Abstract
Study Objectives:
Poor sleep quality and short sleep duration have been associated with elevated risk for several cancer types; however, the relationship between sleep and cancer outcomes has not been well characterized. We assessed the association between pre-diagnostic sleep attributes and subsequent cancer survival within the Women's Health Initiative (WHI).
Methods:
We identified WHI participants in whom a first primary invasive cancer had been diagnosed during follow-up (n = 21,230). Participants provided information on sleep characteristics at enrollment. Cox regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between these pre-diagnostic sleep characteristics and cancer-specific survival for all cancers combined and separately for common cancers. Analyses were adjusted for age, study arm, cancer site, marital status, income, smoking, physical activity, and time to diagnosis.
Results:
No individual pre-diagnostic sleep characteristics were found to be significantly associated with cancer survival in analyses of all cancer sites combined; however, women who reported short sleep duration (≤ 6 h sleep/night) combined with frequent snoring (≥ 5 nights/w experienced significantly poorer cancer-specific survival than those who reported 7–8 h of sleep/night and no snoring (HR = 1.32, 95% CI: 1.14–1.54). Short sleep duration (HR = 1.46, 95% CI: 1.07–1.99) and frequent snoring (HR = 1.34, 95% CI: 0.98–1.85) were each associated with poorer breast cancer survival; those reporting short sleep combined with frequent snoring combined had substantially poorer breast cancer survival than those reporting neither (HR = 2.14, 95% CI: 1.47–3.13).
Conclusions:
Short sleep duration combined with frequent snoring reported prior to cancer diagnosis may influence subsequent cancer survival, particularly breast cancer survival.
Citation:
Phipps AI, Bhatti P, Neuhouser ML, Chen C, Crane TE, Kroenke CH, Ochs-Balcom H, Rissling M, Snively BM, Stefanick ML, Treggiari MM, Watson NF. Pre-diagnostic sleep duration and sleep quality in relation to subsequent cancer survival. J Clin Sleep Med 2016;12(4):495–503.
Keywords: cancer, sleep duration, snoring, survival
INTRODUCTION
Poor sleep quality has been associated with an elevated risk for several types of cancer.1–8 Specific mechanisms underlying these risks have not been fully elucidated, although sleep curtailment has been suggested to trigger inflammatory processes,9 contribute to the production of reactive oxygen species and oxidative DNA damage,10 and alter immune function.11 Frequent snoring, as an indicator of possible obstructive sleep apnea, may contribute to increased levels of oxidative stress and systemic inflammation stemming from intermittent hypoxia.12,13 Such mechanisms may also have implications for cancer survival. However, while associations between indicators of sleep and cancer incidence have previously been documented, few studies have explored a possible relationship between sleep and cancer survival.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Poor sleep quality and short sleep duration have been associated with elevated risk for several cancer types; however, the relationship between sleep and cancer outcomes has not been well characterized. We assessed the association between pre-diagnostic sleep attributes and subsequent cancer survival within the Women's Health Initiative (WHI).
Study Impact: To our knowledge, this is the first study to evaluate the relationship between sleep patterns before cancer diagnosis and subsequent cancer survival. Findings from this study suggest that short sleep duration may be associated with poor breast cancer survival.
To date, epidemiologic studies of sleep and cancer survival have focused on sleep assessments collected after cancer diagnosis.14–21 In particular, previous studies have reported that post-diagnostic actigraphy-based measures of sleep efficiency and sleep quality are independent predictors of overall survival in women with advanced breast cancer19 and in patients with metastatic colorectal cancer,20 respectively. Using biomarkers to assess the slope of circadian cycling, other studies have suggested poorer survival in lung,22 renal cell,21 and breast15 cancer patients with flattened daily fluctuations in cortisol levels relative to those with steeper diurnal cortisol slopes. Although evidence from such studies suggests an important relationship between circadian rhythms and cancer prognosis, inference is limited by concerns of reverse causality, as measurements of sleep collected post-diagnostically could be affected by disease severity and treatment.
Using data from the Women's Health Initiative (WHI), we assessed the association between baseline measures of sleep duration, snoring, other sleep patterns, and survival among women with a subsequent diagnosis of cancer during study follow-up.
METHODS
Study Population
The WHI is a longitudinal study of postmenopausal women composed of three overlapping randomized controlled trials (CT) and an observational study (OS). WHI recruitment protocols and eligibility criteria have been described in detail elsewhere.23 Briefly, postmenopausal women between the ages of 50–79 y were enrolled between October 1, 1993, and December 31, 1998, from 40 participating centers across the United States. Women with a predicted survival less than 3 y, and those unlikely to remain in the same geographic area for at least 3 y, were excluded from participation. Additional eligibility criteria pertaining to competing risks, likely adherence, and safety were imposed for women interested in participating in the WHI CT; women not meeting these additional eligibility criteria, or who were not interested in participating in the CT, were given the opportunity to enroll in the WHI OS. All women provided written informed consent for participation at the time of enrollment and, additionally, for the WHI Extension periods (2005–2010 and 2010–2015). Human Subjects Review Committees at all participating institutions approved the study protocol.
In total, 161,808 women enrolled in the WHI. The current analysis was restricted to participants who were cancer-free at enrollment and with a subsequent diagnosis of an invasive primary cancer during study follow-up (n = 27,180), excluding those with cancer diagnoses based on death certificates only (n = 2,125), those who reported a history of cancer at the time of study enrollment (n = 2,724), those without a date of last known follow-up (n = 7), those with an unknown cause of death (n = 549), those with a diagnosis of an invasive cancer within 6 mo of baseline data collection (n = 495), and those who died within 1 y of study enrollment (n = 49). Women who did not provide information on any sleep habits at the time of baseline data collection were also excluded (n = 63). The analytic study sample thus included 21,230 women.
Case Identification and Follow-up for Survival Outcomes
Cancer diagnoses were self-reported by study participants at regular follow-up interviews, and were verified locally by WHI physician adjudicators based on a review of medical records and pathology reports.24 Women in whom multiple invasive cancers had been diagnosed at different anatomic sites during follow-up (n = 1,329) were classified according to the site of their first primary cancer diagnosed during the study period. Vital status was ascertained through regular follow-up with study participants and surrogates, and through regular linkage to the National Death Index. Cause of death was determined based on central adjudication of death certificates. Ascertainment of cancer diagnoses and vital status was conducted through August 2014.
Data Collection for Sleep Patterns
Information on demographic factors, medical history, physical activity, height, weight, and sleep patterns were collected at study enrollment via self-administered questionnaires. Ten sleep questions were included in the WHI baseline assessment, including items related to sleep duration, falling asleep during quiet activity, napping, sleep medication use, and snoring, as well as five items that constitute the validated WHI Insomnia Rating Scale (WHIIRS)25: trouble falling asleep, waking up at night, waking earlier than planned, trouble falling back to sleep after waking, and restfulness of sleep. An overall insomnia burden score was calculated based on components from the WHIIRS. All sleep items have previously been used in studies of other health outcomes, including incidence of breast,26 colorectal,27 thyroid,5 and endometrial cancers.4 Given that baseline data collection preceded cancer diagnosis for all women included in this analysis, we refer to these measures of sleep habits as pre-diagnostic measures.
Statistical Analysis
We used Cox proportional hazards regression to evaluate the association between self-reported pre-diagnostic sleep characteristics and cause-specific survival after cancer diagnosis, with the time axis based on time since the first primary invasive cancer diagnosis during study follow-up. Primary analyses focused on cause-specific survival; the endpoint was defined as death attributed to cancer at the site of a participant's first primary cancer during the study period. Women who died from other causes were censored at the time of death. We evaluated associations with individual and composite sleep variables (e.g., WHIIRS, combined sleep duration and snoring) for all cancer cases combined. We also conducted parallel analyses of sleep characteristics stratified by cancer site for five of the six most common causes of cancer death within the analytic population: breast cancer, lung cancer, colorectal cancer, ovarian cancer, and non-Hodgkin's lymphoma (NHL). Pancreatic cancer accounted for more deaths than ovarian cancer and NHL, but was not considered separately as it was anticipated that the very high fatality rate of pancreatic cancer would likely limit the effect of pre-diagnostic external factors (e.g., sleep patterns) on survival as compared to cancers with more variable prognosis. Proportional hazards assumptions were verified by testing for a nonzero slope of the scaled Schoenfeld residuals on ranked failure times.28
Regression models were adjusted for age at enrollment (continuous), marital status, household income, smoking history, and recreational physical activity; baseline hazards were also stratified on age at enrollment (5-y categories), study arm, and cancer site. Categorizations used for these variables are illustrated in Table 1. We also adjusted for the time lag between baseline data collection and cancer diagnosis (continuous); the median lag was 7.5 y (interquartile range, 4.0–11.9 y). We explored further adjustment for educational history, race/ethnicity, alcohol consumption, coffee consumption, dietary caffeine intake (including coffee, tea, and soda), body mass index (BMI), waist circumference, and time since menopause; however, point estimates changed by less than 10% with the addition of these variables to the analytic model. Although women with missing data on model covariates were excluded from primary analyses (n = 2,203), we conducted exploratory analyses using multiple imputation29,30 to account for missing data. Imputation models included all variables listed in Table 1 and all variables included in the primary analytic models. Iterative rounds of imputation (n = 10) were performed using the mi command in STATA SE version 13.1 (College Station, TX).
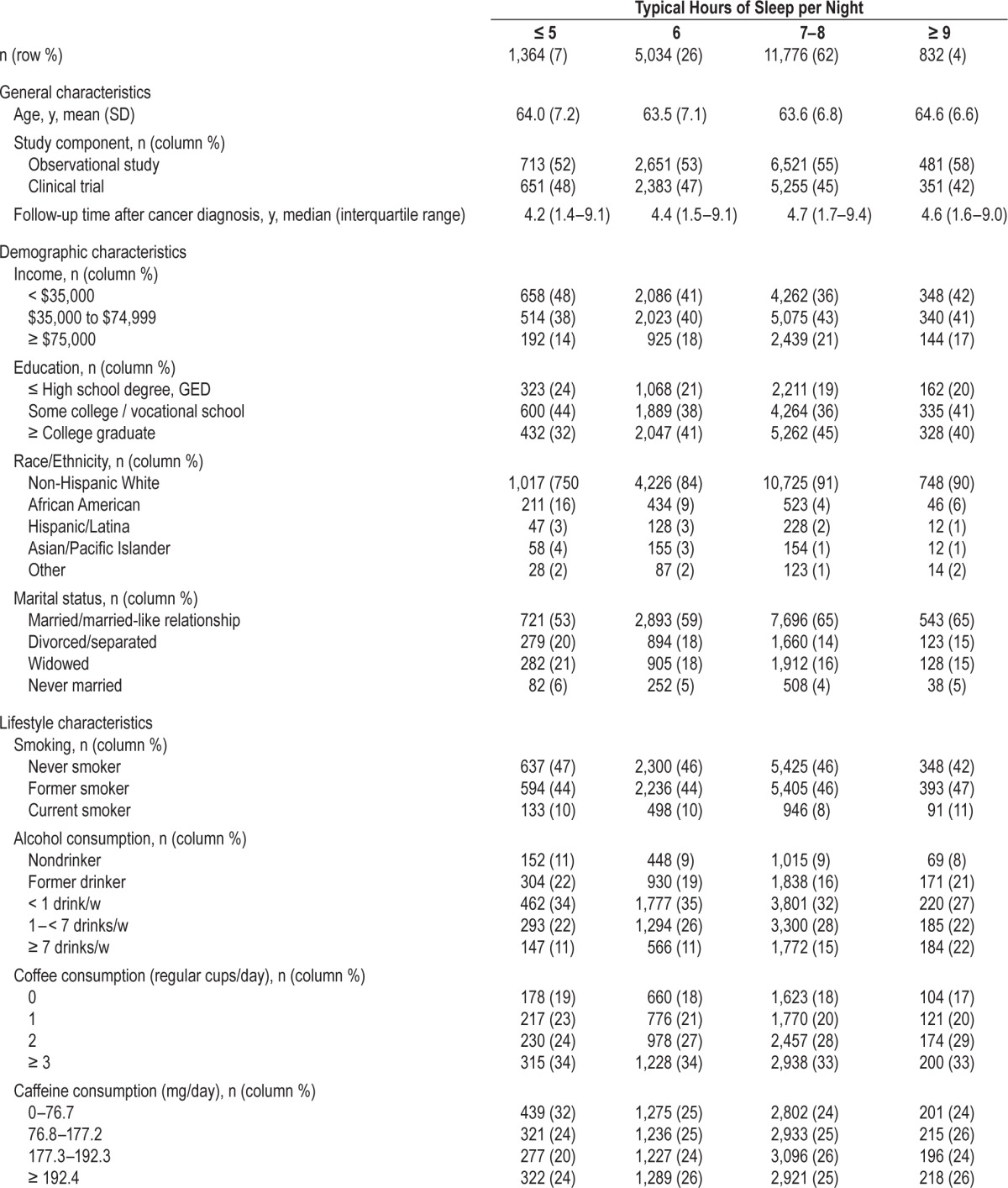
Table 1.
Characteristics of participants at study entry across levels of baseline (pre-diagnostic) sleep duration.
In addition to primary analyses, we conducted sensitivity analyses restricted to women diagnosed with their first primary invasive cancer less than 10 y after baseline (n = 13,406), women with a diagnosis less than 5 y after baseline (n = 6,605), non-Hispanic white participants (n = 18,690), and African American participants (n = 1,352). We also conducted analyses stratified by BMI at enrollment (< 25, 25–29.9, ≥ 30 kg/m2).
RESULTS
Attributes of the analytic study population are provided in Table 1. Most participants (62%) reported receiving 7–8 h of sleep/night. In comparison to this predominant group, participants who reported ≤ 5 h of sleep/night had, on average, a lower household income, less education, higher BMI and waist circumference, were more likely to be nonwhite or unmarried, were less likely to consume ≥ 1 alcoholic beverage/w, were less physically active, and had experienced menopause less recently. Approximately half the study participants were unaware of their snoring habits (n = 9,712); these individuals were more likely to be unmarried, were slightly older, and had lower income relative to those who were able to report their snoring patterns, but were otherwise similar with respect to demographic characteristics, lifestyle factors, and sleep patterns (not shown).
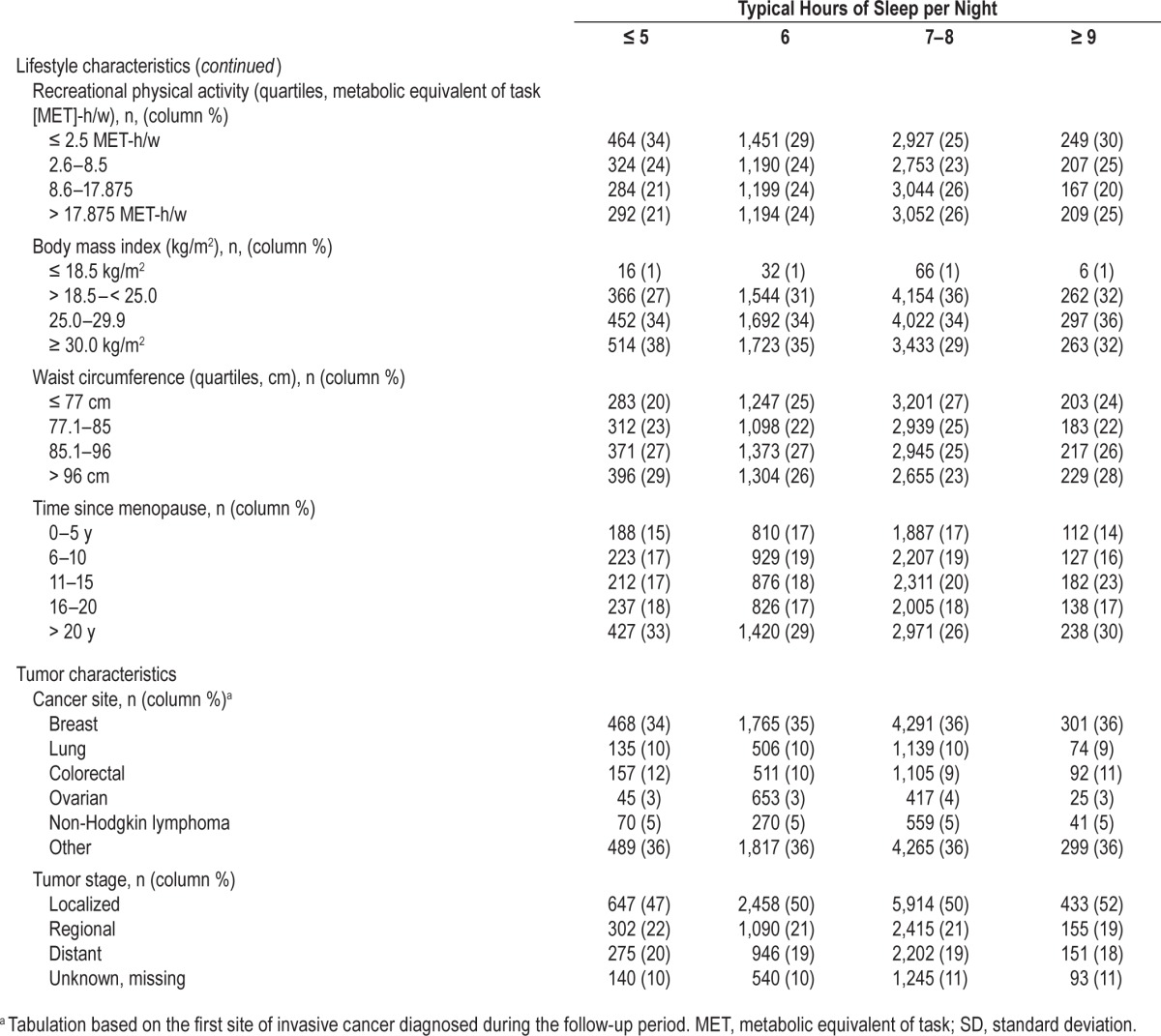
In primary analyses of cause-specific survival, no associations were noted with respect to insomnia burden level (i.e., WHIIRS summary score), sleep quality, frequency of daytime napping, or use of medications or alcohol as sleep aides, regardless of multivariable adjustment (Table 2). Modest associations with poorer survival were noted for individuals who reported ≤ 5 h of sleep/night (hazard ratio [HR] = 1.13, 95% confidence interval [CI]: 1.01–1.26 relative to 7–8 h/night) or frequent snoring (HR = 1.22, 95% CI: 1.09–1.36 for snoring ≥ 5 nights/w versus not in the past 4 w) in age-adjusted analyses; however, both associations were attenuated and non-significant after multivariable adjustment.
Table 2.
Association between pre-diagnostic sleep habits and cause-specific survival after cancer diagnosis.
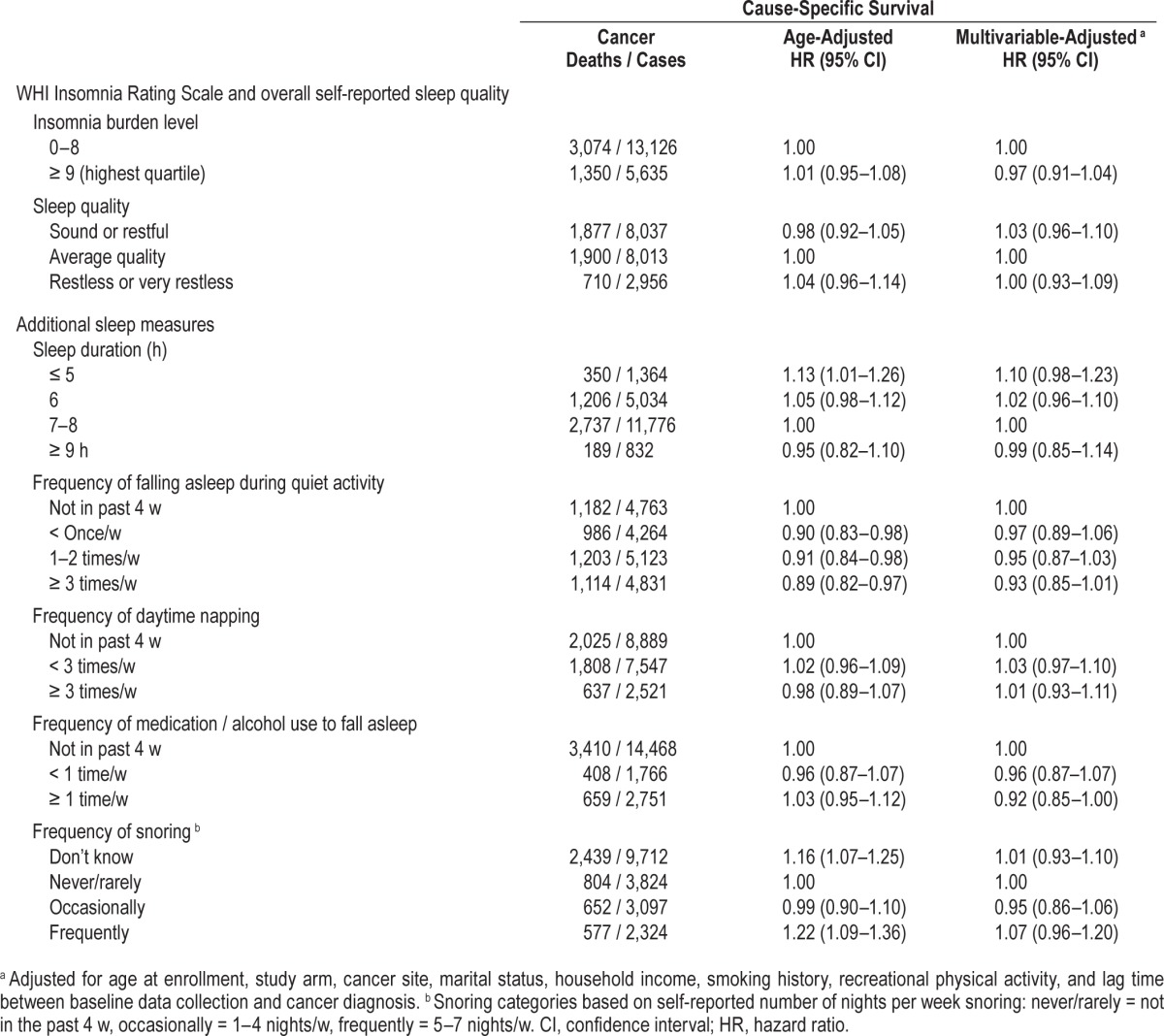
In multivariable-adjusted analyses conducted within strata defined by cancer site (Table 3), short sleep duration was associated with significantly poorer breast cancer-specific survival (HR = 1.46, 95% CI: 1.07–1.99 relative to 7–8 h/night) and was suggestively, but not significantly associated with poorer ovarian cancer (HR = 1.30, 95% CI: 0.85–1.98) and lung cancer survival (HR = 1.18, 95% CI: 0.95–1.47). Frequent snoring was also modestly, but not significantly associated with poorer survival for women with breast cancer (HR = 1.34, 95% CI: 0.98–1.85), colorectal cancer (HR = 1.33, 95% CI: 0.94–1.88), and NHL (HR = 1.35, 95% CI: 0.83–2.21).
Table 3.
Association between pre-diagnostic sleep habits and cause-specific survival after cancer diagnosis by cancer site.
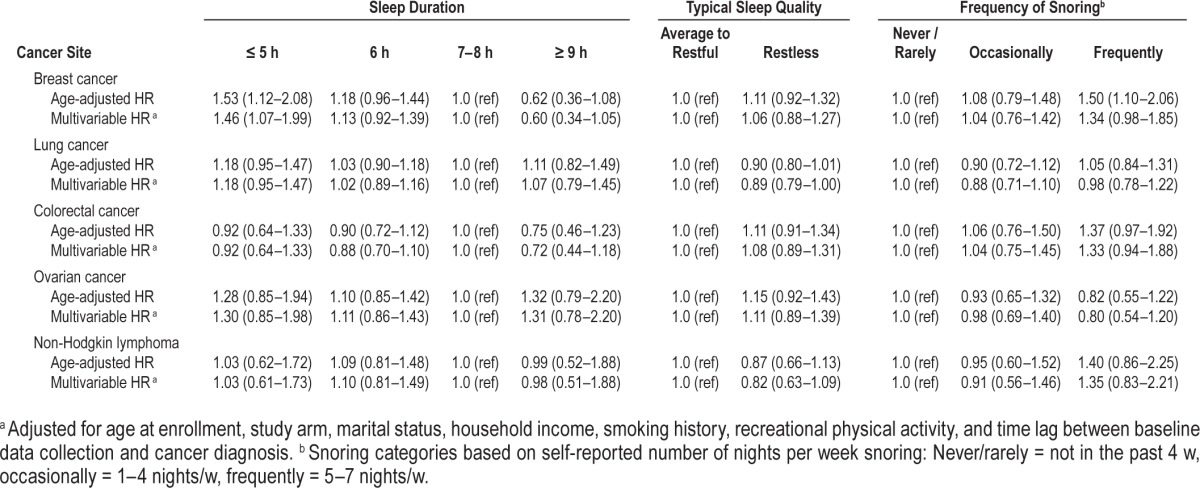
In analyses combining sleep duration and snoring variables, women who reported short sleep duration (≤ 6 h/night) combined with frequent snoring (≥ 5 nights/w) experienced significantly poorer cause-specific survival relative to women who received 7–8 h of sleep/night and were not frequent snorers (HR = 1.32, 95% CI: 1.14–1.54) (Figure 1). This association was particularly pronounced in women with breast cancer (HR = 2.14, 95% CI: 1.47–3.13) and, to a lesser extent, those with lung cancer (HR = 1.37, 95% CI: 0.98–1.91) (Figures 2 and 3). No other combinations of snoring and sleep duration were significantly associated with survival. Women who reported a long sleep duration combined with a lack of snoring appeared to have the most favorable prognosis; however, very few women reported sleeping more than 8 h/night and, as such, results for these categories were based on small numbers.
Figure 1. Cause-specific survival in relation to typical sleep duration and survival among cancer case participants combined.
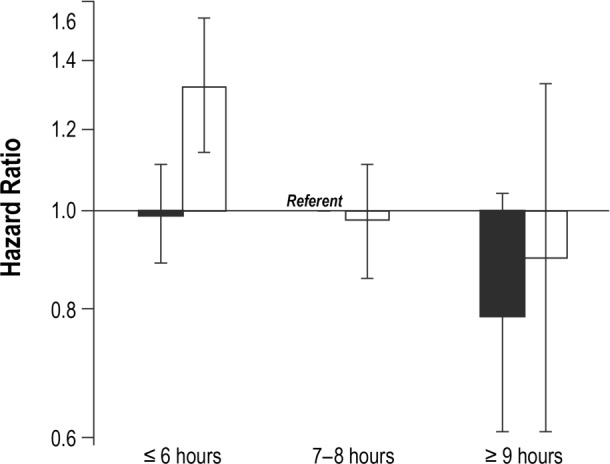
Solid black = never/rarely snore (< 5 nights/w); hatched black = frequent snorers (≥ 5 nights/w).
Figure 2. Cause-specific survival in relation to typical sleep duration and survival among women with breast cancer.
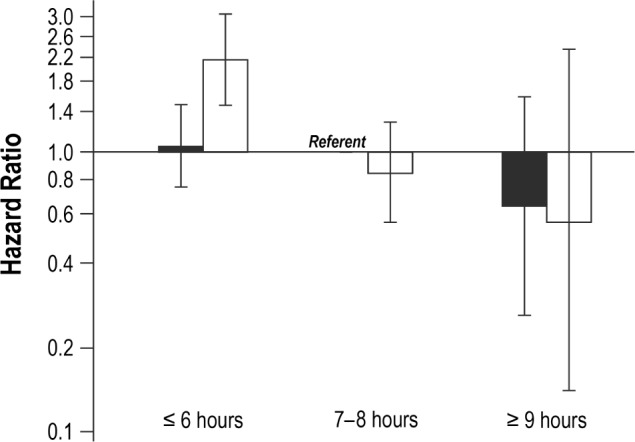
Solid black = never/rarely snore (< 5 nights/w); hatched black = frequent snorers (≥ 5 nights/w).
Figure 3. Cause-specific survival in relation to typical sleep duration and survival among women with lung cancer.
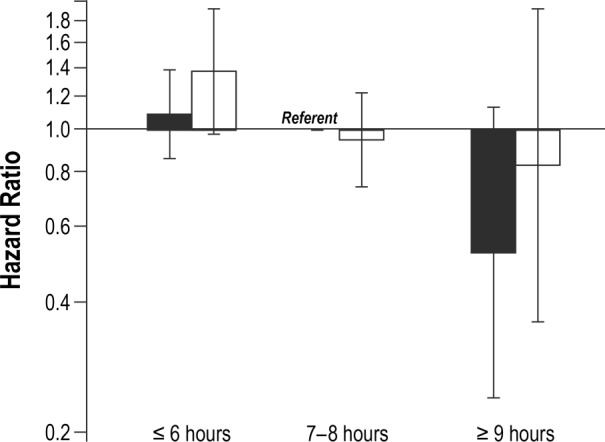
Solid black = never/rarely snore (< 5 nights/w); hatched black = frequent snorers (≥ 5 nights/w).
Associations were almost identical in analyses that excluded women with a cancer diagnosis ≥ 5 y or ≥ 10 y after baseline data collection (results not shown). No differences in associations were noted when stratified by BMI categories (not shown). Likewise, analyses restricted to non-Hispanic white women, analyses restricted to African American women, and analyses using multiple imputation to account for missing covariate data all yielded results very similar to those in the primary analysis (results not shown).
DISCUSSION
In this large, prospective cohort, women who reported short sleep duration combined with frequent snoring prior to cancer diagnosis experienced significantly poorer cancer-specific prognosis. Moreover, among women in whom invasive breast cancer has been diagnosed, short pre-diagnostic sleep duration and frequent snoring were each suggestively associated with subsequent poorer cause-specific survival, and the combination of these attributes was statistically significantly associated with poorer prognosis. To our knowledge, our study represents the first investigation of pre-diagnostic sleep patterns in relation to cancer survival.
Attributes of sleep and circadian rhythms, including poor sleep quality, short sleep duration, sleep apnea, and exposure to light at night have previously been associated with an elevated risk for several types of cancer.1–7,31,32 On the basis of evidence in support of such associations, the International Agency for Research on Cancer (IARC) has classified shiftwork that involves circadian disruption as a probable human carcinogen (Group 2A).33 Suggested mechanisms underlying these associations include an adverse effect on inflammatory pathways. In experimental studies, sleep loss has been shown to result in upregulation of proinflammatory cytokines and increased activation of nuclear factor (NF)-κB.34,35 Observational studies have also noted elevated levels of inflammatory biomarkers in individuals with disrupted sleep due to shiftwork or insomnia,36 and increased levels of NF-κB in individuals with sleep apnea.37,38 Inflammation, in turn, has long been hypothesized as a contributing factor in the development and progression of cancer.39
Support for the biologically plausible role of sleep in cancer survival has also been provided by several mouse models of sleep and circadian disruption.40–44 In one such model, Hakim et al.40 recently demonstrated that chronic sleep disturbance contributed to accelerated tumor growth and invasiveness. Other mouse models have linked chronic jetlag and sleep deprivation with shortened cancer survival,41–43 and induced intermittent hypoxia (simulating the physiological effects of sleep apnea) with significantly increased tumor growth45 and rate of lung metastases.44 To date, however, epidemiologic studies into the relationship between sleep and cancer survival in humans have been limited in scope, size, and number.
Previous studies using actigraphy- and biomarker-based assessments of sleep collected after cancer diagnosis have shown reduced sleep efficiency and disrupted circadian rhythms to be associated with poorer prognosis.15,16,19–22 In one such analysis, Palesh et al.19 found actigraphy-based sleep efficiency, defined as the ratio of total sleep time to total sleep time plus wake after sleep onset, to be a positive predictor of six-year overall survival among women with advanced breast cancer (HR = 0.94, 95% CI: 0.91–0.97), independent of other known prognostic factors. Similarly, Lévi et al.20 found poor sleep quality after diagnosis to be an independent predictor of prognosis in patients with metastatic colorectal cancer: median overall survival was 21.6 versus 11.9 mo for patients with dichotomy index above versus below 97.5%, where dichotomy index was defined as the proportion of minutes during sleep time with a recorded activity score less than the median activity score during awake time.
There are several possible reasons why our observed null findings in primary analyses differ from positive findings noted in previous studies. In particular, our assessment of sleep patterns was based on self-report. Self-reported measures may be subject to measurement error,46 although previous studies have indicated that sleep duration is adequately approximated via self-report.47,48 Objective actigraphy- and biomarker-based assessments are also subject to measurement error, but offer greater specificity toward underlying mechanisms. Our finding that the combined report of snoring and short sleep duration was more strongly associated with cancer prognosis than the individual sleep measures may represent the capture of poorer sleep patterns not otherwise detected in the isolated items of the sleep survey. It is also plausible that snoring could enhance the adverse effects of short sleep duration by fragmenting sleep and adversely impacting sleep quality. Additionally, in contrast to previous studies, we used information on sleep patterns collected before cancer diagnosis. Although the use of pre-diagnostic measures of sleep is a major strength of our study, one limitation of these measures is that sleep patterns can change with time and age, such that patterns reported at long intervals before cancer diagnosis could be less relevant to prognosis than those reported closer to diagnosis. However, we conducted sensitivity analyses excluding women with a cancer diagnosis ≥ 5 y or ≥ 10 y after data collection and found no difference in results. Last, our primary analyses were based on women with a variety of cancer types and stages at diagnosis, whereas previous studies have been cancer site-specific and many have focused on those with advanced tumors.
In subanalyses stratified by cancer site, we found short sleep duration combined with frequent snoring to be associated with poorer cause-specific survival among women with invasive breast cancer. The biological mechanisms responsible for such a site-specific association are unclear. However, steroid sex hormones, widely suggested to play a role in breast cancer etiology and progression,49 have been implicated in sleep patterns and disorders.50–52 In particular, progesterone levels are significantly lower in individuals with severe obstructive sleep apnea relative to those with mild or no apnea,51 as progesterone increases the activity of the upper airway dilator muscles. Thus, it is possible that the observed poorer prognosis associated with short sleep duration and snoring among women with breast cancer could reflect a differing distribution of hormone-receptor status according to sleep attributes. Although small numbers precluded stratified analyses in women with breast cancer according to hormone receptor status, the proportion of women who reported short sleep duration was lower among those with hormone receptor-positive tumors (32%) than in those with hormone receptor-negative tumors (37%; not shown).
This analysis was limited by the use of self-reported measures of sleep patterns collected several years before cancer diagnosis (median time lag = 7.5 y). Additionally, small numbers precluded some cancer site-specific analyses, and analyses stratified by tumor attributes; thus, heterogeneity in the association between sleep patterns and survival may have obscured certain relationships. Last, given that the WHI was restricted to postmenopausal women, these results may not be generalizable to younger women or to men with cancer.
Although there are limitations to the sleep measures evaluated, one important advantage of our pre-diagnostic sleep assessment is that our results are unlikely to be influenced by reverse causality. In contrast, findings reported from studies based on post-diagnostic assessments of sleep may reflect the effects of cancer-induced physiological factors, treatment side effects, or cancer-related anxiety and depression, all of which may be independently associated with disease prognosis. Additional strengths of this study include its large overall size, prospective design, and completeness of follow-up. To date, few studies have explored the relationship between sleep and cancer survival, and no prior studies have considered the role of pre-diagnostic sleep in this regard. This analysis thus contributes to a sparse literature, and provides suggestive evidence that short sleep duration and frequent snoring prior to cancer diagnosis may have an effect on subsequent cancer survival, particularly breast cancer survival.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the National Cancer Institute at the National Institutes of Health (K07 CA172298 to A.I.P.). The Women's Health Initiative program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, United States Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the generous contributions of women who participated in the Women's Health Initiative, and the contributions of Women's Health Initiative investigators. In particular, we acknowledge the following scientific contributors: Program Office (National Heart, Lung, and Blood Institute, Bethesda, Maryland)— Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller; Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA)—Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; Women's Health Initiative Memory Study (Wake Forest University School of Medicine, Winston-Salem, NC). A full list of investigators who have contributed to the Women's Health Initiative science is provided at: https://www.whi.org/researchers/Documents Write a Paper/WHI Investigator Long List.pdf
ABBREVIATIONS
- BMI
body mass index
- CI
confidence interval
- CT
randomized controlled trial
- HR
hazard ratio
- IARC
International Agency for Research on Cancer
- MET
metabolic equivalent of task
- NHL
non-Hodgkin's lymphoma
- OS
observational study
- WHI
Women's Health Initiative
- WHIIRS
WHI Insomnia Rating Scale
REFERENCES
- 1.McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res. 2006;15:241–9. doi: 10.1111/j.1365-2869.2006.00523.x. [DOI] [PubMed] [Google Scholar]
- 2.Kakizaki M, Kuriyama S, Sone T, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99:1502–5. doi: 10.1038/sj.bjc.6604684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 2008;29:1244–8. doi: 10.1093/carcin/bgn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sturgeon SR, Luisi N, Balasubramanian R, Reeves KW. Sleep duration and endometrial cancer risk. Cancer Causes Control. 2012;23:547–53. doi: 10.1007/s10552-012-9912-2. [DOI] [PubMed] [Google Scholar]
- 5.Luo J, Sands M, Wactawski-Wende J, Song Y, Margolis KL. Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women's Health Initiative. Am J Epidemiol. 2013;177:42–9. doi: 10.1093/aje/kws193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the busselton health study cohort. J Clin Sleep Med. 2014;10:355–62. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Zheng T, Holford TR, Boyle P, Zhang Y, Dai M. Light at night and breast cancer risk: results from a population-based case-control study in Connecticut, USA. Cancer Causes Control. 2010;21:2281–5. doi: 10.1007/s10552-010-9653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Giovannucci EL, Wu K, et al. Associations of self-reported sleep duration and snoring with colorectal cancer risk in men and women. Sleep. 2013;36:681–8. doi: 10.5665/sleep.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 10.Noguti J, Andersen ML, Cirelli C, Ribeiro DA. Oxidative stress, cancer, and sleep deprivation: is there a logical link in this association? Sleep Breath. 2013;17:905–10. doi: 10.1007/s11325-012-0797-9. [DOI] [PubMed] [Google Scholar]
- 11.Irwin M, Mascovich A, Gillin JC, Willoughby R, Pike J, Smith TL. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom Med. 1994;56:493–8. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Qian XH, Zhou W, et al. Time-dependent inflammatory factor production and NFkappaB activation in a rodent model of intermittent hypoxia. Swiss Med Wkly. 2011;141:w13309. doi: 10.4414/smw.2011.13309. [DOI] [PubMed] [Google Scholar]
- 13.Oyama J, Yamamoto H, Maeda T, Ito A, Node K, Makino N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin Cardiol. 2012;35:231–6. doi: 10.1002/clc.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mormont MC, Waterhouse J, Bleuzen P, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6:3038–45. [PubMed] [Google Scholar]
- 15.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 16.Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–92. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Palesh OG, Collie K, Batiuchok D, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol Psychol. 2007;75:37–44. doi: 10.1016/j.biopsycho.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sephton SE, Lush E, Dedert EA, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30(Suppl):S163–70. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Palesh O, Aldridge-Gerry A, Zeitzer JM, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37:837–42. doi: 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levi F, Dugue PA, Innominato P, et al. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiol Int. 2014:1–10. doi: 10.3109/07420528.2014.924523. [DOI] [PubMed] [Google Scholar]
- 21.Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One. 2012;7:e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17:321–8. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 23.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 24.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–8. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 25.Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women's Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15:137–48. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 26.Vogtmann E, Levitan EB, Hale L, et al. Association between sleep and breast cancer incidence among postmenopausal women in the Women's Health Initiative. Sleep. 2013;36:1437–44. doi: 10.5665/sleep.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao L, Duan Z, Sangi-Haghpeykar H, Hale L, White DL, El-Serag HB. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br J Cancer. 2013;108:213–21. doi: 10.1038/bjc.2012.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therneau TM, Grambsch PM. New York, NY: Springer; 2000. Modeling survival data: extending the Cox model. [Google Scholar]
- 29.Groenwold RH, Donders AR, Roes KC, Harrell FE, Jr, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol. 2012;175:210–7. doi: 10.1093/aje/kwr302. [DOI] [PubMed] [Google Scholar]
- 30.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 31.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–32. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, Oh J. Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;138:291–301. doi: 10.1007/s10549-013-2433-1. [DOI] [PubMed] [Google Scholar]
- 33.International Agency for Research on Cancer, World Health Organization Painting, firefighting, and shiftwork. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2007:98. [PMC free article] [PubMed] [Google Scholar]
- 34.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 35.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motivala S, Irwin MR. Sleep and immunity: cytokine pathways linking sleep and health outcomes. Curr Dir Psychol Sci. 2007;16:21–5. [Google Scholar]
- 37.Htoo AK, Greenberg H, Tongia S, et al. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath. 2006;10:43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 38.Yamauchi M, Tamaki S, Tomoda K, et al. Evidence for activation of nuclear factor kappaB in obstructive sleep apnea. Sleep Breath. 2006;10:189–93. doi: 10.1007/s11325-006-0074-x. [DOI] [PubMed] [Google Scholar]
- 39.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 40.Hakim F, Wang Y, Zhang SX, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74:1329–37. doi: 10.1158/0008-5472.CAN-13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maragno-Correa JM, Patti CL, Zanin KA, et al. Sleep deprivation increases mortality in female mice bearing Ehrlich ascitic tumor. Neuroimmunomodulation. 2013;20:134–40. doi: 10.1159/000346201. [DOI] [PubMed] [Google Scholar]
- 42.Filipski E, King VM, Li X, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94:690–7. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- 43.Filipski E, Delaunay F, King VM, et al. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–85. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 44.Almendros I, Montserrat JM, Torres M, et al. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol. 2013;186:303–7. doi: 10.1016/j.resp.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Almendros I, Montserrat JM, Ramirez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur J Respir J. 2012;39:215–7. doi: 10.1183/09031936.00185110. [DOI] [PubMed] [Google Scholar]
- 46.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175–83. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 48.Hauri PJ, Wisbey J. Wrist actigraphy in insomnia. Sleep. 1992;15:293–301. doi: 10.1093/sleep/15.4.293. [DOI] [PubMed] [Google Scholar]
- 49.Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol. 2003;87:1–25. doi: 10.1016/s0960-0760(03)00390-x. [DOI] [PubMed] [Google Scholar]
- 50.Teran-Perez G, Arana-Lechuga Y, Esqueda-Leon E, Santana-Miranda R, Rojas-Zamorano JA, Velazquez Moctezuma J. Steroid hormones and sleep regulation. Mini Rev Medicinal Chem. 2012;12:1040–8. doi: 10.2174/138955712802762167. [DOI] [PubMed] [Google Scholar]
- 51.Stavaras C, Pastaka C, Papala M, et al. Sexual function in pre- and post-menopausal women with obstructive sleep apnea syndrome. Int J Impotence Res. 2012;24:228–33. doi: 10.1038/ijir.2012.20. [DOI] [PubMed] [Google Scholar]
- 52.Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31:1339–49. [PMC free article] [PubMed] [Google Scholar]


