Abstract
Background:
Autoimmune encephalitis associated with antibodies against γ-aminobutyric acid B receptor (GABAB R) in patients with limbic encephalitis (LE) was first described in 2010. We present a series of Han Chinese patients for further clinical refinement.
Methods:
Serum and cerebrospinal fluid (CSF) samples from patients referred to the program of encephalitis and paraneoplastic syndrome of Peking Union Medical College Hospital were tested with indirect immunofluorescence. Clinical information of patients with anti-GABAB R antibody positivity was retrospectively reviewed, and descriptive statistical analysis was performed.
Results:
All eighteen anti-GABAB R antibody-positive cases had limbic syndromes, and electroencephalogram (EEG) or neuroimaging evidence fulfilled the diagnostic criteria of LE. Four patients had additional antibodies against Hu in serum and one had anti-N-methyl-d-aspartate receptor antibody in both sera and CSF. Seventeen (17/18) patients presented with new-onset refractory seizure or status epileptics. Twelve (12/18) patients had memory deficits, 11 (11/18) patients had personality change, 7 (7/18) patients had disturbance of consciousness, and 3 (3/18) patients showed cerebellar dysfunction. One patient with LE had progressive motor and sensory polyneuropathy. Lung cancer was detected in 6 (6/18) patients. Ten (10/18) patients showed abnormality in bilateral or unilateral mediotemporal region on magnetic resonance imaging. Ten (10/18) patients had temporal lobe epileptic activity with or without general slowing on EEG. Seventeen patients received immunotherapy and 15 of them showed neurological improvement. Four patients with lung cancer died within 1–12 months due to neoplastic complications.
Conclusions:
Our study demonstrates that most Han Chinese patients with anti-GABAB R antibody-associated LE have prominent refractory epilepsy and show neurological improvement on immunotherapy. Patients with underlying lung tumor have a relatively poor prognosis. Testing for anti-GABAB R antibodies is necessary for patients with possible LE or new-onset epilepsy with unknown etiology.
Keywords: Anti-γ-aminobutyric Acid B Receptor Antibody, Autoimmune, Limbic Encephalitis, Seizure
INTRODUCTION
Autoimmune encephalitis associated with autoantibodies to synaptic proteins of neurons is increasingly recognized. Timely diagnosis of these autoimmune diseases is critical for neurological practice because of the effective treatment options.[1,2] Among the spectrum of autoantibodies associated with limbic encephalitis (LE), antibodies to γ-aminobutyric acid B receptor (GABAB R) were first described by Lancaster et al. in 2010.[3] Anti-GABAB R antibodies have been revealed by their discoverers as diagnostic biomarkers and causative autoantibodies in some cases with LE.[3,4,5] GABAB Rs are mainly located in the presynaptic and postsynaptic regions of hippocampus, thalamus and cerebellum. The main function of these receptors is to inhibit the activities of neurons.[6,7,8] In this study, in order to describe characteristics of this rare disorder in Chinese patients, we respectively reviewed the clinical data, cerebrospinal fluid (CSF), electroencephalogram (EEG), magnetic resonance imaging (MRI) findings, and outcomes in patients with anti-GABAB R antibody-associated LE.
METHODS
Patients and registrations
The patients with encephalitis of unknown etiology, or suspected autoimmune encephalitis, or suspected paraneoplastic syndrome (PNS) (including patients with LE, nonfocal encephalitis, encephalomyelitis, and cerebellar dysfunction) were enrolled for antibody detection in the program of encephalitis and PNS. Sera, CSFs, and sera-CSFs pairs from 4320 patients from medical centers across the mainland of China were test for antineuronal antibodies between September 2012 and September 2014 in the Department of Neurology, Peking Union Medical College Hospital (PUMCH). Clinical information was obtained from questionnaires filled out by the referring neurologists and telephone interviews. Informed consent for identification of antineuronal antibodies was obtained from each patient. The study was approved by the Ethics Committee of PUMCH. For the patients with positive results in autoantibody testing, the clinical information was retrospectively collected and reviewed by the neurological experts in the team.
Detection of antineuronal antibodies
Commercially available postfixed sagittal mouse brain sections and cell-based assays in the form of biochips (Euroimmun, Lübeck, Germany) were used. The biochips consist of human embryonic kidney (293) cells transfected with plasmids encoding the following antigens (subsequent fixation with the substances given in brackets): GABAB R, N-methyl-d-aspartate receptor (NMDAR, consisting of NR1 subunits only), leucine-rich glioma inactivated 1 protein (LGI1), contactin-associated protein-like 2 (CASPR2), and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR). The protocol for indirect immunofluorescence followed the instructions given by Euroimmun (FA 112d-1, immunoglobulin G [IgG]): Serum samples were diluted to 1:10 before incubation, the buffer was the phosphate buffer solution, the secondary system consisted of an anti-human IgG antibody conjugated with DyLight 594 at a dilution of 1:100 (Euroimmun: Anti-human IgG, goat, conjugated with FITC). The stained biochips were examined under a fluorescence microscope (Eurostar 3 Plus, Euroimmun, Germany). The decision if antibody was present in the tested material was made by one of the two investigators (Dr. Guan H and Ren H) using the signal of the surrounding (supposedly negative) fields as respective negative controls.
LE was defined according to the diagnostic criteria (modified from PNS Euronetwork): Acute or subacute or seizure, short-term memory loss, confusion, and psychiatric symptoms suggesting the involvement of the limbic systems. Definite diagnosis must also include either neuropathological, EEG, or neuroradiological evidence of the involvement of the limbic system.
Statistical analysis
Statistical analysis was performed using SPSS 11.5 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to analyze clinical data, such as median value and percentage.
RESULTS
Antineuronal antibody findings
We found 18 patients harboring anti-GABAB R antibodies (besides this, 404 patients were identified as being positive for antibodies to the following other antigens: NMDAR n = 360; LGI1 n = 35; CASPR2 n = 6; and AMPAR n = 3). In all of the 18 patients, CSF-serum pairs were available. Anti-GABAB R antibodies were both found in the serum and CSF of 16 patients. Anti-GABAB R antibodies were only present in the serum but not in the CSF in 1 case (case No. 2). Anti-GABAB R antibodies were only present in the CSF but not in the serum in one case (case No. 7). All GABAB R antibody-positive cases had limbic syndromes and EEG or imaging evidences that fulfilled the diagnostic criteria of LE. Four patients had additional antibodies against Hu in serum, and one had anti-NMDAR antibody in the serum and CSF.
Patients
A summary of the clinical information of these patients is shown in Tables 1 and 2. Eighteen patients were all Han Chinese. The median age was 56 years (range: 44–65 years). The male/female ratio was 13/5. The median time from symptom onset until the diagnosis was 28 days (range: 11 days to 18 weeks).
Table 1.
Clinical characteristics of 18 patients with anti-GABABR antibody-associated LE
| Sex | Age (years) | Duration (days) | Behavior changes | Memory deficits | Seizure | Consciousness disturbance | Speech impairment | Ataxia | Sleep disorder | ICU |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 60 | 50 | + | + | + | − | + | − | − | − |
| Female | 53 | 28 | + | + | + | + | − | − | − | + |
| Male | 61 | 13 | − | + | + | + | − | + | − | + |
| Male | 60 | 110 | + | + | + | + | − | − | + | − |
| Male | 61 | 24 | + | + | + | + | − | + | − | + |
| Male | 61 | 84 | − | + | + | + | − | − | − | − |
| Female | 59 | 14 | − | + | + | − | − | − | − | − |
| Male | 44 | 11 | + | − | + | + | − | − | − | − |
| Male | 58 | 126 | + | + | − | − | − | − | + | − |
| Male | 65 | 42 | + | − | + | − | − | − | − | − |
| Male | 49 | 14 | − | − | + | − | − | − | − | − |
| Male | 65 | 56 | + | + | + | − | − | − | − | − |
| Male | 56 | 28 | + | − | + | − | − | − | − | + |
| Female | 45 | 11 | + | + | + | − | − | − | − | − |
| Female | 50 | 13 | − | − | + | + | + | + | − | − |
| Male | 57 | 60 | − | − | + | − | + | − | + | − |
| Male | 54 | 13 | − | + | + | − | − | − | − | − |
| Female | 57 | 27 | + | + | + | − | − | − | − | − |
LE: Limbic encephalitis; ICU: Intensive Care Unit; LE: Limbic encephalitis; GABABR: γaminobutyric acid B receptor. +: Positive sign; –: Negative sign.
Table 2.
Diagnostic test, treatment and outcome of 18 patients with anti-GABABR antibody-associated LE
| GABABR antibodies in serum | GABABR antibodies in CSF | Additional antibodies | CSF pressure (mmH2O) | CSF WBC (/mm3) | CSF cytology | MRI: T2/FLAIR abnormality | EA on EEG | Slowing on EEG | Tumor | Immunotherapy | mRS before/after treatment/follow-up | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | + | − | 180 | 9 | L | Bilateral MT | + | − | − | IVIg | 3 | 1 | 1 |
| + | − | − | 200 | 0 | L | Bilateral MT | + | − | − | Steroids | 3 | 1 | 1 |
| + | + | − | 180 | 37 | L-N | Bilateral MT | − | + | − | IVIg + steroids | 5 | 2 | 2 |
| + | + | − | 120 | 3 | UR | Unilateral MT | − | + | − | ND | 3 | 3 | 3 |
| + | + | Hu | 180 | 6 | L | UR | ND | ND | LC | IVIg | 5 | 6 | 6 |
| + | + | Hu | 145 | 5 | L | UR | + | − | LC | IVIg + steroids | 3 | 0 | 0 |
| − | + | Hu, NMDAR | 350 | 40 | L-N | Bilateral MT | + | − | LC | IVIg + steroid | 3 | 1 | 6 |
| + | + | − | 140 | 38 | L | Bilateral MT | ND | ND | IVIg + steroids | 4 | 2 | 1 | |
| + | + | Hu | 180 | 1 | UR | Bilateral MT | UR | UR | LC | IVIg | 4 | 4 | 6 |
| + | + | − | 120 | 7 | L | UR | ND | ND | LC | IVIg | 4 | 2 | 6 |
| + | + | − | ND | 1 | ND | UR | − | + | − | ND | 3 | 1 | 1 |
| + | + | − | 140 | 1 | UR | Unilateral MT | + | + | − | IVIg | 3 | 1 | 1 |
| + | + | − | 230 | 10 | L | UR | + | + | − | IVIg | 5 | 2 | ND |
| + | + | − | 200 | 14 | L | UR | − | + | − | Steroids | 4 | 2 | ND |
| + | + | − | 300 | 63 | L-N | Bilateral MT | + | + | − | Steroids | 5 | 1 | 0 |
| + | + | − | 120 | 1 | UR | UR | ND | ND | − | IVIg | 3 | 1 | 1 |
| + | + | − | 50 | 80 | L-N | UR | − | − | LC | steroids | 4 | 2 | 2 |
| + | + | − | 50 | 1 | UR | Unilateral MT | + | + | − | IVIg + steroids | 4 | 2 | 2 |
LE: Limbic encephalitis; CSF: Cerebrospinal fluid; FLAIR: Fluid-attenuated inversion recovery; EA: Apileptic activity; EEG: Electroencephography; mRS: Modified Rankin score; L: Lymphocytic pleocytosis; L-N: Lymphocytic-neutrophilic pleocytosis; UR: Unremarkable; MT: Mesial temporal lobe; ND: Not done or no date available; NMDAR: N-methyl-d-aspartate receptor; LC: Lung cancer; IVIg: Intravenous immunoglobulin; GABABR: γ-aminobutyric acid B receptor; MRI: Magnetic resonance imaging; WBC: White blood cell; +: Positive result; –: Negative result.
Clinical presentation
All the 18 patients presented with a clinical feature of LE. Among them, seventeen (94.4%) patients presented with new-onset seizure and 16 (88.9%) patients presented with seizure as the initial symptom. All the 17 patients eventually developed generalized tonic-clonic seizures while one of them had partial complex seizure with multiple pure motor episodes (myoclonic jerks of the right leg initially which was interpreted as focal seizures) before progression to generalized tonic-clonic seizures. None of the patients in our series achieved seizure-free on their initial anti-epileptic medication. Four patients developed generalized status epilepticus refractory to multiple anticonvulsants before the referring autoantibody test. Twelve (66.7%) patients had memory deficits and 11 (61.1%) patients had a personality change, confusion or hallucinations. Seven (38.9%) patients had a disturbance of consciousness. Speech problems or aphasia were observed in 4 (22.2%) patients. Three (16.6%) patients showed cerebellar dysfunction. Two of them presented with gait and stance ataxia, one with limb ataxia and intentional tremor. One patient with LE had progressive motor and sensory polyneuropathy (with positive anti-Hu antibodies and lung cancer).
Before the final diagnosis of anti-GABAB R encephalitis, the patients’ neurological function reached a modified Rankin score (mRS) of 3–5 (median: 4). Four (22.2%) patients were admitted to Intensive Care Unit including 3 patients requiring mechanical ventilation.
Lung cancer was detected in 6 (33.3%) patients, with small-cell lung cancer (SCLC) pathologically confirmed in three of them. The neurological syndrome preceded the diagnosis of lung cancer in all of these patients. However, in 2 patients, the searching for tumors was inadequate due to patients’ will or resource problems.
None of the patients had the systemic autoimmune disease on immunological screening tests, besides one patient with evidence of Hashimoto thyroiditis. Only 1 patient had mild hyponatremia (132 mmol/L) without evidence of syndrome of inappropriate secretion of antidiuretic hormone.
MRI studies demonstrated abnormalities in mesial temporal regions on T2-weighted and fluid-attenuated inversion recovery in 10 (55.6%) patients (seven bilateral, three unilateral) [Figure 1a and b] without obvious enhancement. The other 8 patients had normal MRI. EEG was available from 12 patients: 8 had temporal lobe epileptic activity with or without general slowing; the others were unremarkable, although they did not undergo long-term or continuous monitoring.
Figure 1.
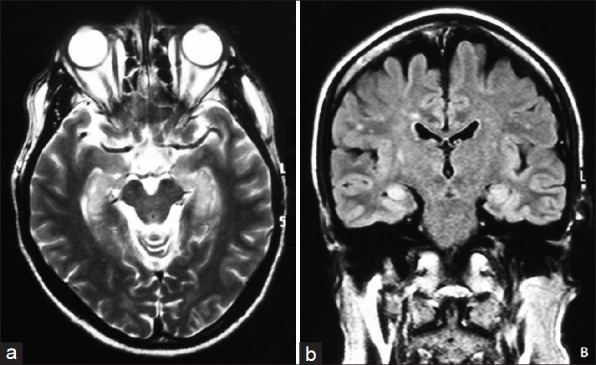
Magnetic resonance imaging of limbic encephalitis associated with anti-GABAB receptor antibodies. (a) T2-weighted magnetic resonance imaging of a patient with γ-aminobutyric acid B receptor abs and limbic encephalitis show increased signal in the mesial temporal lobes; (b) Fluid-attenuated inversion recovery magnetic resonance imaging show increased signal in the mesial temporal lobes.
Cerebrospinal fluid studies
All patients in our series underwent CSF tests. Opening pressure was high (>180 mmH2 O) in 5 (27.8%) cases. White blood cell was elevated (>4 cells/mm3) in 12 (66.7%) cases (range: 1–80/mm3, median: 14/mm3). Results for CSF cytology were available in 10 patients. Lymphocytic pleocytosis revealed by cytology was found in 6/10 patients and lymphocytic-neutrophilic pleocytosis in 4/10 patients [Figure 2]. Protein concentration of CSF was elevated (>450 mg/L) in five cases (range: 233.5–1000.0 mg/L, median: 350 mg/L). Oligoclonal bands were found in 5 of 8 (62.5%, 5/8) patients with available data. Extensive microbiology studies were negative in all cases.
Figure 2.
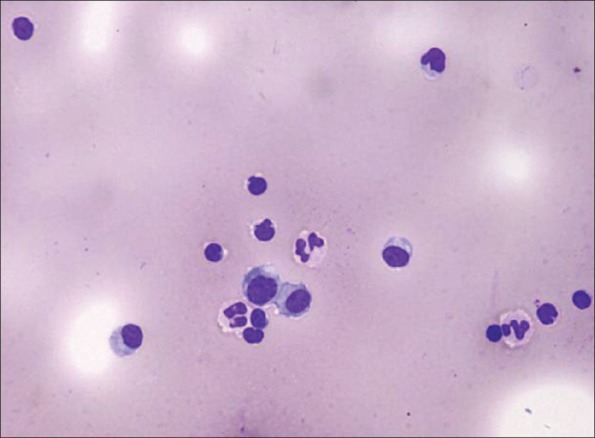
Cerebrospinal fluid cytology of a patient with γ-aminobutyric acid B receptor antibodies show mild lymphocytic-neutrophilic pleocytosis (May-Granwald-Giemsa stain, original magnification ×200).
Treatment and follow-up
Seventeen patients received immunotherapy (steroids and/or intravenous Ig). Fifteen (88.2%, 15/17) patients showed complete or partial neurological relief to immunotherapy with improved mRS (median: 1, range: 0–2) while the other 2 patients, with lung cancer, had no neurological improvement on immunotherapy. Three of the six patients with lung cancer received anti-tumor treatment (chemotherapy, 2 additionally radiation therapy). Two patients without lung cancer only received anti-epileptic medication without immunotherapy until the last follow-up: One was stable and the other improved partially. Four patients, all with lung cancer, died within 1–12 months due to the neoplastic complication. Autopsy was not available. One patient (case No. 4) received immunotherapy and was neurologically stable with a moderate disability (mRS: 3). None of the tumor-negative patients had progression or died at last follow-up. The follow-up period in 16 patients covered a median of 8 months (range: 1–21 months), while 2 patients failed to obtain further follow-up after discharge.
DISCUSSION
Surface receptor antibodies are highly specific and sensitive diagnostic markers for autoimmune encephalitis that often responds to immunotherapy.[1] One of these autoantibodies was found to be against the GABAB R by Lancaster et al. in 2010.[3] We report 18 patients with LE associated with GABAB R antibodies, representing the largest case series of Chinese patients with this disorder reported to date. The presence of anti-GABAB R antibody is rarer in autoimmune encephalitis when comparing with NMDAR antibody as indicated by this study. There are only dozens of cases reported since its first report in 2010.
Our study confirms that most patients with anti-GABAB R antibodies develop LE with prominent epilepsy, which is usually refractory to multiple anti-epileptic agents and shows response to immunotherapy.[3,4,9] In the series where these antibodies were first reported by Lancaster et al., all the 15 patients had LE and prominent seizures.[3] In fact, most patients with this disorder present initially with new-onset refractory generalized tonic-clonic, partial complex seizure or status epilepticus. The epileptic patients usually have temporal lobe epileptic activity with or without general slowing on EEG studies.[3,4] Memory deficits, personality change and speech problems are typical signs of LE, while MRI and PET indicate mesial temporal involvement in most cases.[4,5]
Cerebellar dysfunction, including ataxia and opsoclonus, presented as the initial symptom before the development of LE in some cases.[4,10] The occurrence of cerebellar symptoms, including opsoclonus,[11] is not surprising given the high density of expression of GABAB R in the cerebellum.[7] The peripheral nerve involvement in one case of our series may be classified as anti-Hu-associated polyneuropathy and unrelated to anti-GABAB R antibodies pathologically. Considering the wide anatomical distribution of GABAB R, it is reasonable to assume that with further recognition and systematic screening more cases with atypical presentation or additional syndromes may be found.[4,12]
Anti-GABAB R antibodies exist in patients with LE, with or without underlying tumor. According to previous studies, approximately 50% of cases are associated with lung cancer, especially SCLC.[3,4] Identification of these antibodies should prompt a search for lung cancer. The cases with SCLC usually have a poor response to immunotherapy and carry a poor prognosis.[3,4,13] This was confirmed by our studies since all 4 deceased patients in our series had lung cancer. However in our series, only one third of the patients had an underlying tumor, which may result from inadequate tumor screening and follow-up. Although the previous reports have shown higher prevalence of tumor, more nonparaneoplastic cases are likely to be identified as this disorder becomes more widely recognized.
Anti-antibodies in patients with LE can be accompanied by other autoantibodies with different clinical implications. These autoantibodies include anti-Hu, NMDAR, voltage-gated calcium channel (VGCC), glutamic acid decarboxylase 65 (GAD65), Sex determining region Y-box 1 (SOX1) and amphiphysin antibodies, as previously reported.[3,14] The additional onconeuronal antibodies, such as anti-Hu antibodies, may be associated with the underlying tumor rather than with the LE.[15] However, the presence of additional autoantibodies may be related to additional paraneoplastic neurological syndromes and may modify the neurologic phenotype. In our series, 1 patient with additional anti-Hu antibodies had severe sensory and motor neuropathy that might belongs to classical anti-Hu related PNS. Anti-Hu-associated encephalomyelitis and anti-amphiphysin-associated encephalomyelitis with gait ataxia has been previously reported in patients with positive anti-GABAB R antibodies.[4,13] The patient with additional anti-NMDAR antibodies showed prominent psychiatric symptoms, and the patient with GAD65 antibodies developed refractory seizures.[4,14] In patients with anti-GABAB R associated encephalitis, the co-occurrence of VGCC antibodies has been previously reported.[5] These antibodies cause paraneoplastic Lambert-Eaton myasthenic syndrome by reduction of presynaptic VGCCs.[16] The type of classical onconeuronal antibodies varied depending on the presence or absence of underlying tumor, and detection of these antibodies may lead to the identification of cancer and suggest a clinical prognosis. For example, all of the 4 patients with anti-Hu antibodies in our series had an underlying lung cancer and 3 of them had a rapid clinical progression to death. Additional thyroid peroxidase antibody or Hashimoto thyroiditis has been reported since the initial study of anti-GABAB R antibody-positive LE and LGI1 antibody-positive LE.[3,4,5] The so-called steroid-responsive encephalitis or Hashimoto encephalitis might need reclassification due to the discovery of the novel anti-neuronal antibodies.
Our studies have two main limitations. The small number of patients in our cohort and relatively short period of follow-up prevented us from assessing the long-term outcomes. Moreover, we were unable to test antibody titres and other anti-neuronal antibodies, such as anti-SOX1 antibodies and anti-ZIC4 antibodies, due to the working procedure of our laboratory and unavailability of additional antibody test panel.
In conclusion, our study confirms that most Han Chinese patients with anti-GABAB R antibodies develop LE with refractory epilepsy, which is potentially responsive to immunotherapy. The patients with underlying lung tumor have a relatively poor prognosis. Anti-GABAB R antibodies in patients with LE can be accompanied by additional autoantibodies with different clinical implications. The diagnosis of this rare disorder is important and testing for anti-GABAB R antibodies is necessary for patients with possible LE or new-onset epilepsy with unknown etiology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Graus F, Saiz A, Lai M, Bruna J, López F, Sabater L, et al. Neuronal surface antigen antibodies in limbic encephalitis: Clinical-immunologic associations. Neurology. 2008;71:930–6. doi: 10.1212/01.wnl.0000325917.48466.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graus F, Dalmau J. Paraneoplastic neurological syndromes. Curr Opin Neurol. 2012;25:795–801. doi: 10.1097/WCO.0b013e328359da15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: Case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, et al. Encephalitis and GABAB receptor antibodies: Novel findings in a new case series of 20 patients. Neurology. 2013;81:1500–6. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeffery OJ, Lennon VA, Pittock SJ, Gregory JK, Britton JW, McKeon A. GABAB receptor autoantibody frequency in service serologic evaluation. Neurology. 2013;81:882–7. doi: 10.1212/WNL.0b013e3182a35271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billinton A, Ige AO, Wise A, White JH, Disney GH, Marshall FH, et al. GABA(B) receptor heterodimer-component localisation in human brain. Brain Res Mol Brain Res. 2000;77:111–24. doi: 10.1016/s0169-328x(00)00047-4. [DOI] [PubMed] [Google Scholar]
- 7.Luján R, Shigemoto R. Localization of metabotropic GABA receptor subunits GABAB1 and GABAB2 relative to synaptic sites in the rat developing cerebellum. Eur J Neurosci. 2006;23:1479–90. doi: 10.1111/j.1460-9568.2006.04669.x. [DOI] [PubMed] [Google Scholar]
- 8.Benarroch EE. GABAB receptors: Structure, functions, and clinical implications. Neurology. 2012;78:578–84. doi: 10.1212/WNL.0b013e318247cd03. [DOI] [PubMed] [Google Scholar]
- 9.Hainsworth JB, Shishido A, Theeler BJ, Carroll CG, Fasano RE. Treatment responsive GABA(B)-receptor limbic encephalitis presenting as new-onset super-refractory status epilepticus (NORSE) in a deployed U.S. soldier. Epileptic Disord. 2014;16:486–93. doi: 10.1684/epd.2014.0702. [DOI] [PubMed] [Google Scholar]
- 10.Jarius S, Steinmeyer F, Knobel A, Streitberger K, Hotter B, Horn S, et al. GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J Neuroimmunol. 2013;256:94–6. doi: 10.1016/j.jneuroim.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Bataller L, Rosenfeld MR, Graus F, Vilchez JJ, Cheung NK, Dalmau J. Autoantigen diversity in the opsoclonus-myoclonus syndrome. Ann Neurol. 2003;53:347–53. doi: 10.1002/ana.10462. [DOI] [PubMed] [Google Scholar]
- 12.Mundiyanapurath S, Jarius S, Probst C, Stöcker W, Wildemann B, Bösel J. GABA-B-receptor antibodies in paraneoplastic brainstem encephalitis. J Neuroimmunol. 2013;259:88–91. doi: 10.1016/j.jneuroim.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Dogan Onugoren M, Deuretzbacher D, Haensch CA, Hagedorn HJ, Halve S, Isenmann S, et al. Limbic encephalitis due to GABAB and AMPA receptor antibodies: A case series. J Neurol Neurosurg Psychiatry. 2015;86:965–72. doi: 10.1136/jnnp-2014-308814. [DOI] [PubMed] [Google Scholar]
- 14.Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. 2011;76:795–800. doi: 10.1212/WNL.0b013e31820e7b8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol. 2004;56:715–9. doi: 10.1002/ana.20269. [DOI] [PubMed] [Google Scholar]
- 16.Dogan Onugoren M, Rauschka H, Bien CG. Conjoint occurrence of GABAB receptor antibodies in Lambert-Eaton myasthenic syndrome with antibodies to the voltage gated calcium channel. J Neuroimmunol. 2014;273:115–6. doi: 10.1016/j.jneuroim.2014.05.011. [DOI] [PubMed] [Google Scholar]


