Abstract
Study Objectives:
To assess positive airway pressure (PAP) therapy adherence in commercial motor vehicle (CMV) drivers presenting to a sleep center.
Methods:
A retrospective chart review of 120 drivers evaluated for obstructive sleep apnea OSA and 53 initiated on PAP therapy in a single sleep center over a one-year period (2012); PAP therapy data were collected up to 1 year.
Results:
Early PAP usage best predicted adherence up to 1 year (p < 0.0001) compared to patient factors, OSA disease characteristics, and treatment elements analyzed. The proportion of participants adherent to therapy was 68.0% at 1 week, decreasing to 39.6% at 1 year, with 31.1% lost to follow-up by 1 year. In the group categorized based on adherence at week 1, 80.6% were adherent at 1 month, decreasing to 52.8% at 1 year. For the group non-adherent at 1 week, 29.4% were adherent at 1 month, decreasing to 11.7% at 1 year. Participants were predominantly male (75.8%), middle-aged (median 50.5 years), and African American (71.7%). Of those referred to the sleep center, 86.7% had OSA (median apnea-hypopnea index [AHI] or respiratory event index [REI] 20.1), with 51.0% of the OSA group having an AHI or REI > 20 and initiating PAP therapy.
Conclusions:
Early PAP utilization patterns predicted one year adherence for our CMV driver population within a sleep clinic setting. OSA testing of these CMV drivers after occupational health referral identifies high proportions of undiagnosed OSA, with approximately half requiring PAP therapy based on current published treatment recommendations.
Citation:
Colvin LJ, Dace GA, Colvin RM, Ojile J, Collop N. Commercial motor vehicle driver positive airway pressure therapy adherence in a sleep center. J Clin Sleep Med 2016;12(4):477–485.
Keywords: obstructive sleep apnea, commercial motor vehicle, driver, sleep apnea, positive airway pressure, continuous positive airway pressure, adherence, Federal Motor Carrier Safety Administration
INTRODUCTION
Obstructive sleep apnea (OSA) is associated with sleepiness, fatigue, diabetes, hypertension, serious cardiovascular disorders, and increased vehicular crash risk.1–8 Consensus-based expert panels recommend comprehensive OSA screening and assessment protocols for commercial motor vehicle (CMV) drivers.9–11 Protocols employing more broad OSA risk factor assessment have been shown to increase detection of undiagnosed and previously diagnosed OSA in occupational settings compared to current federal requirements.12–17 OSA treatment recommendations, including minimum adherence standards for positive airway pressure (PAP) therapy utilization, are also provided within expert panel publications.9–11 A detailed overview of these recommendations is available in a separate article.18
Continuous positive airway pressure (CPAP) therapy has been shown to reduce crash risk in general driving populations.6,19 Within clinical and research settings, adherence to positive airway pressure (PAP) therapy is a recognized challenge both in achieving desirable outcomes for therapy usage and defining criteria for sufficient amount and frequency of use.20–23 PAP therapy adherence studies vary widely in their reported percentages of patients with successful adherence, ranging from 30% to 80% in clinical research populations.20,24,25 Although some variation can be attributed to differences in criteria used to define optimal adherence, these estimates still suggest suboptimal PAP therapy utilization.20,24 When considering safety-sensitive professionals such as CMV drivers, the goal to attain optimal adherence is of particular importance.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Expert consensus panels recommend OSA assessment and treatment for CMV drivers; however, scant data on PAP therapy adherence in CMV drivers have been published. This study evaluates PAP therapy adherence for CMV drivers within a sleep center clinical environment.
Study Impact: Without studies of PAP adherence specifically in CMV driving populations, sleep specialists must extrapolate from published studies in clinical patients. This study provides data on PAP adherence specifically in the CMV driver population.
PAP adherence predictors studied in clinical populations can be categorized broadly as patient factors, OSA disease characteristics and treatment elements.20,21,25 Overall, it appears that these factors do not, by themselves, fully predict PAP therapy adherence. Rather, studies suggest psychologically based factors such as health beliefs, attitudes, knowledge, and early adherence behavior patterns also play a significant role20,21,25; PAP therapy adherence patterns observed within as little as one week can predict future therapy use.26–29 Interventions based on health behavior change concepts appear to have a significant impact in promoting adherence compared to educational and technologic enhancements alone.20,21,24,25,30,31
There are few published data on CPAP therapy initiation and utilization specifically in CMV operators, and no studies report above average adherence; we located only two published studies. In one study of commercial bus drivers in Hong Kong, from a population of 85 drivers with OSA, 25 were referred for evaluation with 9 using CPAP therapy, averaging 4.5 h use after 3 months.32 In a second study conducted within the United States, of the 20 participants with OSA, 4 provided documentation of CPAP use with 1 driver achieving more than 4 hours per night.13
METHODS
Figure 1 provides an overview of the study population and distribution characteristics. For this study, a retrospective chart review of 120 consecutive adult CMV drivers referred by a commercial driver medical examiner (CDME) who underwent new evaluation between January 1, 2012, and December 31, 2012, in a sleep center located in the midwestern region of the United States was performed.
Figure 1. Study population diagram.
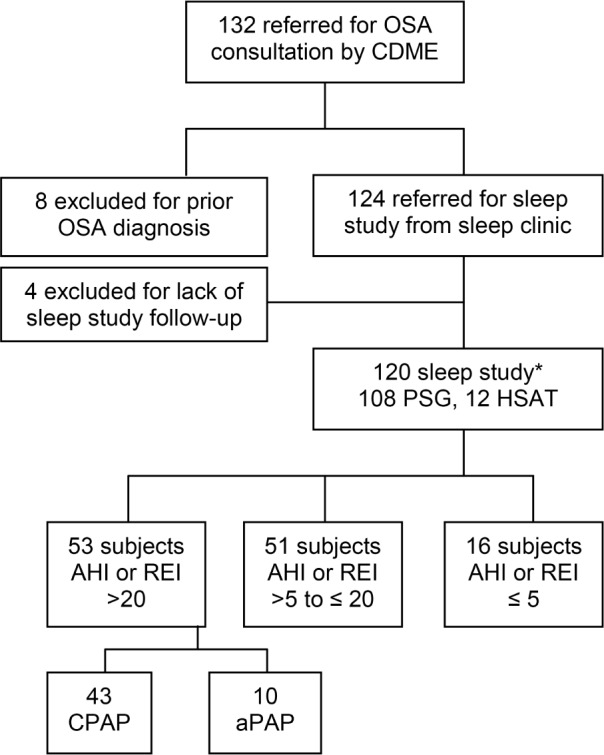
*Initial sleep study performed, confirmatory PSG performed for 2 subjects with REI ≤ 5 reported by HSAT.
Patients were referred by the CDME based on findings from OSA clinical screening based on consensus recommendations.9–11 Of 132 patients referred, 12 were excluded for a prior diagnosis of OSA (n = 8) or if the recommended sleep study was not completed (n = 4). For those recommended to initiate PAP therapy (n = 53), collection of treatment adherence data continued for up to 1 year after initiation, when available. All participants on PAP therapy had 1 week of data available; however, progressive increases in participants lost to follow-up were noted when querying electronic adherence data which were available for 1 year in only 36 participants (68.0%). Study approval was granted by the University of Missouri-Saint Louis Institutional Review Board.
Clinical Assessment Data
Participants provided their demographics including date of birth, gender, and ethnicity and answered the Epworth Sleepiness Scale (ESS) and medical history questionnaires via paper forms. One participant declined to provide their ethnicity and was excluded from any analysis involving ethnicity. Clinical evaluation was performed by one provider (LC) obtaining the entire physical examination including height, weight, vital signs, and neck circumference (to the nearest 0.5 inch) and a detailed review of the medical history provided on written questionnaires with verbal verification and clarification. Hypertension history was identified by patient report or clinical assessment. The Federal Motor Carrier Safety Administration (FMCSA) commercial driver medical certification form completed in the occupational health clinic was not available for review by study personnel.
Sleep center data reviewed included forms, questionnaires completed by the participant, and notes completed by the provider. Scanned or typed notes were available within the electronic health record (EHR). PAP adherence data were acquired directly from the device manufacturer software, allowing retrieval of all historical adherence data from the PAP device. Data loss occurred in participants who did not complete testing or for those on PAP therapy who became lost to follow-up subsequent to the 1-week visit. Otherwise, there was no additional loss of clinical data stored in the EHR.
Sleep Study Data
Sleep studies were performed by an American Academy of Sleep Medicine (AASM) accredited sleep center. Both attended in-lab polysomnography (PSG) and unattended “home” sleep apnea testing (HSAT) was included, as this reflected clinical practice at the time; HSAT was offered as an alternative diagnostic tool based on financial, medical insurance, or employer-based factors. PSG testing was performed in the center, attended by a sleep technologist using standard clinical PSG recording protocols. HSAT was performed away from the sleep center including locations such as the participant's home, a hotel, or their truck sleeper-cab. HSAT devices were dispensed to the participant after in-person instruction and chain-of-custody device application. U.S. Food and Drug Administration (FDA)-approved type III HSAT devices (MediByte, Braebon, Ogdensburg, NY, or ApneaLink, ResMed, San Diego, CA) were utilized to monitor nasal pressure via nasal pressure transducer, oxygen saturation and heart rate via finger arterial pulse oximetry, and chest movements with respiratory inductance plethysmography belts. For HSAT, recording time began when nasal pressure and pulse oximetry sensors were placed by the participant at bedtime and appropriate waveforms observed; the patient was instructed to remove the device upon awakening, terminating the recording time.
OSA severity was assessed using apnea-hypopnea index (AHI) derived from PSG or respiratory event index (REI) derived from HSAT (events per hour); both are reported as disordered breathing events per hour of sleep (PSG) or recording (HSAT). The REI term adopted by the AASM was chosen for this analysis to describe the sleep disordered breathing index calculated from a type III HSAT device that does not include electroencephalogram (EEG)-derived total sleep time.1 For PSG, apneas and hypopneas were scored per AASM scoring rules using ≥ 4% oxygen desaturation criteria in addition to nasal flow and sleep arousal criteria associated with hypopnea scoring.32 For HSAT, hypopneas were scored with ≥ 4% oxygen desaturation.
OSA was defined by an AHI or REI ≥ 5 obstructive disordered breathing events per hour. When a confirmatory PSG was performed for negative HSAT studies, the PSG findings were utilized for determining diagnostic categorization. Participants with AHI > 20 on PSG had CPAP titration during the initial testing per protocol; if they were unable to initiate or complete CPAP titration due to factors such as intolerance or time constraints, they generally returned for a second sleep study to initiate CPAP. Participants with REI > 20 on HSAT were generally initiated on aPAP therapy. It was possible, but not common, for in-lab PSG to have home aPAP initiation, or HSAT to have in-lab CPAP titration study based on either insurance coverage and/or participant preference.
PAP Therapy Adherence Data
Although a driver diagnosed with less significant OSA severity (AHI or REI ≥ 5 to 20) could elect to initiate PAP therapy, the factors influencing the decision to initiate or maintain PAP therapy varied widely; within this group at the time of our study, treatment was not generally considered an absolute requirement to maintain medical certification by referring CDMEs. Therefore, this group was excluded from adherence analysis, focusing on participants with an AHI or REI > 20 for which treatment was considered a requirement to maintain medical certification by the referring CDMEs. Participants were offered surgical evaluation as an alternative treatment option; however, all participants chose to initiate PAP therapy.
PAP therapy utilization and adherence data were obtained and analyzed directly within the machine manufacturer software (EncoreAnywhere, Philips Respironics, Pittsburgh, PA; EasyCare Online, ResMed, San Diego, CA; or Infosmart Web, Fisher & Paykel, Irvine, CA). Adherence was assessed at 1 week, 1, 3, and 6 months, and 1 year after PAP equipment was dispensed. Adherence was defined according to the minimum standard recommended by 2 FMCSA expert advisory panels (≥ 4 h/24 h time period for ≥ 70% of days).15,16 The time points assessed included those reflected by regional CDME clinical practice standards for medical certification extensions (issuing 1-month, 3-month, or 1-year extensions) and recommendations to the FMCSA to require a minimum of 1 week adherence.10,11 Although one recommendation10 occasionally references > 4 h while also listing ≥ 4 h, because all PAP device software utilized for this study report ≥ 4 h usage data, this threshold was used in our study.
Usual care in clinical practice was followed, therefore aPAP machine settings could remain unchanged from the initial prescription, adjusted, or converted to CPAP settings anytime during the 1-year follow-up period based on discretion of the treating sleep provider. Similarly, mask style, machine manufacturer, and use of comfort features such as humidification or pressure relief followed usual care in the clinical setting. In general, humidification was provided as a standard option, and pressure relief was reserved as an occasional add-on feature by the sleep provider or scripted order after CPAP titration when indicated. Modems were utilized for approximately 3 months after initialization of therapy unless cost-prohibitive to the patient or durable medical equipment (DME) company. Automated adherence data transmission via modem discontinued after approximately 3 months per DME company protocols; subsequent data capture occurred via home, sleep center, or DME office download. Although clinic follow-up was routinely scheduled and encouraged, data downloads could occur separately from scheduled clinic follow-up visits within the first year. Only PAP machines with objective adherence data recording and reporting were utilized.
A participant was considered lost to follow-up when electronic adherence data were no longer available. In comparing PAP adherence, the analysis tests were completed with those lost to follow-up (i.e., no electronic adherence data available) included and excluded to assess the potential impact of each scenario. Given that our setting was a sleep clinic, where there is potential for natural attrition in a CMV population, this approach allows analysis of adherence behaviors for those continuing to return to the sleep center for care.
Statistical Analysis
Data extracted from the scanned and typed documentation within the EHR and commercially available PAP machine data analysis software were manually entered into a computer database.
All data were presented as number (percent) or median (25th, 75th percentile). Comparisons between characteristics and OSA diagnosis and severity categories were conducted using Fisher exact test (categorical variables) or Wilcoxon rank sum test (measured variables).
CPAP adherence data were assessed with logistic regression models, where adherence category was the dependent variable and each characteristic of interest was the dependent variable. Observations from each adherence time point were included in the models, so generalized estimating equations were used to account for within-patient correlation. Separate logistic regression models including characteristic by time interaction terms were run in order to assess whether the relationship between adherence and each characteristic varied over time.
All analyses were conducted using SAS® (version 9.4, Cary, North Carolina).
RESULTS
Population Descriptions and Sleep Study Findings
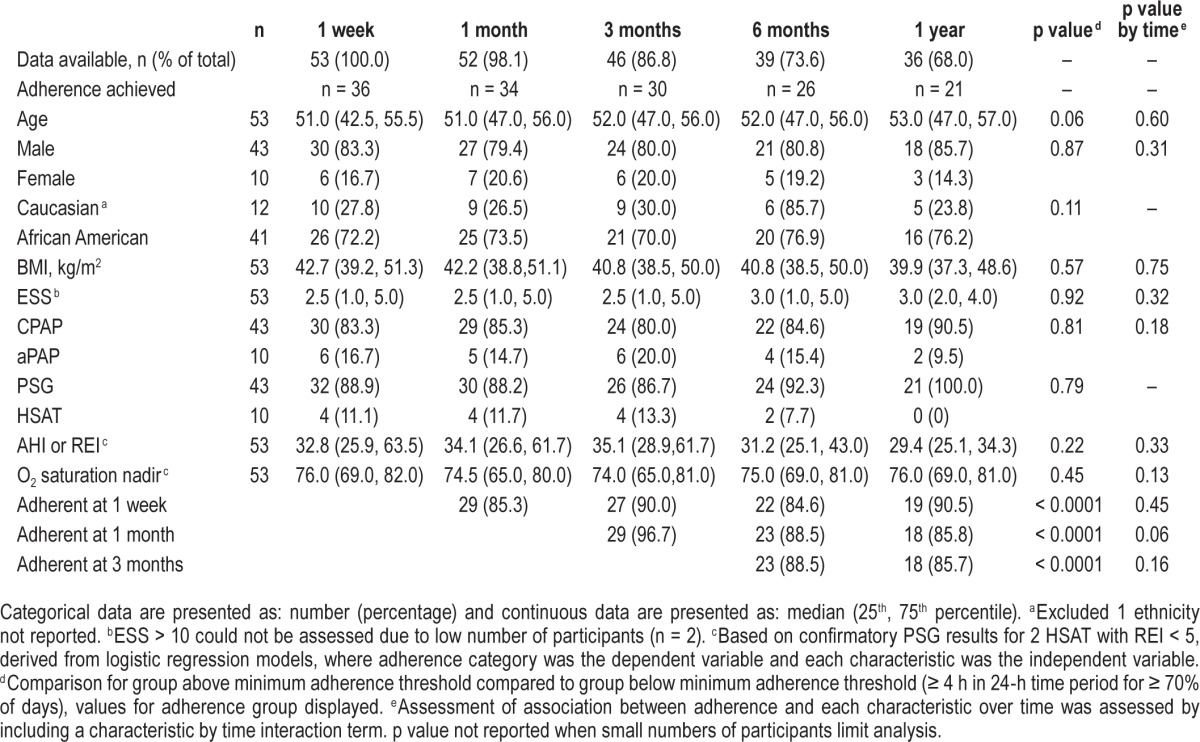
In 1 year, 120 participants had OSA clinical and sleep study assessment (Figure 1). Based on the diagnostic testing, 104 (86.7%) were diagnosed with OSA. For those with AHI or REI > 20 events/h (44.2% of all and 51.0% of OSA-positive participants), CPAP was prescribed for 81.1% and aPAP for 18.9%. Initially, 90.0% were referred for PSG while 10.0% had HSAT. Two participants who had a negative HSAT required PSG; one had an AHI of 3.6 and the other 9.6 events per hour.
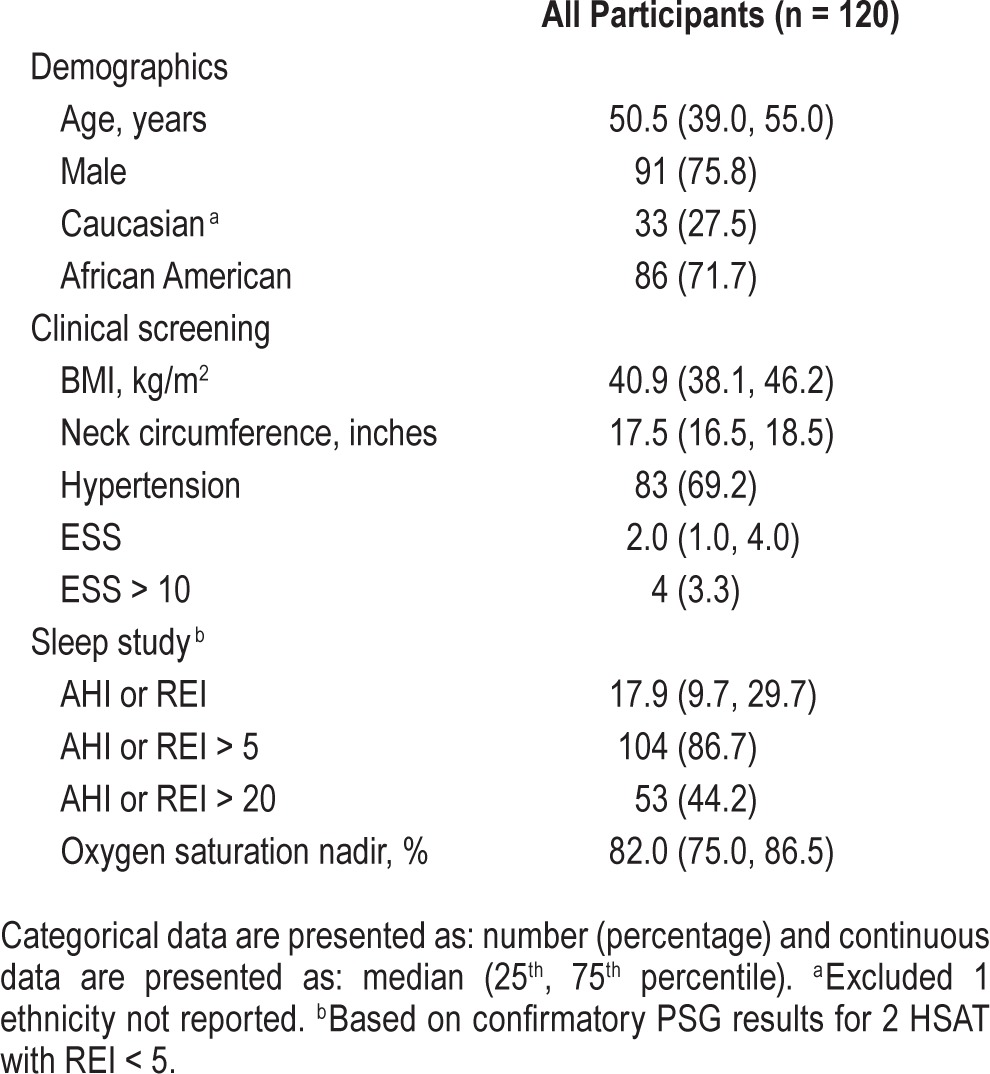
Demographic, clinical evaluation, and sleep study characteristics are presented in Table 1. The population was predominantly middle-aged (range 23–67), African American, and male. As expected, given the OSA screening protocols in use, the population was obese (BMI range 30.2 to 76.1 kg/m2) with large neck circumferences (range 14–24 inches) and hypertension. ESS median scores were low (range 0–12), with only a small number (n = 4) indicating a score > 10. Influenced by the high proportion of OSA positive participants, the AHI or REI median indicated moderate severity (range 0.0–135.7) with abnormal nadir oxygen saturations (range 51.0% to 91.0%).
Table 1.
Demographic, clinical assessment and sleep study characteristics.
Comparison analyses were performed to assess differences among groups differentiated by absence or presence of OSA, severity of OSA, initial sleep testing performed, and gender. In general, few differences were noted among the various groups compared. Comparison of those with or without OSA and categorized by OSA severity are provided in Table 2. Additional analyses were performed comparing groups by initial sleep test type and by gender utilizing the parameters listed in Table 2 (not presented in tables).
Table 2.
Comparison by OSA diagnosis and severity.
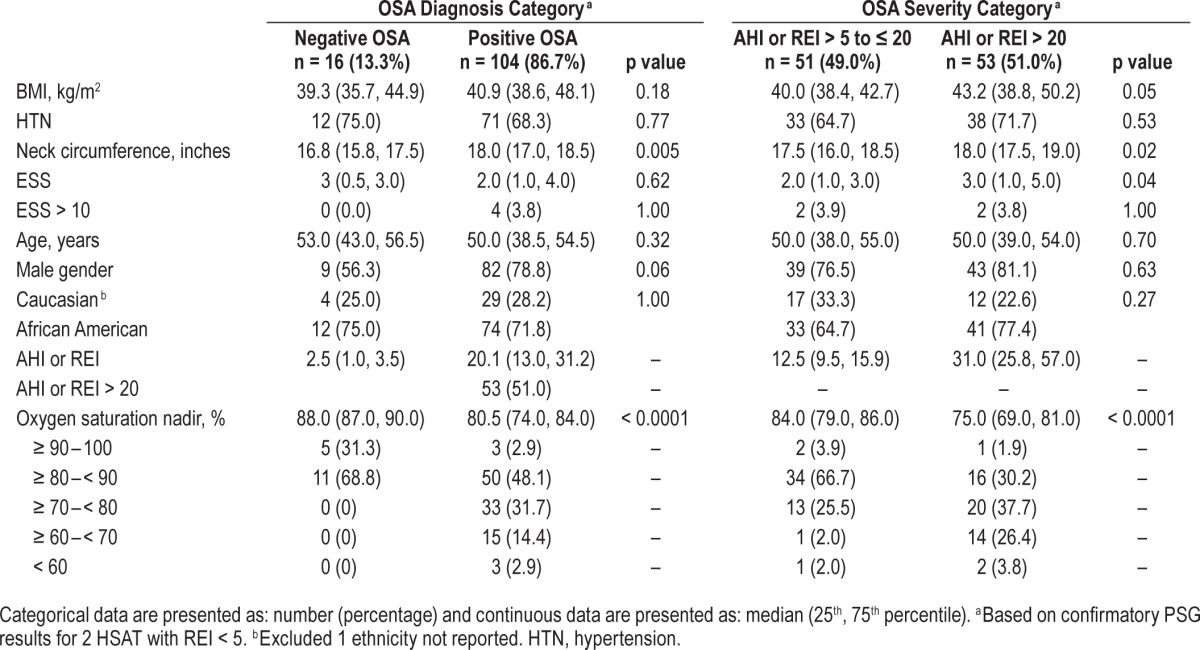
When comparing absence and presence of OSA groups, there was no significant difference other than neck circumference (p = 0.005). Within the positive OSA group, approximately half had an AHI or REI > 20 events/hour. Comparing those with OSA differentiated by OSA severity, the parameters showing statistically significant differences included neck circumference (p = 0.02), ESS (p = 0.04), and oxygen saturation nadir (p < 0.0001). The group with AHI or REI > 20 recorded lower median oxygen saturation nadirs by almost 10 percentage points compared to those with the AHI or REI in the 5 to 20 range. For initial testing type (PSG versus HSAT), no significant difference was noted for any parameters other than ethnicity (p = 0.02) with 26 Caucasians having PSG (78.8%) and 7 undergoing HSAT (21.2%) compared to 81 African Americans having PSG (94.2%) and 5 undergoing HSAT (5.8%). For gender, no significant differences were noted other than neck circumference (p < 0.0001) with the male median value reported as 18.0 inches (25th percentile 17.5 and 75th percentile 19.0) compared to the female median value 16.0 inches (25th percentile 15.0 and 75th percentile 16.5).
PAP Therapy Adherence Analysis
Figure 2 includes the proportion of all drivers at each time point who were adherent, non-adherent or lost to follow-up. From 1 week to 1 year, the proportion of adherent drivers progressively decreased from 68.0% to 39.6% while the non-adherent proportion increased from 32.1% to 41.7% with the loss of 17 participants at 1 year (33.1%).
Figure 2. PAP adherence proportions including subjects lost to follow-up.
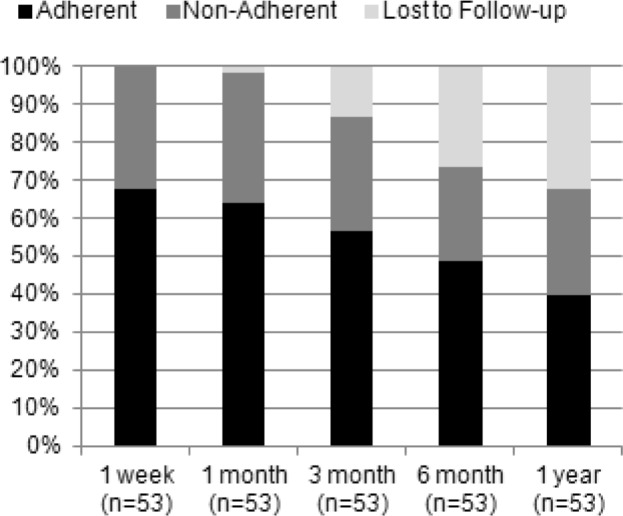
Figure 3 displays the adherent and non-adherent groups in Figure 2 with those lost to follow-up (no electronic adherence data available) removed at each time point. As there was no loss to follow-up at week 1, 68.0% were again adherent at 1 week, with 58.3% of those with electronic data available adherent at 1 year and all time points staying within this range.
Figure 3. PAP adherence proportions excluding subjects lost to follow-up.
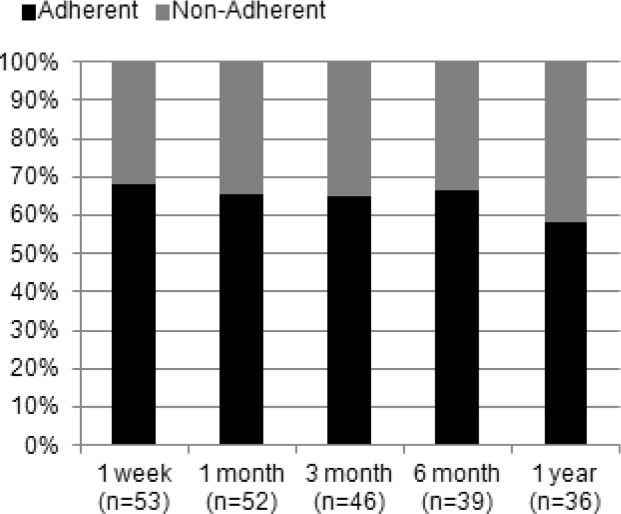
Figures 4 and 5 categorized participants based on their adherence at 1 week with lost-to follow up participants included in the denominator for calculations. In Figure 4, the adherent group is represented; at 1 month, 80.6% were adherent decreasing to 52.8% by 1 year. The group non-adherent at week 1 is represented in Figure 5; the proportion of those adherent at 1 month was 29.4% decreasing to 11.7% (n = 2) by 1 year.
Figure 4. Follow-up adherence for subjects adherent at week 1.
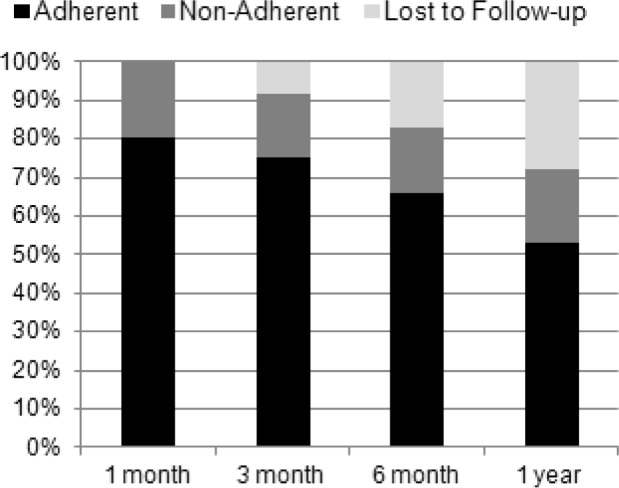
Figure 5. Follow-up adherence for subjects not adherent at week 1.
In Table 3, participants were analyzed assessing the association between the listed characteristics with adherence as well as an assessment of any variation in the effect over time. Similar to previous tables, demographic, clinical assessment, and sleep study parameters were included with the addition of PAP type, sleep study type and early PAP adherence. In this population, only early adherence (at time points 1 week, 1 month, and 3 months) predicted PAP adherence overall (p < 0.0001) with no variation in this effect over time. Performing the same analysis, assuming non-adherence for those lost to follow-up (data not shown), yielded similar results with small exceptions. Age showed a significant difference overall (p = 0.03); however, this did not vary with time (p = 0.57). A similar strong effect was noted for early adherence at 1 week (p = 0.0002), 1 month (p < 0.0001), and 3 months (p = 0.002) with a significant variation over time noted at 1 month only (p = 0.0069).
Table 3.
Participants achieving PAP adherence: demographic, clinical assessment and sleep study characteristics over time, excluding participants lost to follow-up.
DISCUSSION
Compared to prior studies13,32 representing a total of 14 CMV drivers on CPAP, our study includes a much larger number of CMV drivers on PAP therapy and provides a more detailed analysis of PAP adherence patterns and predictors. This study focused on assessment of PAP adherence over 1 year in CMV drivers, but also provided an opportunity to assess clinical and diagnostic parameters of a CMV driver population specifically within a sleep center environment after referral by a CDME.
PAP Therapy Adherence
When considering PAP adherence in a CMV population, it begs the question of which method of adherence promotion may have more impact, the “carrot” or the “stick.” Within CMV populations, the “stick” is the focus, yet in this study, acceptable adherence levels were not observed in a potentially high stakes environment.
PAP therapy utilization patterns (as early as 1 week) provided reasonable prediction of future PAP usage compared to other parameters assessed, consistent with findings in clinical research populations.26–29 The group adherent at 1 week showed much higher proportions of adherent participants at subsequent time points compared to those participants who were non-adherent at 1 week. For non-adherent participants at week 1, the number adherent at 1 year in our study was quite low (11.7%). Based on these findings, taken in context with findings in clinical research populations, the role of behavior in maintaining PAP therapy adherence appears to be notable and deserves further study within CMV driver populations.
In our safety-sensitive population, the poor PAP adherence rates observed and high loss to follow-up in a sleep center setting were particularly disappointing. Approximately one-third of participants failed to maintain sufficient PAP therapy usage to even meet the low bar of at least 4 hours for at least 70% of 24-hour time periods. Although not studied specifically in CMV drivers, scientific evidence suggests a dose-response effect from CPAP in clinical patients, suggesting there may be more to gain from additional usage above minimum standards.20,21,25,36 Policies for extending medical certification should be based on early PAP therapy adherence measured objectively, with shorter intervals for temporary certification extensions until minimum adherence is attained. For those identified as having poor adherence early, there may be a role for psychologically based interventions; however, exactly how these impact a CMV driver population remains untested, and research continues in this area for clinical populations.37
It is reassuring to note that the type of initial sleep testing performed (PSG versus HSAT) or the PAP therapy modality initiated (CPAP versus aPAP) did not significantly impact adherence in our population. Diagnostic testing decision-making has shifted away from the direct control of the ordering provider due to influences from health insurance policies, employer-based testing programs, or patient factors. In cases where the clinical assessment identifies the driver as having a high pretest probability of OSA, being limited to HSAT as a first step may be acceptable. However, due to the limitations of HSAT, it is not reliable to “rule-out” OSA.
The significance PAP adherence data loss had on our study is difficult to assess. Factors such as normal attrition within the CMV driver population likely contribute; however, other factors including changes in employer-based OSA screening program contractual agreements can impact follow-up patterns. Taking the perspective of the sleep specialist, the behaviors of those continuing to return to for data download and clinic follow-up may be the primary focus. However, for the CDME or employer, this may be a different issue if it affects driver productivity and retention. Further study of PAP adherence behaviors in a setting that allows better capture of follow-up participants would be beneficial.
OSA Diagnosis after Clinical Screening
Our study found 86.7% of those evaluated based on CDME referral had undiagnosed OSA; a similar finding to prior studies in occupational settings reporting 79% to 100% of drivers with a positive clinical screen had OSA.11–13,17 Over half of those diagnosed with OSA met requirements to initiate PAP therapy based on OSA severity indices alone (AHI or REI > 20).
There were only few statistically significant differences among groups when assessing demographic, clinical, and testing parameters compared by OSA diagnosis, OSA severity, sleep study type, or gender. Most of these differences could be accounted for based on known clinical differences within the populations (e.g., neck circumference, ESS, and oxygen saturation nadir). Given the general similarity of these groups in the absence of sleep study results, the utilization of objective sleep testing as part of the risk assessment of the CMV driver is essential. The difference in testing attributed to ethnicity is difficult to explain without assessing for other influential factors that may contribute to this finding which are beyond the scope of this study. However, we hypothesize, based on general knowledge of our CMV driver population, some influence may be due to employer-based factors influencing medical insurance policies for testing coverage which can favor one sleep study type over another. Racial demographics within companies vary and may be associated with policies favoring PSG versus HSAT or vice versa.
Concern has been expressed about those who may have OSA that escape detection when applying the screening criteria proposed by the joint task force (JTF) or FMCSA consensus panels.34 This is an important concern, yet, it is currently impractical to objectively test all CMV drivers due to resource limitations.35 Therefore, having a method that helps to identify the “low hanging fruit” while balancing effectiveness with accessibility and cost is an important step forward. The standard of medical care both within occupational and clinical settings should be to screen for OSA in CMV drivers using comprehensive screening protocols. It is again important to note that given the technical limitations of HSAT, subsequent confirma-tory PSG is recommended for any non-diagnostic HSAT.
Limitations
As a retrospective uncontrolled study, data available and collected from standard clinical and testing protocols may have limitations including loss of patients, loss of data and data inaccuracies when data collection is occurring as part of clinical care. Because this study relied on assessment of those CMV drivers already referred to the sleep center by a CDME, our findings may be limited by referral and scheduling bias. The study relied on the criteria applied by the CDME to the driver and the authors cannot assess the CDME patterns of referral compared to those not referred for sleep apnea clinical evaluation.
During the time frame of this study, many factors were exerting influence on elements of OSA screening, testing and treatment in occupational health environments. These include employer-based OSA screening initiatives, transient behaviors for commercial drivers seeking medical certification, medical insurance adoption of home sleep apnea testing, and changes to medical insurance programs through the Affordable Care Act. The full impact of these variables cannot be assessed but could be considered a moving target at times based on our experience.
Because our study assessed patterns and practices within a clinical environment, we could not control the testing or PAP therapy types utilized, nor did we have a protocol for addressing participants lost to follow-up beyond standard clinical practices. With a dedicated research team, it may be possible to better address data loss issues; however, this would be weighed against the potential for losing CMV drivers from analysis when specifically recruited for research purposes.
For those initiated on PAP therapy, there was a progressively increasing rate of loss to follow-up in the year monitored after treatment initiation. The exact impact this may have cannot be assessed within this study. However, it is important to note that some element of loss to follow-up may be found within an occupational health population.
CONCLUSIONS
Based on our study findings, consideration should be given to early assessment of PAP therapy utilization, even as early as 1 week after therapy initiation. When a CMV driver is not achieving minimum adherence recommendations, close monitoring is recommended to observe for changes in this behavioral pattern to suggest successful adaptation to PAP therapy as evidenced by routine and sufficient use to meet minimum adherence threshold recommendations.
Assuming we move beyond the current climate of federal impotence in establishing standards for OSA screening, assessment, and treatment, continued research is needed to better understand the relationship between OSA identification, treatment and safety outcomes. While estimates suggest high prevalence of OSA within CMV drivers, not all of these drivers are involved in crashes. Better tools are needed to identify the subgroup at higher risk of driving performance impairment to focus more resources on this group and guide decisions regarding maintaining a driver in or out of service during evaluation. But, the benefit of screening and testing is limited when treatment itself is imperfect. Therefore, sleep specialists, CDMEs, and regulators need to consider PAP adherence specifically within the CMV population and the challenges to successful treatment that may present.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Ojile has received research support from Fisher-Paykel and Phillips. Dr. Collop has received royalties from Up-To-Date and honoraria from Best Doctors. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Mark Muehlbach, PhD, DABSM, for his assistance with this project.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- aPAP
auto-titrating positive airway pressure
- BMI
body mass index
- CDME
commercial driver medical examiner
- CMV
commercial motor vehicle
- CPAP
continuous positive airway description
- DME
durable medical equipment
- EHR
elecronic health record
- EEG
electroencephalography
- ESS
Epworth Sleepiness Scale
- FDA
Food and Drug Administration
- FMCSA
Federal Motor Carrier Safety Administration
- HSAT
home sleep apnea test
- JTF
Joint Task Force
- MVC
motor vehicle crash
- O2
oxygen
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PSG
polysomnogram
- REI
respiratory event index
- SAS
Statistical Analysis System
REFERENCES
- 1.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Tregear SJ, Tiller M, Fontarrosa J, Price N, Akafomo C. McLean, VA: Manila Consulting Group; 2007. Executive summary obstructive sleep apnea and commercial vehicle driver safety [Internet] [cited 2015 Feb 11]. Available from: http://ntl.bts.gov/lib/30000/30100/30187/Final_Sleep_Disorders_Evid_Report_Vol._1.pdf. [Google Scholar]
- 3.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Ellen RB, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Son-Hing M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2:193–200. [PubMed] [Google Scholar]
- 5.Hiestand D, Phillips B. Obstructive sleep apnea syndrome: assessing and managing risk in the motor vehicle operator. Curr Opin Pulm Med. 2011;17:412–8. doi: 10.1097/MCP.0b013e32834b96a4. [DOI] [PubMed] [Google Scholar]
- 6.Karimi M, Hedner J, Häbel H, Nerman O, Grote L. Sleep apnea related risk of motor vehicle accidents is reduced by continuous positive airway pressure: Swedish traffic accident registry data. Sleep. 2015;38:341–9. doi: 10.5665/sleep.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard ME, Desai AV, Grunstein RR, et al. Sleepiness, sleep-disordered breathing, and accident risk factors in commercial vehicle drivers. Am J Respir Crit Care Med. 2004;170:1014–21. doi: 10.1164/rccm.200312-1782OC. [DOI] [PubMed] [Google Scholar]
- 8.Meuleners L, Fraser ML, Govorko MH, Stevenson MR. Obstructive sleep apnea, health-related factors, and long distance heavy vehicle crashes in Western Australia: a case control study. J Clin Sleep Med. 2015;11:413–8. doi: 10.5664/jcsm.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartenbaum N, Collop N, Rosen IM, et al. Sleep apnea and commercial motor vehicle operators. J Occup Environ Med. 2006;48:S4–37. doi: 10.1097/01.jom.0000236404.96857.a2. [DOI] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Czeisler CA, George CF, Guilleminault C, Pack AI. Washington DC: 2008. Obstructive sleep apnea and commercial driver safety. [cited 2015 Feb 11]. Available from: http://www.fmcsa.dot.gov/sites/fmcsa.dot.gov/files/docs/Sleep-MEP-Panel-Recommendations-508.pdf. [Google Scholar]
- 11.Parker DR, Hoffman BH. Washington DC: Federal Motor Carrier Safety Administration; 2012. Motor carrier safety advisory committee and medical review board task 11-05 report. [cited 2015 Feb 11]. Available from: http://mcsac.fmcsa.dot.gov/Reports.htm. [Google Scholar]
- 12.Talmage JB, Hudson TB, Hegmann KT, Thiese MS. Consensus criteria for screening commercial drivers for obstructive sleep apnea: evidence of efficacy. J Occup Environ Med. 2008;50:324–29. doi: 10.1097/JOM.0b013e3181617ab8. [DOI] [PubMed] [Google Scholar]
- 13.Parks PD, Durand G, Tsismenakis AJ, Vela-Bueno A, Kales S. Screening for obstructive sleep apnea during commercial driver medical examinations. J Occup Environ Med. 2009;51:275–82. doi: 10.1097/jom.0b013e31819eaaa4. [DOI] [PubMed] [Google Scholar]
- 14.Xie W, Chakrabarty S, Levine R, Johnson R, Talmage JB. Factors associated with obstructive sleep apnea among commercial motor vehicle drivers. J Occup Environ Med. 2011;53:169–73. doi: 10.1097/JOM.0b013e3182068ceb. [DOI] [PubMed] [Google Scholar]
- 15.Washington DC: U.S. Department of Transportation; Federal Motor Carrier Safety Administration, Department of Transportation, 49 C.F.R., Sect. 390.5, Definitions. [n.d.; cited 2015 Feb 11]. Available from: http://www.fmcsa.dot.gov/regulations/title49/section/390.5. [Google Scholar]
- 16.U.S. Federal Motor Carrier Safety Administration. Washington DC: U.S. Department of Transportation; Medical examination report for commercial driver fitness determination. [updated 2014, Mar 19; cited 2015 Feb 11]. Available from: http://www.fmcsa.dot.gov/sites/fmcsa.dot.gov/files/docs/Medical_Examination_Report_for_Commercial_Driver_Fitness_Determination_649-F%286045%29.pdf. [Google Scholar]
- 17.Berger M, Varvarigou V, Rielly A, Cxeisler CA, Malhotra A, Kales SN. Employer-mandated sleep apnea screening and diagnosis in commercial drivers. J Occup Environ Med. 2012;54:1017–25. doi: 10.1097/JOM.0b013e3182572e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colvin LJ, Collop NA. Commercial motor vehicle driver obstructive sleep apnea screening and treatment in the United States: an update and recommendation overview. J Clin Sleep Med. 2016;12:113–25. doi: 10.5664/jcsm.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33:1373–80. doi: 10.1093/sleep/33.10.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future directions. Indian J Med Res. 2010;131:245–58. [PMC free article] [PubMed] [Google Scholar]
- 21.Sawyer AM, Gooneratne NS, Marcus CL. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–56. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai-Coetzer CL, Lou YM, Antic NA, Zhang XL, Chen BY, He QY. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep. 2013;36:1929–37. doi: 10.5665/sleep.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balachandran JS, Yu X, Wroblewski K, Moklesi B. A brief survey of patients' first impression after CPAP titration predicts future CPAP adherence: a pilot study. J Clin Sleep Med. 2013;9:199–205. doi: 10.5664/jcsm.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wickwire EM, Lettieri CJ, Cairns AA, Collop N. Maximizing positive airway pressure adherence in adults. Chest. 2013;144:680–93. doi: 10.1378/chest.12-2681. [DOI] [PubMed] [Google Scholar]
- 26.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over first three months of treatment. Sleep. 1997;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 27.Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5:229–40. doi: 10.1080/15402000701264005. [DOI] [PubMed] [Google Scholar]
- 28.Budhiraja R, Partheserathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30:320–4. [PubMed] [Google Scholar]
- 29.Strohl KP, Brown DB, Colop NC, George C, Gunstein R, Han F. An official American Thoracic Society clinical practice guideline: sleep apnea, sleepiness and driving risk in noncommercial drivers. Am J Respir Crit Care Med. 2013;187:1259–66. doi: 10.1164/rccm.201304-0726ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aloia MS. Understanding the problem of poor CPAP adherence. Sleep Med Rev. 2011;15:341–2. doi: 10.1016/j.smrv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Olsen S, Smith S, Oei T, Douglas J. Health belief model predicts adherence to CPAP before experrience with CPAP. Eur Respir. 2008;32:710–17. doi: 10.1183/09031936.00127507. [DOI] [PubMed] [Google Scholar]
- 32.Hui DS, Ko FW, Chan JK, et al. Sleep-disordered breathing and continuous positive airway pressure compliance in a group of commercial bus drivers in Hong Kong. Respirology. 2006;11:723–30. doi: 10.1111/j.1440-1843.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 33.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. [Google Scholar]
- 34.Platt AB, Wick LC, Hurley S, et al. Hits and misses: screening commercial drivers for obstructive sleep apnea using guidelines recommended by a joint task force. J Occup Environ Med. 2013;55:1035–40. doi: 10.1097/JOM.0b013e318298fb0e. [DOI] [PubMed] [Google Scholar]
- 35.Hartenbaum NP, Phillips B, Collop N. Response to “hits and misses: screening commercial drivers for obstructive sleep apnea using guidelines recommended by a joint task force” by Platt et al. J Occup Environ Med. 2014;56:119–21. doi: 10.1097/JOM.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 36.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai AY, Fong DY, Lam JC, Weaver T, Ip MS. The efficacy of a brief motivational enhancement education program on CPAP adherence in OSA. Chest. 2014;146:600–10. doi: 10.1378/chest.13-2228. [DOI] [PubMed] [Google Scholar]


