Abstract
Study Objectives:
Nasal positive airway pressure (nPAP) for treatment of pediatric obstructive sleep apnea (OSA) is a widespread therapy that currently lacks longitudinal data describing how mask pressure impacts the developing facial skeleton. This retrospective cohort study compared midfacial growth in pediatric patients with underlying craniofacial conditions diagnosed with OSA who were compliant vs. noncompliant with nPAP therapy, and explored correlations between demographic, medical, and sleep variables with annual rate of facial change.
Methods:
Records from Seattle Children's Hospital's Craniofacial Center and Sleep Disorders Center were reviewed to identify patients prescribed nPAP for OSA with serial cephalographic images obtained during routine clinical care for concomitant craniofacial diagnosis. Lateral cephalometric analysis was used to determine mean annual change in midfacial structures from T1 (pre-nPAP) to T2 (post-nPAP) in compliant vs. noncompliant subjects. Compliance was indicated by nPAP usage of > 20 h/week for > 6 months.
Results:
50 subjects were compliant with nPAP therapy (mean age 10.42 years) for an average of 2.57 years, and 50 subjects were noncompliant (mean age 8.53 years). Compliant subjects experienced negative mean annual change (retrusion) of the midface compared to forward growth seen in noncompliant subjects (SNA: −0.57° vs. 0.56°), counterclockwise rotation of palatal plane (SN-PP: −1.15° vs. 0.09°), and upper incisor flaring (U1-SN: 2.41° vs. −0.51°).
Conclusions:
Pressure to the midface from compliant nPAP use may alter normal facial growth. Cephalometric findings indicate a greater need for collaboration between sleep medicine physicians and orthodontists to monitor midfacial growth during nPAP treatment.
Citation:
Roberts SD, Kapadia H, Greenlee G, Chen ML. Midfacial and dental changes associated with nasal positive airway pressure in children with obstructive sleep apnea and craniofacial conditions. J Clin Sleep Med 2016;12(4):469–475.
Keywords: adolescent, airway obstruction, cephalometry, dentition, masks, retrospective studies, sleep, sleep apnea, obstructive, sleep disorders, snoring
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep disorder characterized by upper airway obstruction, abnormal respiratory patterns, and fragmented sleep. The prevalence of OSA in children ages 2–6 years is around 2% to 5%, and estimates for primary snoring are as high as 17%.1–3 Untreated OSA is associated with early and significant morbidity, including neurocognitive deficits and decreased academic performance, behavioral and mood difficulties, cardiovascular impact including hypertension, hypercoagulability, and cardiac dysfunction, and chronic systemic inflammation with metabolic abnormalities.3–5 Thus, treatment of OSA is imperative for maximizing a child's developmental potential and overall health. Adenotonsillectomy to remove large tonsils and adenoids is a first-line treatment for children with OSA.3 Children with underlying craniofacial differences, such as midface hypoplasia, may experience airway obstruction even with normal-sized tonsils and adenoids due to the retruded position of the maxilla or mandible. These children may therefore also benefit from adenotonsillectomy to achieve a patent airway.
If the underlying craniofacial condition is very severe, adenotonsillectomy may not be sufficient to resolve OSA. Positive airway pressure (PAP) has become an increasingly common choice of therapy when adenotonsillectomy is unsuccessful in treating pediatric OSA, and has been deemed a second-line therapy for nonsurgical candidates, including those with obesity and underlying neurologic comorbidities.3,6,7 PAP treats OSA by applying positive pressure via an external mask, creating a pneumatic stent in the upper airway to prevent airway collapse. Efficacy of nasal mask PAP (nPAP) is dependent on creating an airtight seal around the nasal interface, placing a substantial amount of pressure on the surrounding tissue and bones.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Prolonged application of orthopedic forces from a nasal positive airway pressure (nPAP) mask used for treatment of pediatric obstructive sleep apnea (OSA) could cause midfacial retrusion in the developing facial skeleton. Since midface retrusion can contribute to decreased airway space, there is a need understand the midfacial effects of nPAP treatment and explore correlations between demographic, medical, and sleep variables with degree of facial change.
Study Impact: Children who were compliant with nPAP for 2.5 years demonstrated facial and dental changes that could exacerbate overall upper airway constriction, potentially worsen sleep symptoms and result in increased therapy with PAP or orthognathic surgery. Sleep specialists should collaborate with their orthodontic colleagues to monitor children undergoing nPAP therapy for signs of midface retrusion, counterclockwise tipping of the palatal plane, and flaring of the maxillary incisors.
The prolonged application of force to the facial skeleton can alter the magnitude and direction of skeletal growth. Such force from a nPAP mask has been associated with midfacial retrusion in growing children in a few case reports and case series.8–10 Children with an underlying tendency for midface deficiency, such as from a craniofacial syndrome or operated cleft lip/palate, would likely display relative midface retrusion even without nPAP usage.11–13 Nasal mask pressure may enhance this underdeveloped appearance by inhibiting midface growth or by actively pushing midfacial structures backward during the growth phase. Normal maxillary growth results in forward and downward movement of the midface (away from cranial base) in all children; therefore, any evidence of active midface retrusion over time reflects the negative pressure effect of the nPAP mask.14 Ironically, midface retrusion may lead to a more narrowed upper airway and potentially worsen the inherent OSA that nPAP is attempting to treat. For children with underlying craniofacial differences, this may further complicate already challenging medical care. This highlights the need to document the long-term effects of nPAP therapy, as the very treatment being prescribed for OSA may result in decreased airway space and the need for high-risk reconstructive surgical repair, at significant personal and medical costs. This study attempts to characterize and quantify the physical effects of nPAP therapy to the growing midface and maxillary dentition in a sample of children with OSA and craniofacial conditions. Objective cephalographic measurements at baseline and follow-up time points are used to compare subjects who were compliant with nPAP therapy with those who were noncompliant.
METHODS
In this retrospective cohort study approved by the Seattle Children's Hospital IRB, medical records were reviewed for patients treated between 2012 and 2014 at both the Craniofacial Center and Sleep Disorders Center of Seattle Children's Hospital. Medical and surgical histories and diagnostic testing/ imaging results were also extracted.
Inclusion criteria for all subjects were as follows: (1) age 0–18 years; (2) diagnosis by a sleep specialist of OSA confirmed by polysomnography (PSG); (3) prescription of PAP via a nasal mask, either continuous or bilevel, per usual clinical practice at the Sleep Disorders Center at Seattle Children's Hospital; (4) medical-grade CT scan or lateral cephalogram at T1 (time-point after starting nPAP and within 12 months of initial PSG) and T2 (> 6 months after T1). For subjects noncompliant with nPAP, T1 refers to a timepoint after nPAP was initially prescribed. Since it is rare for children to have diagnostic facial imaging unless they are undergoing orthodontic treatment or treatment for a craniofacial anomaly, all subjects with primary craniofacial diagnoses were included. Exclusion criteria were as follows: (1) maxillary orthognathic surgical treatment (Le-Fort 1, 2, or 3) occurring between T1 and T2; (2) poor quality imaging; and (3) insufficient nPAP compliance data.
Sleep Variables
All subjects underwent diagnostic PSG as part of their clinical care. PSG was performed at Seattle Children's Sleep Disorders Center, an accredited pediatric-specific facility. PSG lasted ≥ 6 h and was performed in a private darkened room free of distraction with a parent or guardian present. The following physiologic parameters were monitored: electroencephalogram, electro-oculogram, submental and anterior tibialis electro-myograms, electrocardiogram, oronasal airflow measured by thermistor and pressure transducer, expired end-tidal and transcutaneous carbon dioxide, oxygen saturation via pulse oximeter, and thoracic and abdominal movement. All data were recorded into a computer-based acquisition and analysis program (XLTEK, Natus Oakville, Ontario, Canada or Rembrandt, Buffalo, NY), scored by certified technicians, and interpreted by a board certified sleep medicine physician in accordance with AASM guidelines, including updates after 2012.15,16
Determination of compliance with nPAP was based on objective real-time data retrieved from an online database (EncoreAnywhere v 2.23.5.3, Philips Respironics) as well as documentation in the medical record. Subjects were considered compliant if their record demonstrated nPAP usage ≥ 4 h/ night on 70% of nights (∼20 h/week) for ≥ 6 months.17 Those who were not compliant with nPAP remained in the study as comparative control participants. There was insufficient data to reliably collect information on total hours of nPAP usage or average mask pressure, and hence was not included in this study.
Cephalometric Analysis
Medical-grade CT scans that were obtained in Digital Imaging and Communications in Medicine (DICOM) format were converted to 2-dimensional lateral cephalograms using Dolphin Imaging 3D software (Dolphin Imaging, Chatsworth, CA). Digital film cephalograms from a single image source were also used when CT imaging was not available for a given time point. All images were calibrated for image size standardization, randomized, and traced with Dolphin Imaging software by a single blinded operator (S.R.). Twenty-five images were re-measured to calculate intra-observer error. Standard cephalometric landmarks were used and a custom analysis was generated using measurements relevant to the anterior-posterior and vertical position of the maxilla and anterior cranial base, and to the inclination of the palate and maxillary incisors (Figure 1). The following measurements were chosen for analysis:
SNA (degrees): anteroposterior projection of anterior maxilla relative to anterior cranial base
S-N (mm): length of anterior cranial base
ANS-PNS (mm): length of maxilla
SN-PP (degrees): angulation of palatal plane relative to anterior cranial base
Ba-S-N (degrees): degree of flexure of cranial base
A-SN7 (mm): “effective maxillary height,” vertical distance from anterior maxilla to the SN7 (7° below S-N) line
A-SN7⊥ (mm): “effective maxillary length,” anteroposterior distance from anterior maxilla to a line perpendicular to the SN7 line
U1- SN (degrees): inclination of upper incisor relative to anterior cranial base
U1-PP (degrees): inclination of upper incisor relative to palatal plane
Since the nPAP mask has direct contact with the bridge of the nose and the maxilla, cephalometric measurements were chosen that specifically isolate the position of the maxilla (A-point, anterior nasal spine, posterior nasal spine) and upper incisor (U1) in reference to the anterior cranial base (sella and nasion) (Figure 1).
Figure 1. Points used for cephalometric analysis.
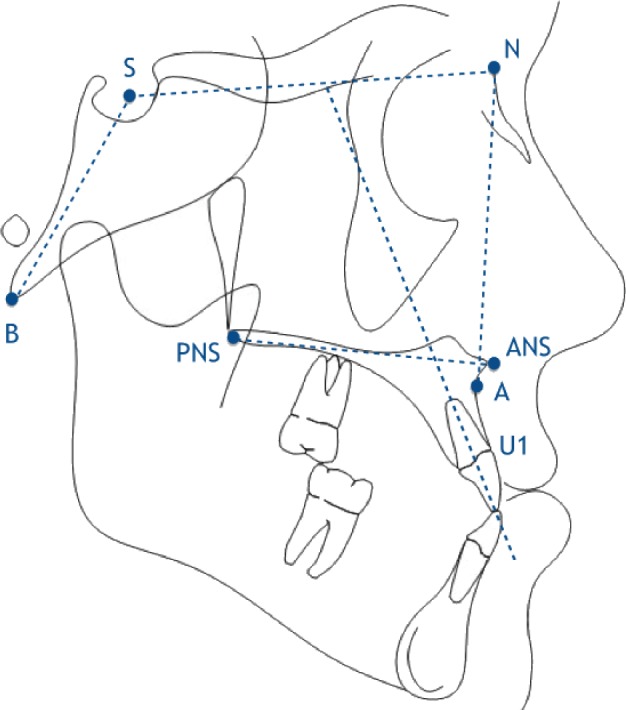
A, subspinale; ANS, anterior nasal spine; B, basion; N, nasion; PNS, posterior nasal spine; S, sella; U1, upper incisor.
A-SN7 and A-SN7⊥ are measurements found in the literature that correspond to the vertical and horizontal position of A-point in relation to the cranial base. Using the SN7 and SN7⊥ lines provides a cephalometric axis from which the anterior aspect of the maxilla (A-point) can be plotted as if on an x,y grid (Figure 2). Mandibular measurements were not included due to inconsistent imaging (mandible not fully captured or CT taken under general anesthesia with mouth open).
Figure 2. A-point plotted by A-SN' and A-SN'perp.
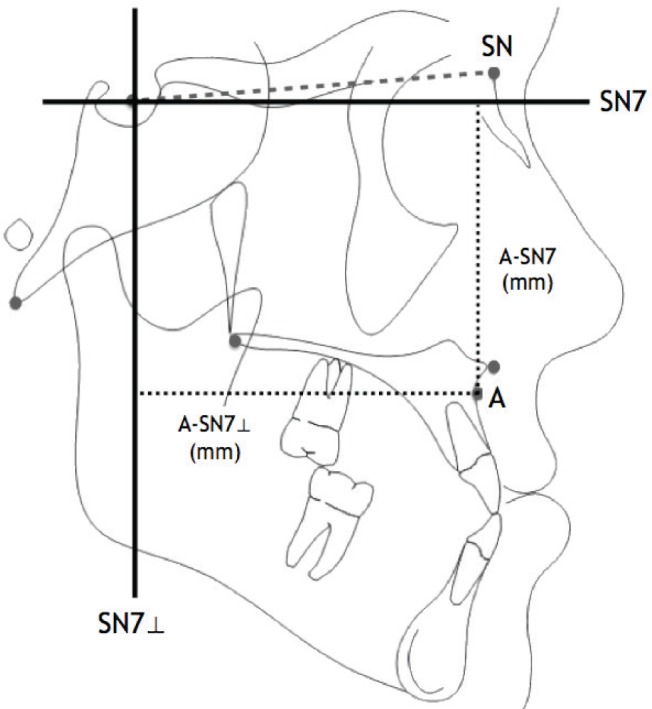
SN7, line 7 degrees below S-N line; SN7⊥, line perpendicular to SN7; A-SN7, “effective maxillary height” (vertical distance between A and SN7 in mm); A-SN7⊥, “effective maxillary length” (horizontal distance between A and SN7⊥ in mm).
For all of the midface measurements chosen, expected rate of growth is a positive value (trajectory away from cranial base).14 Thus, results expressed as a negative value, or a smaller value when compared to the control group, represent midfacial structures being pushed backward or restricted in their growth relative to cranial base over time. Results are shown as annual rates of change, i.e., millimeters or degrees of change per year, for each measurement. For measurements involving the inclination of the maxillary incisors, patients with braces at any point between T1 and T2 were excluded.
Statistical Analysis
Data was analyzed using SPSS software (version 13.00; SPSS, Chicago, IL). Baseline characteristics and imaging results were compared using t-tests between compliant and noncom-pliant groups. Comparisons were adjusted for age, gender, and primary craniofacial diagnosis. Further analyses of cephalometric changes were done by age groups (< 8 years, 8–15 years, > 15 years). Multivariate regression analysis was used to relate change in cephalometric measurements to underlying severity of OSA and body mass index (BMI). For this descriptive study, significance was set at p < 0.05.
RESULTS
Of approximately 1,800 children who were treated with nPAP at Seattle Children's Hospital between 2012 and 2014, 217 were also followed in the Craniofacial Clinic. Of these, 98 (45.2%) were considered compliant with nPAP and 119 (54.8%) were noncompliant (data not shown). Reasons for noncompliance included: patient unable to tolerate nPAP (n = 49); weekly nPAP usage less than compliance criteria (n = 28); nPAP used compliantly < 6 months (n = 23); patient did not start nPAP for undocumented reason (n = 10), adenotonsillectomy after nPAP prescription (n = 4); tracheotomy performed within 6 months of nPAP prescription (n = 3); oral appliance prescribed within 6 months of nPAP prescription (n = 2). 3D or 2D imaging at T1 and T2 was available for 136 subjects. Thirty-six subjects were excluded from this study (n = 18 for maxillary surgery between T1 and T2; n = 13 for inadequate record of compliance, n = 5 for poor quality radiographs). Final data analyses and results were based on the remaining 100 subjects.
Baseline demographic and sleep study characteristics revealed that severity of OSA was similar between the 2 groups, and that the only statistical differences between the compliant and noncompliant groups were: (1) age at T1—compliant subjects were 10.42 years old on average, and noncompliant subjects were 8.53 years old (p < 0.05) (Table 1); (2) total arousal index—compliant subjects experienced mean 24.85 cortical arousals/h, and noncompliant subjects experienced mean 17.04 arousals/h (Table 2). There was fairly even distribution of underlying diagnoses in this cohort and a low prevalence of pre-PAP orthognathic surgeries (Table 1). Nearly all subjects in both groups underwent pre-PAP adenotonsillectomy (data not shown). Follow-up time between images was 2.57 years (± 1.17) for the compliant group and 2.45 years (± 1.26) for the noncompliant group. Differences between absolute cephalo-metric measurements at T1 in the compliant and noncompliant groups reflect differences in average age of the groups, but are not significantly different overall (Table 3).
Table 1.
Medical history variables.
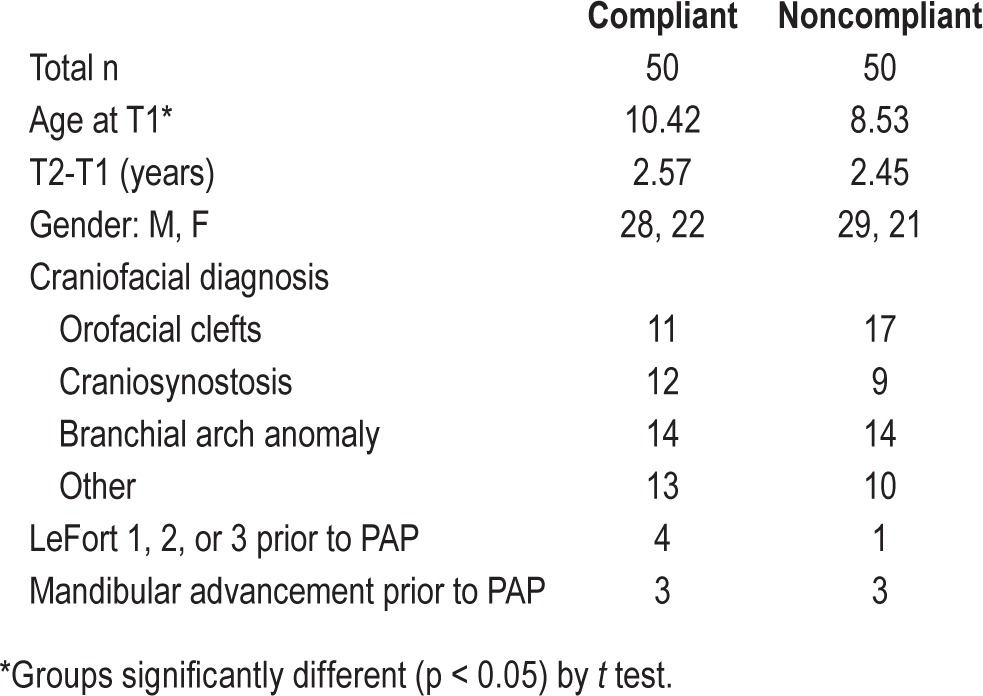
Table 2.
Sleep study variables from initial polysomnography (PSG).
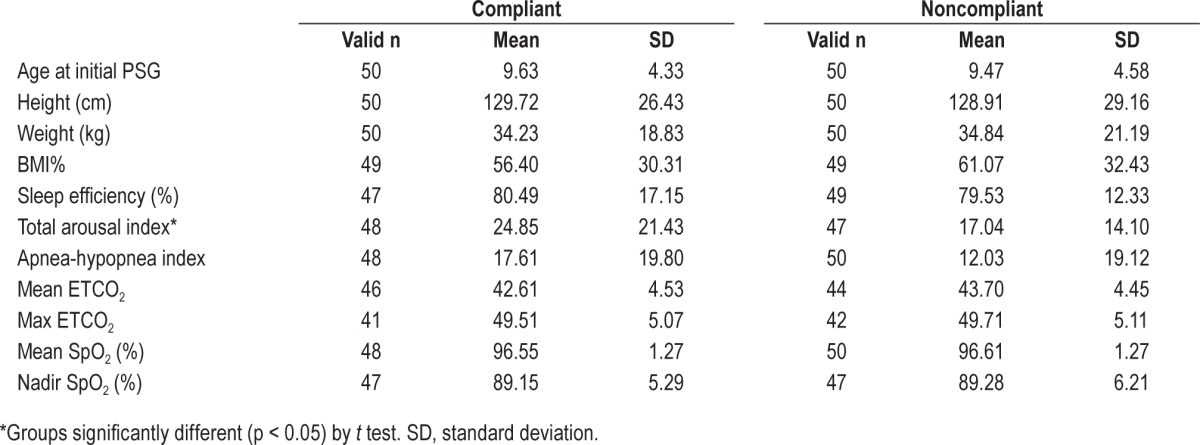
Table 3.
Cephalometric differences at T1 and comparison of growth rates between compliant and noncompliant groups with craniofacial conditions, and standards for normally developing children.
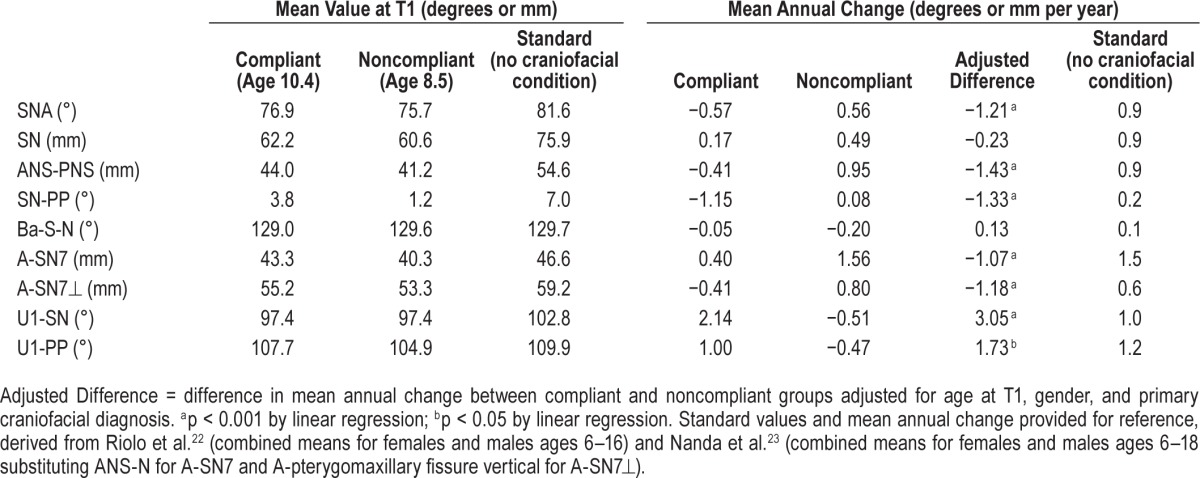
Rates of cephalometric changes for nearly every measurement differed between the 2 groups, with the nPAP compliant group generally showing less positive change over time, consistent with more midface retrusion. Compliant PAP subjects also experienced flaring of the upper incisor as described by increased mean annual change in U1-SN and U1-PP, and counter-clockwise tipping of the palatal plane. Although significant pressure from the nPAP mask has been reported to be exerted on the bridge of the nose (nasion, N point),18 the mean change in cranial base length (S-N) was not different between groups. Twelve compliant subjects and 6 noncompliant subjects were undergoing orthodontic care at one or both time points and therefore incisor measurements were not included in their cephalometric analysis. Intra-observer error was measured to be 0.5°, 0.3 mm, and 0.3 mm for SNA, A-SN7, and A-SN7⊥ by the method of moments estimator.20
Regression analysis was used to test for an interaction between age group and rate of facial change; however, sample size in each age group was not large enough and variability was too high to detect a significant difference between age groups (data not shown). Regression analyses were performed looking at underlying severity of OSA variables and BMI with cephalometric changes, and no significant correlations were found (data not shown). Underlying craniofacial diagnosis was also not shown to correlate to rate of facial change in this study (data not shown).
DISCUSSION
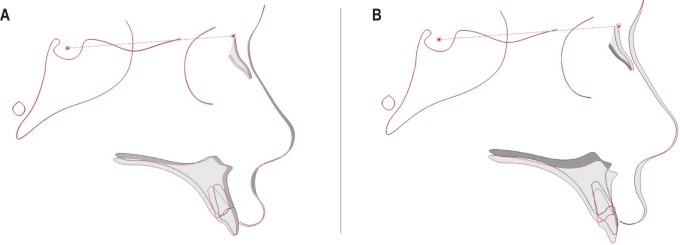
This study adds to a small but growing body of concerning findings evaluating the effects of nPAP use in children, with greater sample size and longer follow-up than prior studies and adding a high-risk cohort of children with underlying craniofacial conditions. Subjects with adequate cephalographic imaging who were compliant with nPAP experienced a significant amount of midface growth restriction greater than 1 mm per year in both the vertical anteroposterior dimensions, mimicking an orthodontic “high-pull headgear effect.”21 Noncompliant controls showed an overall normal pattern of downward and forward midface growth (Figure 3). Decreased ANS-PNS in the compliant group might suggest resorption of the anterior maxilla in response to nPAP mask pressure, although this measurement should be interpreted with caution, as PNS is often a difficult point to reliably identify on a lateral cephalogram. Decreased rate of change in effective maxillary height suggests that nPAP may restrict the normal downward growth of the anterior maxilla, thereby impacting vertical as well as anterior-posterior craniofacial development over time.
Figure 3. Superimpositions of mean baseline and follow-up data.
(A) Compliant. (B) Noncompliant. Average position of skeletal and dental structures at T1 (dark) and T2 (light) for compliant and noncompliant groups, superimposed on sella-nasion line.
In comparison with published standards of pediatric facial growth,14,22,23 the majority of subjects in this study started with relative midface deficiency at T1 resulting from various cranio-facial conditions and experienced worsening of that deficiency with nPAP use. Table 3 summarizes baseline measurements and growth rates in the compliant and noncompliant subjects while providing expected absolute measurements and growth rates over time in typically developing children of similar ages.
There are limited reports in the literature regarding this topic, despite a rapidly increasing prescription rate for nPAP in children.8–10,24,25 A cross-sectional study by Fauroux et al.10 reported facial flattening in 68% and clear maxillary retrusion in 37% of children aged 0–18 years using nPAP, with a stronger association linked to longer nighttime use. Tsuda et al.24 documented measurable maxillary retrusion and remodeling in adults after 2 years of nPAP use using lateral cephalograms, which is remarkable considering the subjects had reached skeletal maturity. Korayem et al.25 evaluated children with and without nPAP therapy at one follow-up time point and identified no significant differences in craniofacial morphology between the groups and found no association between maxillary position and length of nPAP therapy.
This is the first study to reveal maxillary effects of nPAP in children by analyzing baseline and follow-up cephalographic images in a large cohort of both treatment and control subjects of similar baseline demographics, specifically those with craniofacial conditions. In our sample, active midface retrusion was evident in children compliant with nPAP at a rate very similar to that found in adults by Tsuda et al.24 In growing children, the effect is amplified in the context of anticipated positive maxillary growth, as demonstrated by our noncompliant control group. If a child is compliant with nPAP therapy for many years, this inhibition of maxillary skeletal growth may require surgical correction. To fully understand the effects of midface retrusion on airway dimensions and OSA, a 3-dimensional analysis and evaluation of sleep study results are required.
It was hypothesized that nPAP use during periods of rapid facial growth would be most detrimental to the developing midface, and thus reason to suspect that younger children may be more vulnerable to nPAP effects. Chronologic age rather than skeletal age was used in this study and may not be the most accurate determinant of childhood and adolescent growth spurts. Though as a group we did not find worsening rates of retrusion with age alone (data not shown), it is notable that the subject that showed the most negative change in SNA in response to compliant nPAP use was the youngest compliant subject in the sample (male, age 1.05 years at nPAP prescription). Further investigation with larger studies on the effects of age and detailed pressure measurements would lead to better understanding of the vulnerability we suspect is present in younger children with more malleable facial structures.
In addition to demonstrating the associations of nPAP use on midface structures over time, this study highlights the paucity of longitudinal and outcomes data in the literature overall on PAP use in children. Our percentage of compliant nPAP users (45%) is not markedly different than what has been reported, but few data in children are available for comparison. Those compliant in this study were older, and perhaps more tolerant of wearing nPAP while sleeping.
Limitations of this study include its retrospective nature and high variability in underlying craniofacial diagnoses, age, and time between T1 and T2 in this sample. Future research directed at more specific demographic groups might help to identify increased risk in specific populations. Additionally, although a nasal mask interface was initially prescribed to each patient in this study, it is possible that various mask designs were later used, as patients often interact directly with the PAP mask supplier to find a mask that fits comfortably. Another limitation includes variation in image format—although CT was the primary source of imaging, digital cephalograms were also included, which may have contributed to minor error in image size calibration.26 In future studies, baseline and follow-up time points should correspond to the exact commencement and completion of PAP, or a set time after commencement, to provide a more precise baseline analysis and ensure that follow-up analysis precludes any recovery, or “catch-up,” growth of the midface after nPAP is discontinued. PAP pressure (cm H2O) was not consistently recorded in our population and therefore could not be analyzed for correlation to midface retrusion, though this variable should be examined in future research, as it may be a valid proxy for nPAP mask tightness and corresponding midfacial pressure.
PAP is currently the most frequently prescribed therapy for patients with severe OSA who do not respond well to adenotonsillectomy. When used consistently for more than 2 years, nPAP has been shown to cause midfacial retrusion in adults, so it is not surprising that nPAP could impair the developing mid-face of a child or adolescent.24 PAP has, however, been proven to be highly effective in treating OSA in children, and so the potential negative side effects of nPAP on the pediatric mid-face must be weighed against the advantageous effects of PAP, including improvement in daytime sleepiness, temperament, school performance, and reducing the potential for related medical conditions.6,27 When prescribed for children, duration of PAP treatment is usually several years or until airways have enlarged via somatic growth. It may also be lifelong, depending on the underlying associated condition. The clinical significance of potential midface retrusion should be determined for each patient prescribed nPAP. Evaluation and monitoring by an orthodontist throughout nPAP therapy is recommended to detect dental changes, as well as negative skeletal and facial effects that may lead to worsening of potential airway obstruction. Protraction headgear or “facemask,” an orthodontic treatment used to protract the midface and maxillary dentition, may be a viable treatment option for patients showing midface retrusion resulting from nPAP. Further study of different treatment options and advances in PAP mask design may result in alternatives to nPAP therapy for children in the future.
A subsequent prospective study should include description of the pressure setting of the PAP machine, with distinction between continuous and bilevel pressure. Additionally, 3-D analysis of airway volume may determine whether compliant nPAP usage puts patients at risk for overall upper airway constriction that could potentially worsen OSA symptoms and require continued therapy with PAP or orthognathic surgery. 3-D facial imaging and intraoral scanning of the dentition could also be used to document soft tissue and dental changes more precisely in three dimensions and without radiation over multiple time points.
CONCLUSIONS
In this study, 45.2% of all subjects were compliant with nPAP therapy. Craniofacial measures based on cephalometric analysis indicated statistically significant decreases in annual rates of change for all craniofacial measurements related to growth of the midface in compliant vs. noncompliant subjects over an average of 2.5 years of nPAP therapy. Compliant subjects experienced overall maxillary retrusion, counterclockwise tipping of the palatal plane, and flaring of the maxillary incisors. Additional studies are needed to further assess clinical factors associated with nPAP therapy and surrogate endpoints related to pediatric midface development. The preliminary findings of this study indicate a need for greater collaboration between sleep medicine physicians and their orthodontic colleagues to detect facial and dental effects related to nPAP mask pressure over time in children with OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was funded by the University of Washington Orthodontic Alumni Association. The authors have indicated no financial conflicts of interest. This research was completed at the University of Washington, Seattle, WA and Seattle Children's Hospital, Seattle, WA.
ACKNOWLEDGMENTS
We would like to thank Dr. Sue Herring, statistician Chuck Spiekerman, Dr. Carrie Heike, Marcella Blackledge, Judith Iwata, Deborah Kahrimanovic, and Dr. Michael Chiulli for their generous assistance throughout this study. Thank you also to the University of Washington Orthodontic Alumni Association for their financial support.
ABBREVIATIONS
- A
A point
- ANS
anterior nasal spine
- Ba
basion
- BMI
body mass index
- ETCO2
end-tidal carbon dioxide
- N
nasion
- nPAP
nasal mask positive airway pressure
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PNS
posterior nasal spine
- PP
palatal plane
- PSG
polysomnography
- S
sella
- SN
sella-nasion
- SNA
sella – nasion – A point
- SN7
sella-nasion minus 7 degrees
- SN7⊥
sella-nasion minus 7 degrees perpendicular
- SpO2
oxygen saturation of the blood as measured by a pulse oximeter
- T1
timepoint 1
- T2
timepoint 2
- U1
upper incisor
REFERENCES
- 1.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willington AJ, Ramsden JD. Adenotonsillectomy for the management of obstructive sleep apnea in children with congenital craniosynostosis syndromes. J Craniofac Surg. 2012;23:1020–2. doi: 10.1097/SCS.0b013e31824e6cf8. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 4.Toukh M, Pereira EJ, Falcon BJ, et al. CPAP reduces hypercoagulability, as assessed by thromboelastography, in severe obstructive sleep apnoea. Respir Physiol Neurobiol. 2012;183:218–23. doi: 10.1016/j.resp.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Bonuck K, Freeman K, Chervin RD, Xu L. Sleep-disordered breathing in a population-based cohort: behavioral outcomes at 4 and 7 years. Pediatrics. 2012;129:e857–65. doi: 10.1542/peds.2011-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117:e442–51. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 7.Dayyat E, Serpero LD, Kheirandish-Gozal L, et al. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest. 2009;135:1142–9. doi: 10.1378/chest.08-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li KK, Riley RW, Guilleminault C. An unreported risk in the use of home nasal continuous positive airway pressure and home nasal ventilation in children: mid-face hypoplasia. Chest. 2000;117:916–8. doi: 10.1378/chest.117.3.916. [DOI] [PubMed] [Google Scholar]
- 9.Villa MP, Pagani J, Ambrosio R, Ronchetti R, Bernkopf E. Mid-face hypoplasia after long-term nasal ventilation. Am J Respir Crit Care Med. 2002;166:1142–3. doi: 10.1164/ajrccm.166.8.257c. [DOI] [PubMed] [Google Scholar]
- 10.Fauroux B, Lavis JF, Nicot F, et al. Facial side effects during noninvasive positive pressure ventilation in children. Intensive Care Med. 2005;31:965–9. doi: 10.1007/s00134-005-2669-2. [DOI] [PubMed] [Google Scholar]
- 11.Reitsma JH, Ongkosuwito EM, Buschang PH, Prahl-Andersen B. Facial growth in patients with apert and crouzon syndromes compared to normal children. Cleft Palate Craniofac J. 2012;49:185–93. doi: 10.1597/10-021. [DOI] [PubMed] [Google Scholar]
- 12.Shibazaki-Yorozuya R, Yamada A, Nagata S, Ueda K, Miller AJ, Maki K. Three-dimensional longitudinal changes in craniofacial growth in untreated hemifacial microsomia patients with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2014;145:579–94. doi: 10.1016/j.ajodo.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Lu D, Wamalwa P, Li C, Hu H, Zou S. Craniofacial morphology characteristics of operated unilateral complete cleft lip and palate patients in mixed dentition. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e16–25. doi: 10.1016/j.tripleo.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Bjork A, Skieller V. Growth of the maxilla in three dimensions as revealed radiographically by the implant method. Br J Orthod. 1977;4:53–64. doi: 10.1179/bjo.4.2.53. [DOI] [PubMed] [Google Scholar]
- 15.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson A, Quan SF, for the American Academy of Sleep Medicine . 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 17.Billings ME, Kapur VK. Medicare long-term CPAP coverage policy: a cost-utility analysis. J Clin Sleep Med. 2013;9:1023–9. doi: 10.5664/jcsm.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munckton K, Ho KM, Dobb GJ, Das-Gupta M, Webb SA. The pressure effects of facemasks during noninvasive ventilation: a volunteer study. Anaesthesia. 2007;62:1126–31. doi: 10.1111/j.1365-2044.2007.05190.x. [DOI] [PubMed] [Google Scholar]
- 19.Houston WJ. The analysis of errors in orthodontic measurements. Am J Orthod. 1983;83:382–90. doi: 10.1016/0002-9416(83)90322-6. [DOI] [PubMed] [Google Scholar]
- 20.Springate SD. The effect of sample size and bias on the reliability of estimates of error: a comparative study of Dahlberg's formula. Eur J Orthod. 2012;34:158–63. doi: 10.1093/ejo/cjr010. [DOI] [PubMed] [Google Scholar]
- 21.Firouz M, Zernik J, Nanda R. Dental and orthopedic effects of high-pull headgear in treatment of Class II, division 1 malocclusion. Am J Orthod Dentofacial Orthop. 1992;102:197–205. doi: 10.1016/S0889-5406(05)81053-4. [DOI] [PubMed] [Google Scholar]
- 22.Riolo ML. Ann Arbor, MI: Center for Human Growth and Development, University of Michigan; 1974. An atlas of craniofacial growth: cephalometric standards from the university school growth study, the University of Michigan. [Google Scholar]
- 23.Nanda RS, Ghosh J. Longitudinal growth changes in the sagittal relationship of maxilla and mandible. Am J Orthod Dentofacial Orthop. 1995;107:79–90. doi: 10.1016/s0889-5406(95)70159-1. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda H, Almeida FR, Tsuda T, Moritsuchi Y, Lowe AA. Craniofacial changes after 2 years of nasal continuous positive airway pressure use in patients with obstructive sleep apnea. Chest. 2010;138:870–4. doi: 10.1378/chest.10-0678. [DOI] [PubMed] [Google Scholar]
- 25.Korayem MM, Witmans M, MacLean J, et al. Craniofacial morphology in pediatric patients with persistent obstructive sleep apnea with or without positive airway pressure therapy: a cross-sectional cephalometric comparison with controls. Am J Orthod Dentofacial Orthop. 2013;144:78–85. doi: 10.1016/j.ajodo.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Ghoneima A, Albarakati S, Baysal A, Uysal T, Kula K. Measurements from conventional, digital and CT-derived cephalograms: a comparative study. Aust Orthod J. 2012;28:232–9. [PubMed] [Google Scholar]
- 27.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1098–103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]