15 mg) were subjected to dodecyl sulfate, sodium salt-polyacrylamide gel electrophoresis and were transferred onto polyvinylidene fluoride membranes. Membranes were blocked and incubated with a set of primary antibodies (anti-p-IKKα, anti-IKKα/β, anti-IκBα, and anti-p-IκBα, Bioss Inc., USA) overnight at 4°C. The blots were visualized using an ECL detection kit (GE Healthcare, Germany). A densitometric analysis was performed.
Statistical analysis
Data were shown as mean ± standard deviation (SD). Statistical analysis was performed using one-way or repeated measures analysis of variance (ANOVA), followed by Turkey's post-hoc analysis for multiple comparisons and t-test for dependent samples using SPSS 11.5 (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Hyperbaric oxygen/Ginkgo biloba extract 761 improves cognitive and memory capacities in rat model of Alzheimer's disease
Hidden platform trail
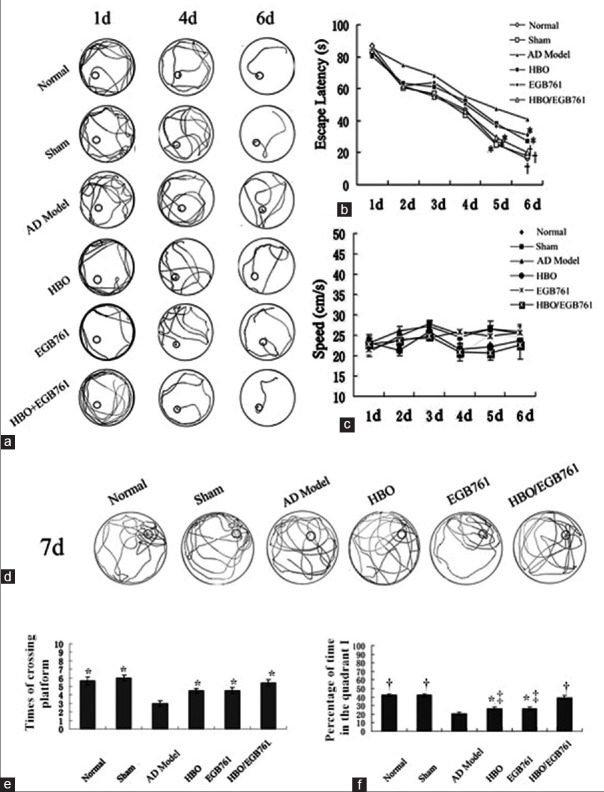
In the hidden platform trial, rats swam along pool wall in the 1st day. However, on the 4th day, the searching behavior performed the cognitive task. In the 6th day, rats almost had a clear goal, their trajectories were nearly single-type [Figure 1a]. The escape latency of HBO/EGB 761 group decreased significantly comparing with AD model group in the 5th day [HBO/EGB 761 group: 22.70 ± 3.08 s vs. AD model: 44.55 ± 3.74 s; F = 25.968, P < 0.05; Figure 1b]. Moreover, in the 6th day, the escape latency decreased significantly in the treatment group (HBO, EGB 761, and HBO/EGB 761 groups) compared to AD model group (HBO: 23.88 ± 3.08 s vs. AD model: 40.55 ± 3.74 s, F = 16.671, P < 0.05; EGB 761: 24.44 ± 2.78 s vs. AD model: 40.55 ± 3.74 s, F = 18.428, P < 0.05; HBO/EGB 761: 19.70 ± 3.08 s vs. AD model: 40.55 ± 3.74 s, F = 69.692, P < 0.01). Moreover, HBO/EGB 761 group had superior effects. No significant difference was observed in the swim speed in the six continuous Morris water maze test [F = 0.886, P > 0.05; [Figure 1c].
Figure 1.
Hyperbaric oxygen (HBO)/Ginkgo biloba extract 761 (EGB 761) improved cognitive and memory capacities of Alzheimer's disease rats. (a) The swimming trajectories of each group in the 6 consecutive training days. (b) The escape latency of each group in the 6 consecutive training days. (c) The swimming speed of each group in 6 consecutive training days. (d) The swimming trajectories of each group in the 7th day. (e) The times of crossing the former platform position of each group. (f) Percentage of time spent in the quadrant I. *P < 0.05, †P < 0.01, versus AD model group; ‡P < 0.05, versus HBO/EGB 761 group.
Probe trail
In the 7th day, rats’ swimming trajectory was depicted in Figure 1d–1f. The trajectory was around the position of quadrant I. Compared with AD model group, HBO, EGB 761, and HBO/EGB 761 groups had longer time in quadrant I to cross the platform [HBO: 4.20 ± 0.29 s vs. AD model: 3.00 ± 0.30 s, F = 8.308, P < 0.05; EGB 761: 4.60 ± 0.48 s vs. AD model: 3.00 ± 0.30 s, F = 8.113, P < 0.05; HBO/EGB 761: 4.30 ± 0.37 s vs. AD model: 3.00 ± 0.30 s, F = 8.308, P < 0.05; Figure 1e]. The percentage of time in quadrant I increased significantly in HBO, EGB 761, and HBO/EGB 761 groups comparing with AD model group [HBO: 26.50 ± 1.60% vs. AD model: 20.33 ± 1.78%, F = 6.621, P < 0.05; EGB 761: 26.50 ± 1.70% vs. AD model: 20.33 ± 1.78%, F = 6.218, P < 0.05; HBO/EGB 761: 41.70 ± 1.94% vs. AD model: 20.33 ± 1.78%, F = 64.26, P < 0.01; Figure 1f]. In this regard, HBO and EGB 761 had synergistic effects (HBO/EGB 761: 41.70 ± 1.94% vs. EGB 761: 26.50 ± 1.70%, F = 36.18, P < 0.05; HBO/EGB 761: 41.70 ± 1.94% vs. HBO: 26.50 ± 1.60%, F = 34.392, P < 0.05).
Hyperbaric oxygen/Ginkgo biloba extract 761 reduces apoptosis
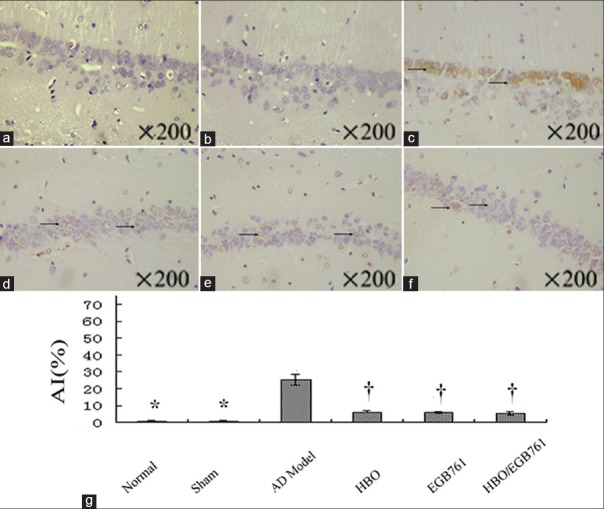
In TUNEL staining [Figure 2a–2f], no positive staining was observed in normal and sham groups, however, a significant amount of positively stained cells was observed in AD model group. In HBO, EGB 761, and HBO/EGB 761 groups, normal hippocampus neurons were surrounded by sporadically positive cells. The AI calculation uncovered a significant difference among these groups [HBO: 5.35 ± 0.25% vs. AD model: 23.50 ± 1.41%, F = 160.110, P < 0.05; EGB 761: 5.19 ± 0.31% vs. AD model: 23.50 ± 1.41%, F = 160.960, P < 0.05; HBO/EGB 761: 4.29 ± 0.44% vs. AD model: 20.33 ± 1.78%, F = 64.260, P < 0.05; Figure 2g].
Figure 2.
Hyperbaric oxygen (HBO)/Ginkgo biloba extract 761 (EGB 761) reduces apoptosis (a–f: TUNEL staining). (a and b) There was no brown precipitate (apoptosis identification) in normal and sham groups. (c) The large visible brown precipitate (arrows) was detected in the AD model group. (d–f) The normal hippocampal neurons were surrounded by the sporadic visible brown precipitate (arrows) in the HBO; EGB 761 and HBO/EGB 761 groups. (g) The apoptotic index showed the significant difference among different groups: AD Model, HBO, EGB 761 and HBO/EGB 761 groups. *P < 0.01, †P < 0.05, versus AD model group. AI: Apoptotic index.
Hyperbaric oxygen/Ginkgo biloba extract 761 activates nuclear factor kappa-B pathway
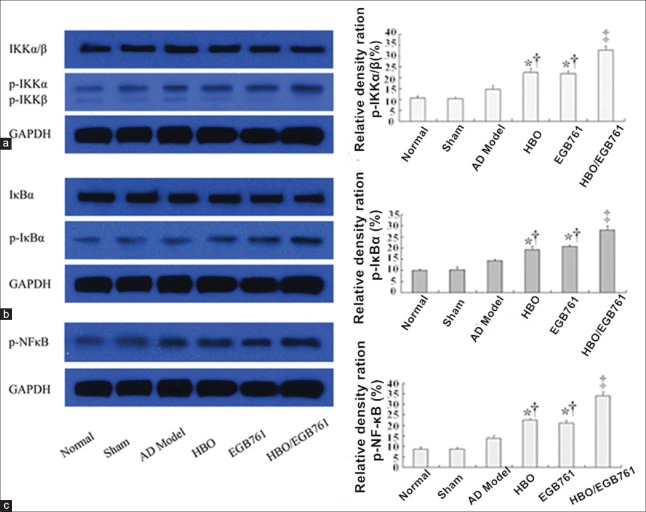
In addition, we went on to investigate whether NF-κB pathway is activated in our models [Figure 3]. This analysis revealed that NF-κB pathway-related proteins such as phospho-IKKα, -IκBα, and NF-κB were slightly induced in model group. However, this difference did not reach statistical significance comparing with the normal, sham group (p-IKKα: Normal: 13.40 ± 1.43% vs. AD model: 11.36 ± 0.35%, F = 0.081, P > 0.05; sham: 11.78 ± 0.37% vs. AD model: 11.36 ± 0.35%, F = 4.050, P > 0.05. p-IκBα: normal: 10.36 ± 0.34% vs. AD model: 11.36 ± 0.81%, F = 0.820, P > 0.05; sham: 10.92 ± 0.84% vs. AD model: 11.36 ± 0.81%, F = 0.640, P > 0.05. NF-κB: normal: 10.64 ± 0.34% vs. AD model: 11.34 ± 0.25%, F = 2.690, P > 0.05; sham: 10.24 ± 0.61% vs. AD model: 11.34 ± 0.25%, F = 0.645, P > 0.05).
Figure 3.
Hyperbaric oxygen (HBO)/Ginkgo biloba extract 761 (EGB 761) activates nuclear factor kappa-B pathway. HBO and EGB 761 significantly increased phosphorylation of IKKα/β (a), IκBα (b), and nuclear factor kappa-B (c). *P < 0.05, ‡P < 0.01, versus AD model group; †P < 0.05, versus HBO/EGB 761 group.
Interestingly, HBO and EGB 761 significantly activated NF-κB pathway in hippocampus neurons. As shown in Figure 3, comparing with the normal, sham and model groups, the expression of NF-κB-related proteins were significantly increased in HBO, EGB 761, and HBO/EGB 761 groups (p-IKKα: HBO: 15.92 ± 0.52% vs. AD model: 11.36 ± 0.359%, F = 52.780, P < 0.05; EGB 761: 16.02 ± 0.35% vs. AD model: 11.36 ± 0.35%, F = 86.230, P < 0.05; HBO/EGB 761: 23.92 ± 0.38% vs. AD model: 11.36 ± 0.35%, F = 576.300, P < 0.01. p-IκBα: HBO: 14.18 ± 0.09% vs. AD model: 11.36 ± 0.35%, F = 60.810, P < 0.05; EGB 761: 14.28 ± 0.14% vs. AD model: 11.36 ± 0.35%, F = 56.164, P < 0.05; HBO/EGB 761: 24.41 ± 0.56% vs. AD model: 11.36 ± 0.35%, F = 126.310, P < 0.01. NF-κB: HBO: 15.00 ± 0.19% vs. AD model: 11.34 ± 0.25%, F = 105.100, P < 0.05; EGB 761: 14.64 ± 0.59% vs. AD model: 11.34 ± 0.25%, F = 53.510, P < 0.05; HBO/EGB 761: 25.01 ± 0.38% vs. AD model: 11.34 ± 0.25% F = 709.690, P < 0.01).
DISCUSSION
Aging, as a risk factor for AD, has been widely investigated. The aging of the nervous system is a physiological process characterized by chronic apoptosis of functional neurons. It is widely accepted that the extracellular accumulation of Aβ in senile plaques is an important event in the pathogenesis of AD.[13] The aggregated Aβ is toxic to neurons both in vitro and in vivo[14] and the overexpression of human amyloid precursor protein is observed in transgenic mouse models of AD,[15] which causes neuritic plaques similar to those seen in AD patients with learning and memory deficits.[16] The transgenic APP mice demonstrated a cognitive decline and were well accepted as very successful AD model.[17,18] Meanwhile, soluble Aβ oligomers can inhibit cognitive function[19] and long-term potentiation in vitro and in vivo.[20] Moreover, the animal models where cognitive impairment and neuropathological signs are induced by intracerebral injection of pre-aggregated Aβ-oligomers are useful models for the early stages of AD,[21] which were used as a good AD model in many studies.[22,23,24]
Our results showed that the excessive deposition of Aβ caused apoptosis in hippocampus neurons, resulting in impairment of memory and learning ability in a rat model of AD. In the interventional groups, HBO/EGB 761 treatment significantly improved the memory and cognitive capacities of AD rats, and they reduced apoptosis in hippocampus neurons. In this respect, HBO and EGB 761 had a synergistic effect. Moreover, we observed that HBO/EGB 761 treatment activated NF-κB pathway in hippocampus neurons. These data was in line with previously published results showing that the activation of NF-κB pathway may have a protective role in AD.[25]
Several studies have demonstrated a correlational link between NF-κB activity and neurodegenerative diseases such as AD, PD, and HD. They proposed that NF-κB activation in neurons had a neuroprotective role in the degenerative process of these diseases.[26] In addition, NF-κB may be involved in the gene long-term memory changing from short-term memory coming from the synaptic signals, which have an important role in signal transduction, memory formation, and neural modeling.[27] Meanwhile, in the pathological conditions, the activation of NF-κB promotes survival neuron survival. For example, NF-κB inhibits apoptosis through regulating apoptosis protein (1APs), anti-oxidative enzymes and inhibition of mitochondrial depolarization, membrane permeability changes, and cytochrome C release.[28] However, there are also evidences indicating that it may cause neuronal death. In TG2576 transgenic mice, the increased NF-κB activation and apoptosis was found in the same cells, implying that NF-κB activation may increase neuronal apoptosis.[29]
Taken together, these findings demonstrated that HBO/EGB 761 ameliorates the cognitive and memory impairment in rat model of AD. The protective effects of HBO/EGB 761 are associated with a reduced apoptosis and NF-κB pathway activation in hippocampus neurons.
Financial support and sponsorship
The study was supported by a grant from the Key Development Project of Medical Science and Technology of Nanjing (No. ZKX13034).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Chauhan V, Chauhan A. Oxidative stress in Alzheimer's disease. Pathophysiology. 2006;13:195–208. doi: 10.1016/j.pathophys.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Mémet S. NF-kappaB functions in the nervous system: From development to disease. Biochem Pharmacol. 2006;72:1180–95. doi: 10.1016/j.bcp.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP. NF-kappaB in the survival and plasticity of neurons. Neurochem Res. 2005;30:883–93. doi: 10.1007/s11064-005-6961-x. [DOI] [PubMed] [Google Scholar]
- 4.Diamond BJ, Shiflett SC, Feiwel N, Matheis RJ, Noskin O, Richards JA, et al. Ginkgo biloba extract: Mechanisms and clinical indications. Arch Phys Med Rehabil. 2000;81:668–78. doi: 10.1016/s0003-9993(00)90052-2. [DOI] [PubMed] [Google Scholar]
- 5.Wang YS, Xu L, Ma K, Wang S, Wang JJ. Protective effects of Ginkgo biloba extract 761 against glutamate-induced neurotoxicity in the cultured retinal neuron. Chin Med J. 2005;118:948–52. [PubMed] [Google Scholar]
- 6.Montes P, Ruiz-Sanchez E, Rojas C, Rojas P. Ginkgo biloba Extract 761: A review of basic studies and potential clinical use in psychiatric disorders. CNS Neurol Disord Drug Targets. 2015;14:132–49. doi: 10.2174/1871527314666150202151440. [DOI] [PubMed] [Google Scholar]
- 7.Cristante AF, Damasceno ML, Barros Filho TE, de Oliveira RP, Marcon RM, da Rocha ID. Evaluation of the effects of hyperbaric oxygen therapy for spinal cord lesion in correlation with the moment of intervention. Spinal Cord. 2012;50:502–6. doi: 10.1038/sc.2012.16. [DOI] [PubMed] [Google Scholar]
- 8.Xiong L, Zhu Z, Dong H, Hu W, Hou L, Chen S. Hyperbaric oxygen preconditioning induces neuroprotection against ischemia in transient, not permanent middle cerebral artery occlusion rat model. Chin Med J. 2000;113:836–9. [PubMed] [Google Scholar]
- 9.Tan J, Zhang F, Liang F, Wang Y, Li Z, Yang J, et al. Protective effects of hyperbaric oxygen treatment against spinal cord injury in rats via toll-like receptor 2/nuclear factor-κB signaling. Int J Clin Exp Pathol. 2014;7:1911–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Hou XY, Liu Y, Zong YY. Different protection of K252a and N-acetyl-L-cysteine against amyloid-beta peptide-induced cortical neuron apoptosis involving inhibition of MLK3-MKK7-JNK3 signal cascades. J Neurosci Res. 2009;87:918–27. doi: 10.1002/jnr.21909. [DOI] [PubMed] [Google Scholar]
- 11.Watson C, Paxinos G. USA: Academic Press; 2004. The Rat Brain in Stereotaxic Coordinates. [DOI] [PubMed] [Google Scholar]
- 12.Huang JH, Huang XH, Chen ZY, Zheng QS, Sun RY. Dose conversion among different animals and healthy volunteers in the pharmacological study (in Chinese) Chin J Clin Pharmacol Ther. 2004;9:1069–72. [Google Scholar]
- 13.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–4. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–64. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 15.Paulson JB, Ramsden M, Forster C, Sherman MA, McGowan E, Ashe KH. Amyloid plaque and neurofibrillary tangle pathology in a regulatable mouse model of Alzheimer's disease. Am J Pathol. 2008;173:762–72. doi: 10.2353/ajpath.2008.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalova IN, Cechalova K, Rehakova Z, Dimitrova P, Ognibene E, Caprioli A, et al. Decrease of dehydrogenase activity of cerebral glyceraldehyde-3-phosphate dehydrogenase in different animal models of Alzheimer's disease. Biochim Biophys Acta. 2007;1770:826–32. doi: 10.1016/j.bbagen.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Liu S, Wang Y, Zhang Q, Zhao W, Wang Z, et al. Effects of curculigoside on memory impairment and bone loss via anti-oxidative character in APP/PS1 mutated transgenic mice. PLoS One. 2015;10:e0133289. doi: 10.1371/journal.pone.0133289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González-Domínguez R, García-Barrera T, Vitorica J, Gómez-Ariza JL. Metabolomic investigation of systemic manifestations associated with Alzheimer's disease in the APP/PS1 transgenic mouse model. Mol Biosyst. 2015;11:2429–40. doi: 10.1039/c4mb00747f. [DOI] [PubMed] [Google Scholar]
- 19.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 20.Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, et al. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–61. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 21.Davis S, Laroche S. What can rodent models tell us about cognitive decline in Alzheimer's disease? Mol Neurobiol. 2003;27:249–76. doi: 10.1385/MN:27:3:249. [DOI] [PubMed] [Google Scholar]
- 22.Fu AL, Zhou CY, Chen X. Thyroid hormone prevents a cognitive deficit in a mouse model of Alzheimer's disease. Neuropharmacology. 2010;58:722–9. doi: 10.1016/j.neuropharm.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, Zhou L, Hou D, Tang J, Sun J, Bondy SC. Paeonol increases levels of cortical cytochrome oxidase and vascular actin and improves behavior in a rat model of Alzheimer's disease. Brain Res. 2011;1388:141–7. doi: 10.1016/j.brainres.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 24.Zhong SZ, Ge QH, Li Q, Qu R, Ma SP. Peoniflorin attentuates Abeta((1-42))-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in the hippocampus of rats. J Neurol Sci. 2009;280:71–8. doi: 10.1016/j.jns.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Cardoso SM, Oliveira CR. Inhibition of NF-kB renders cells more vulnerable to apoptosis induced by amyloid beta peptides. Free Radic Res. 2003;37:967–73. [PubMed] [Google Scholar]
- 26.Smith D, Tweed C, Fernyhough P, Glazner GW. Nuclear factor-kappaB activation in axons and Schwann cells in experimental sciatic nerve injury and its role in modulating axon regeneration: Studies with etanercept. J Neuropathol Exp Neurol. 2009;68:691–700. doi: 10.1097/NEN.0b013e3181a7c14e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freudenthal R, Boccia MM, Acosta GB, Blake MG, Merlo E, Baratti CM, et al. NF-kappaB transcription factor is required for inhibitory avoidance long-term memory in mice. Eur J Neurosci. 2005;21:2845–52. doi: 10.1111/j.1460-9568.2005.04126.x. [DOI] [PubMed] [Google Scholar]
- 28.Sarnico I, Lanzillotta A, Benarese M, Alghisi M, Baiguera C, Battistin L, et al. NF-kappaB dimers in the regulation of neuronal survival. Int Rev Neurobiol. 2009;85:351–62. doi: 10.1016/S0074-7742(09)85024-1. [DOI] [PubMed] [Google Scholar]
- 29.Niu YL, Zhang WJ, Wu P, Liu B, Sun GT, Yu DM, et al. Expression of the apoptosis-related proteins caspase-3 and NF-kappaB in the hippocampus of Tg2576 mice. Neurosci Bull. 2010;26:37–46. doi: 10.1007/s12264-010-6122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]