Abstract
Study Objectives:
Obstructive sleep apnea syndrome (OSAS) patients benefit from continuous positive airway pressure (CPAP) treatment in a dose-response manner. We determined adherence and weight control, as well as their predictors, among long-term CPAP users.
Methods:
Cohort of 1,023 OSAS patients had used CPAP on average of 6.6 ± 1.2 years. BMI was determined at baseline and at follow-up visits. There were 7.4 ± 1.7 BMI and 6.5 ± 1.8 CPAP usage measurements per patient on average. Using the Bayesian hierarchical model, we determined the patients' individual trends of BMI and adherence development. Patients with significantly increasing or decreasing trends were identified at the posterior probability level of > 90%.
Results:
The mean age in the cohort was 55.6 ± 9.8 years, BMI 33.5 ± 6.4 kg/m2, apnea-hypopnea index 33.7 ± 23.1, and CPAP usage 6.0 ± 1.8 h/day. The majority of patients had no significant change in BMI (mean annual weight gain 0.04 ± 0.29 kg/m2) or CPAP adherence (mean annual increase 11.4 ± 7.0 min/day). However, at the individual level, 10% of the patients showed significant annual weight gain (0.63 ± 0.35 kg/m2) during the 5-year follow-up period. At baseline these patients were already more severely obese (mean BMI 40.0 ± 5.9 kg/m2) despite being younger (mean 50.9 ± 9.5 years) than the rest of the cohort.
Conclusions:
In the majority of CPAP-treated OSAS patients, weight did not significantly change but gained slightly slower than in age-matched population in general. However, in 10% of patients, high adherence to CPAP treatment did not prevent the continuation of weight gain. These patients present a high-risk group for OSAS-related multimorbidity later in life.
Citation:
Myllylä M, Kurki S, Anttalainen U, Saaresranta T, Laitinen T. High adherence to CPAP treatment does not prevent the continuation of weight gain among severely obese OSAS patients. J Clin Sleep Med 2016;12(4):519–528.
Keywords: obstructive sleep apnea syndrome, weight control, continuous positive airway pressure therapy, adherence, individual development, observational study
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a major public disorder with a range of harmful consequences to the patients' health and a heavy burden to health care systems. Nasal continuous positive airway pressure (CPAP) is the most effective treatment for OSAS especially among patients with apnea-hypopnea index (AHI) above 15, but the treatment is also recommended in mild OSAS (AHI 5–15) when the patient shows symptoms of excessive daytime sleepiness or has OSAS associated disorders.1,2 CPAP therapy has been shown to improve cognitive performance, consolidate sleep, decrease subjective daytime sleepiness, and possibly prevent cardiovascular events.3–5 Obesity is a major risk factor for OSAS. Proper treatment of OSAS may help in weight control, and thus reduce the severity of OSAS, and ideally lead to resolution of the disease in some individuals.6
Effective use of CPAP has been considered to contribute to weight control among OSAS patients by several potential mechanisms. Firstly, improved daytime vigilance may lead to better motivation to lose weight.7 Secondly, previous studies have shown that OSAS patients usually have increased plasma leptin and ghrelin levels and suffer from leptin resistance. CPAP therapy has been shown to improve leptin resistance and to alter leptin and ghrelin levels in plasma, suggesting patient's improved control of hunger and appetite.8 Contrary to expectations, most of the epidemiological studies published have failed to show the positive effect of regular CPAP use in promoting weight loss.9–11 However, there might be several confounding factors potentially diluting favorable results. Although CPAP treatment is usually lifelong, there are very few long-term effectiveness studies.12 Most published studies have been rather small in patient numbers, especially for women, and the follow-up period has been short.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obesity is the most common cause of obstructive sleep apnea syndrome (OSAS) in adults. According to current evidence, the proper treatment of OSAS with continuous positive airway pressure (CPAP) does not contribute to weight control. This conclusion, however, is mainly based on two publications that both were limited by follow-up of 6 months.
Study Impact: In a 5-year real-world retrospective survey of one pulmonary clinic, the majority of OSAS patients showed no significant change in their weight or adherence to CPAP treatment. However, the minority of patients who gained weight during the treatment were the youngest and most obese patients at baseline; therefore, the main focus of dietary interventions should be on this patient group to prevent the development of obesity-hypoventilation syndrome later in life.
Patients are considered to benefit from CPAP in a dose-response manner.13 CPAP adherence varies from 3 to 7 hours a night between studies.14 The impact of gender and age on CPAP adherence remains controversial.12,15,16 One of the outcome measures of the treatment is its role in patient's weight control. In the present study we explore the individual trends of body mass index (BMI) and adherence during 5-year treatment with CPAP. We hypothesized whether we can recognize (1) heterogeneity between the patients in weight control behavior or adherence to CPAP, and (2) the high-risk patients for weight gain or poor adherence to CPAP during long-term treatment. We studied the hypotheses both at the cohort and at the patient level estimating the uncertainties in observed trends and pinpointing the patients who may benefit from CPAP most in their weight control.
METHODS
Subjects
From the discharge registry of the Turku University Hospital in Finland, we identified 2,489 patients who commenced CPAP treatment for sleep disordered breathing (SDB) during the years 2002–2006. One hundred thirty-three patients were excluded because the original polygraphy recording results could not be verified. From the eligible patients (n = 2,356), 1,064 (45.2%) had been using CPAP treatment regularly for ≥ 5 years, and 1,030 of them had also ≥ 4 follow-up visits. Among these long-term CPAP users, 7 patients had been submitted to bariatric surgery before or during the CPAP treatment and were thus excluded from the study. The remaining 1,023 patients with the mean follow-up period of 6.6 ± 1.2 years were included in the study without further selection (Figure 1). Ethical consideration for the retrospective study was not required.
Figure 1. Flowchart of the study design.
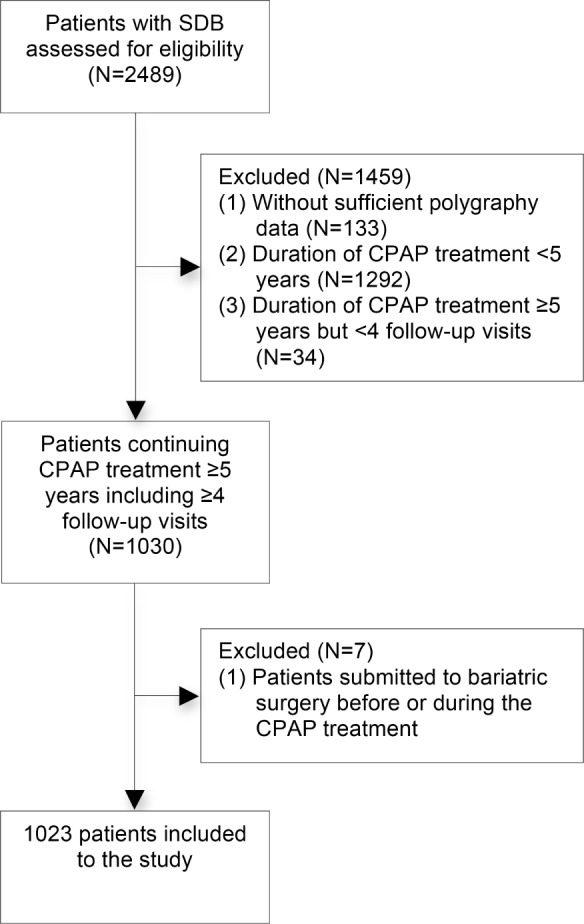
SDB, sleep disordered breathing; CPAP, continuous positive airway pressure.
Diagnosis of OSAS
OSAS was verified by an ambulatory (Embletta, Somnologica Software, Flaga hf. Medical Devices, Reykjavik, Iceland) or by an in-hospital cardiorespiratory polygraphy (Embla, Somnologica Software, Flaga hf. Medical Devices, Reykjavik, Iceland). Electroencephalography was not recorded during the polygraphy. Pulmonologist or clinical neurophysiologist scored the sleep studies. The severity of OSAS was quantified using 4 cutoff points for AHI: < 5 (normal), 5–15 (mild OSAS), 16–30 (moderate OSAS), and > 30 (severe OSAS).17 CPAP treatment was conducted mainly in patients with AHI ≥ 5. However, 138 patients out of the total 2,356 patients (5.9%) had an AHI < 5. Despite the low AHI, these patients had proceeded to CPAP treatment due to symptoms such as snoring, witnessed apneas, or excessive daytime sleepiness, and other clinical findings strongly suggestive for OSAS. Of the 138 patients with AHI < 5, 47 patients had been using the CPAP device for ≥ 5 years (including 4 follow-up visits during the treatment period) and were thus enrolled in the study.
Baseline Comorbidity
Comorbidity at the commencement of CPAP treatment including type 2 diabetes (T2D), impaired fasting glucose (IFG), asthma, chronic obstructive pulmonary disease (COPD), psychiatric disorders (depression, anxiety or psychotic disorder), and smoking status were verified from the electronic medical records.
Follow-up Visits
Follow-up visits occurred approximately 3 times during the first year and after habituation every second year. Mean number of visits during the follow-up period was 7.4 ± 2.1 per patient. Treatment pressure (cm H2O) and CPAP usage hours (h/day) were recorded by inbuilt counter clock of the CPAP device. For each patient, short-term and long-term CPAP usage was determined. Short-term CPAP usage was calculated by using the mean usage hours from the initiation of the treatment either until the first follow-up visit or alternatively until the 6-month follow-up visit when the habituation to treatment took longer period of time. Long-term CPAP usage was the patients' mean usage hours across the whole 5-year follow-up period. BMI was calculated by dividing weight in kilograms by the square of the height in meters (kg/m2). The patients' weight was measured at visits by a nurse. Generally, BMI, CPAP usage hours, and the scores of the self-administered Epworth Sleepiness Scale (ESS)18 and General Health Questionnaire (GHQ-12)19 were determined at follow-up visits and entered in the electronic medical records. Based on these measurements we were able to determine retrospectively OSAS patients' individual trends (slopes) of BMI, adherence, ESS, and GHQ-12 development during the CPAP treatment.
Statistical Analyses
Data analyses were performed using the IBM SPSS Statistics 22.0 (Armonk, NY, USA: IBM Corp.) software package. Continuous variables were expressed as mean values and standard deviations. The one-way analysis of variance (ANOVA) was used to compare the means of ≥ 2 independent groups. Categorical variables were expressed as frequencies and percentages, and the comparison of groups was performed with the χ2 test. Linear relationship between two continuous random variables was assessed by Spearman correlation coefficient.
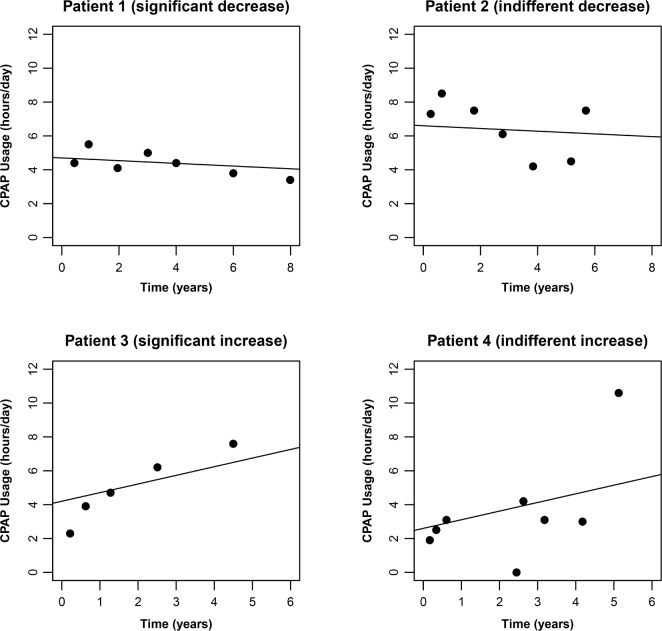
To determine the individual trends for the development of BMI and CPAP adherence across the 5-year follow-up period, we used the Bayesian hierarchical model for linear trend (R Core Team 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). The patient's BMI and CPAP usage measurements were assessed as a function of time starting from the initiation of the treatment. The individual slopes were determined using the linear mixed model. Ascending slope reflected either gaining weight or increasing use of CPAP. Descending slope reflected the contrary. The accuracy of an individual slope is dependent on the number of measurements available and on the within-patient variation between the sequential measurements (Figure 2). To develop the trends of BMI and CPAP usage, there were on average 7.4 ± 1.7 and 6.5 ± 1.8 measurements available per patient, respectively.
Figure 2. CPAP usage.
These examples show that two patients can have similar slope, but the statistical uncertainty associated to the shape of the slope varies due to the number of measurements available and variation between the sequential measurements. Based on the Bayesian hierarchical model at the probability level > 90%, patient 1 had significant decrease and patient 3 significant increase in CPAP usage, while the slopes were indifferent for patient 2 and patient 4.
In order to observe the within-patient variation and to identify the individuals who showed significant loss or gain of weight and significant increase or decrease in CPAP adherence, we used the Monte Carlo simulation. Thus, 5,000 lines were simulated through the measurement points for each patient, and the trend of these lines was evaluated individually at the posterior probability level of > 90% in order to pinpoint the patients with significant change. Patients with significant weight gain or increase in CPAP adherence had > 4,500 ascending slopes out of the total simulated 5,000 slopes (> 90%). Correspondingly, patients with significant weight loss or decrease in CPAP adherence had > 4,500 descending slopes of the total simulated 5,000 slopes (> 90%). Patients with no significant increasing or decreasing change in BMI or adherence had ≤ 4,500 ascending or descending slopes out of the total simulated 5,000 slopes (≤ 90%). Due to within-patient variation, patients with equivalent slope values may have been divided into different subgroups. Subgroups with significant change in BMI (significant gain or loss of weight) or CPAP adherence (significant increase or decrease in CPAP usage) were profiled by univariate logistic regression analysis in order to evaluate the predictors of change. Each covariate was entered into the model separately. Six patients were excluded from the analysis on CPAP adherence due to missing data on short-term CPAP usage.
In addition, individual slope values for BMI ([kg/m2]/year) and CPAP usage ([h/day]/year) were determined for each patient by the linear mixed model in order to illustrate the patients' mean annual change in BMI and adherence during the long-term CPAP treatment. The individual slope values were also determined for the ESS and GHQ-12 score (change of score/year) among patients who had ≥ 3 measurements available (including primary the baseline score or secondary the first score available), e.g., ESS for 948 and GHQ-12 also for 948 patients, respectively. Data on baseline ESS score were available for 977 and data on baseline GHQ-12 for 908 out of the total 1,023 patients. At the cohort level, the mean annual change of the 1,023 OSAS patients in BMI, CPAP adherence, ESS, or GHQ-12 score was illustrated by mean slope values and standard deviations.
RESULTS
Clinical Characteristics of the Cohort at Baseline
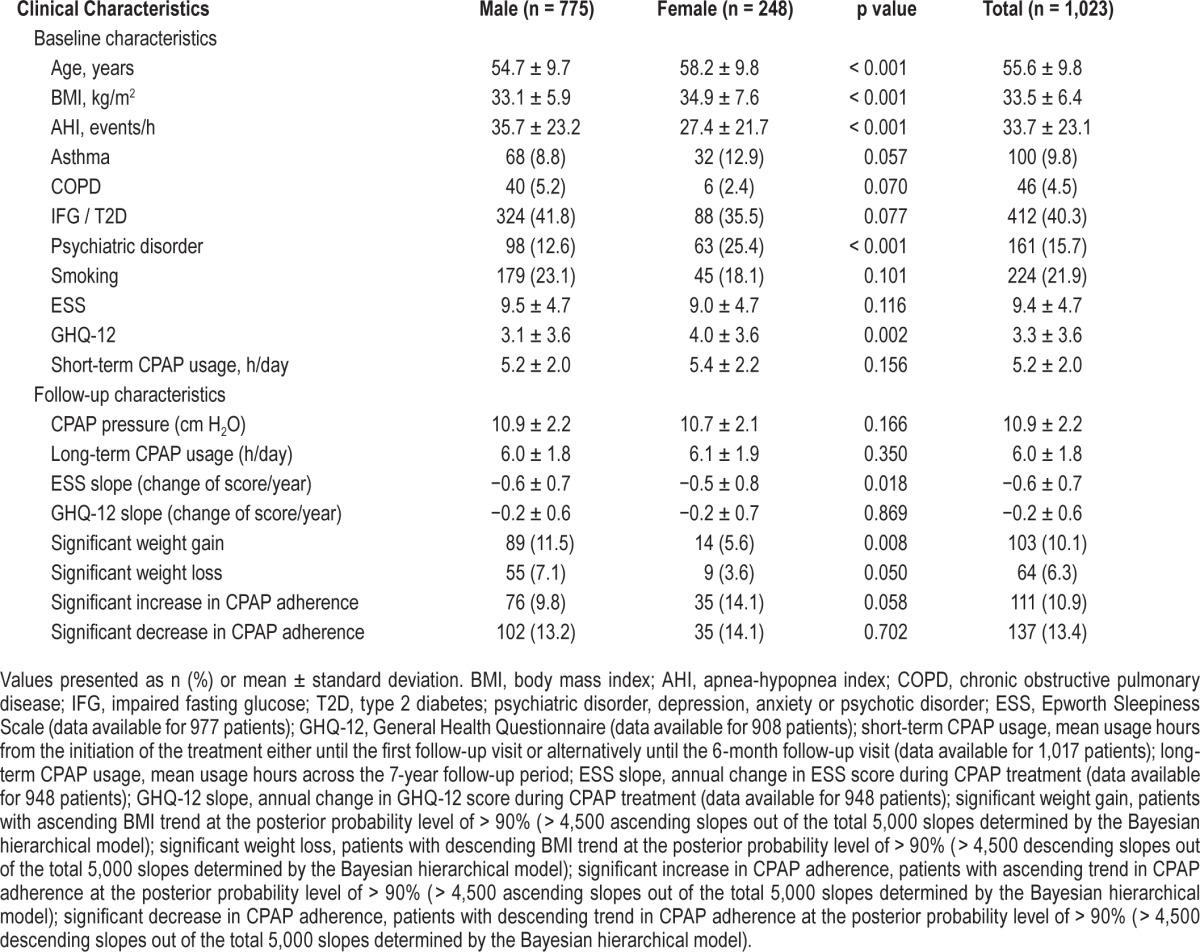
Before treatment was started, all the patients (n = 1,023) were symptomatic (e.g., snoring, witnessed apnea), and 45% of them reported daytime sleepiness (ESS ≥ 10). The mean age was 56 years (Table 1), and 24% of the patients were women (n = 248). Compared to men, women differed in several aspects by being older (58 vs. 55 years, p < 0.001), more obese (BMI 35 vs. 33 kg/m2, p < 0.001), by having milder OSAS (AHI 27 vs. 36, p < 0.001) and more frequently psychiatric disorders (25% vs. 13%, p < 0.001).
Table 1.
Comparison of the clinical characteristics between the male and female CPAP users.
Patients younger than 40 years of age had the highest BMI (36.6 ± 8.6 kg/m2). The difference was significant compared to that among patients aged 40–65 years (33.5 ± 6.3 kg/m2, p = 0.001) and patients older than 65 years (32.5 ± 5.8 kg/m2, p < 0.001). Only 5% of the patients had normal weight (BMI < 25 kg/m2).
The majority of patients (n = 488, 47.7%) had severe OSAS, 269 patients (26.3%) had moderate disease, and 219 patients (21.4%) had mild disorder. In 4.6% (n = 47) of the patients, AHI was < 5. AHI correlated with patients' BMI (R2 = 0.24, p < 0.001), CPAP pressure (R2 = 0.31, p < 0.001) and long-term usage hours (R2 = 0.11, p = 0.001), but not with age (R2 = −0.01, p = 0.7), baseline ESS (R2 = 0.05, p = 0.1), or GHQ-12 (R2 = −0.04, p = 0.3) score. The mean ESS and GHQ-12 slopes of the cohort were both descending (ESS slope −0.6 ± 0.7 and GHQ-12 slope −0.2 ± 0.6 change of score per year) suggesting that for the majority of patients, daytime sleepiness and psychological distress reduced during the CPAP treatment.
Adherence to CPAP Treatment
The mean use of CPAP was 5.2 h/day at the beginning of the treatment and the mean long-term usage was 6.0 ± 1.8 h/day. Using the Bayesian hierarchical model, we determined the mean annual change in CPAP adherence during the 5-year follow-up period. At the cohort level the mean annual change in daily CPAP usage was 10.9 ± 12.0 min, showing a slightly ascending trend (Figure 3). At the individual level, the majority of patients (75.8%) showed no significant change in their CPAP adherence (annual increase of 11.4 ± 7.0 min per day) (Table 2, Figure 4). Eleven percent had, however, significant annual increase (30.4 ± 8.0 min/day), and 13% significant annual decrease (8.1 ± 8.5 min/day) in CPAP adherence.
Figure 3. CPAP adherence.
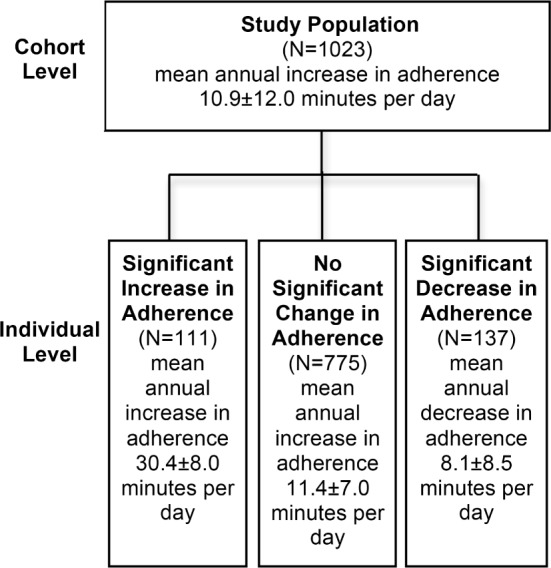
Flow diagram of change in CPAP adherence during 5-year treatment with CPAP at the cohort and at the individual level. Patients with significantly ascending (significant increase in adherence) or descending (significant decrease in adherence) trend in CPAP adherence were identified at the posterior probability level > 90%.
Table 2.
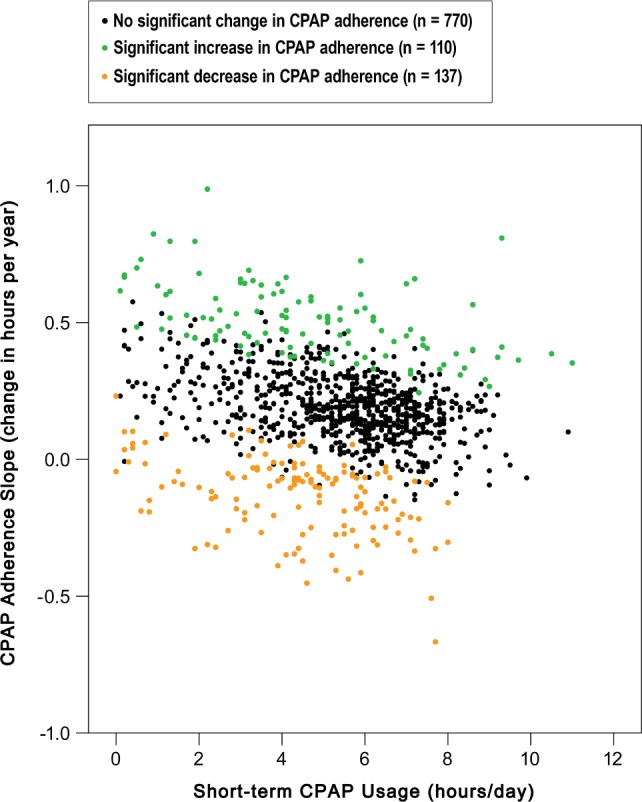
Clinical characteristics associated with significant increase or decrease in CPAP adherence during the 5-year follow-up period determined by univariate logistic regression analysis.
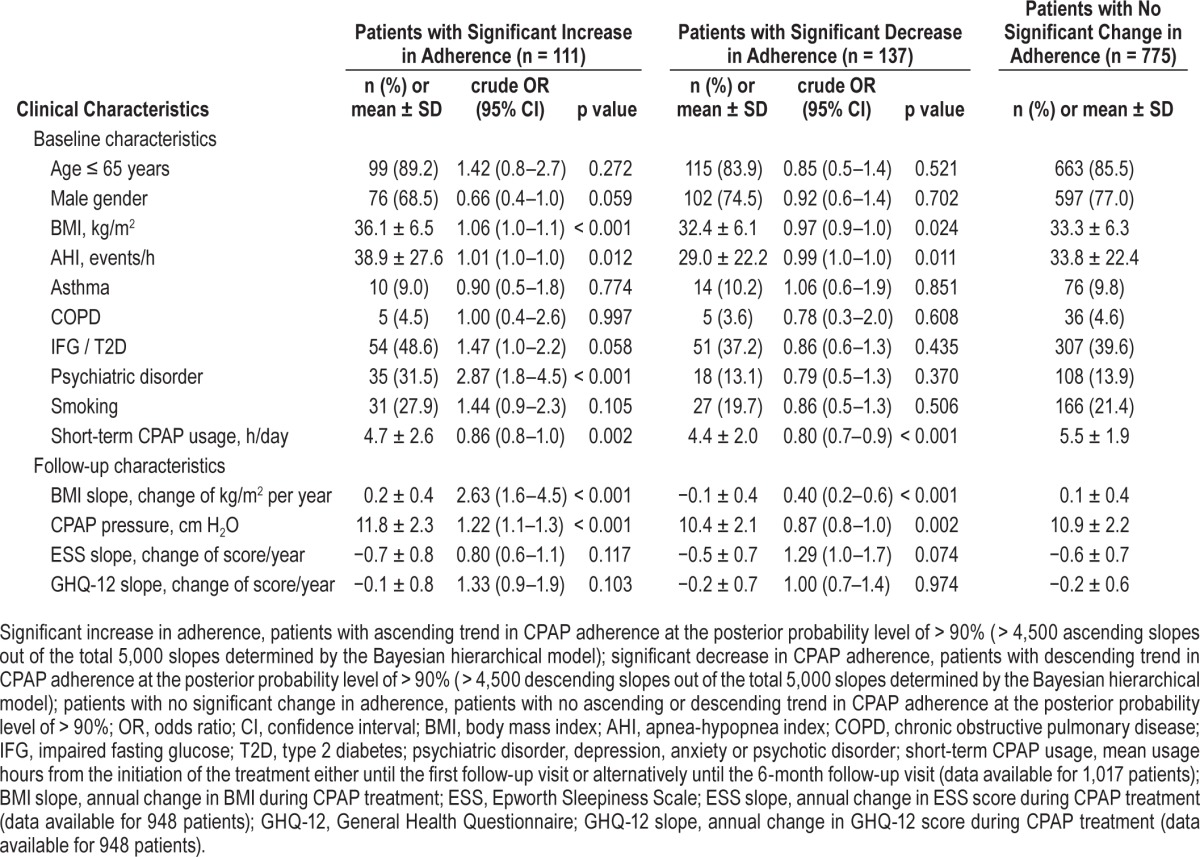
Figure 4. CPAP adherence slope.
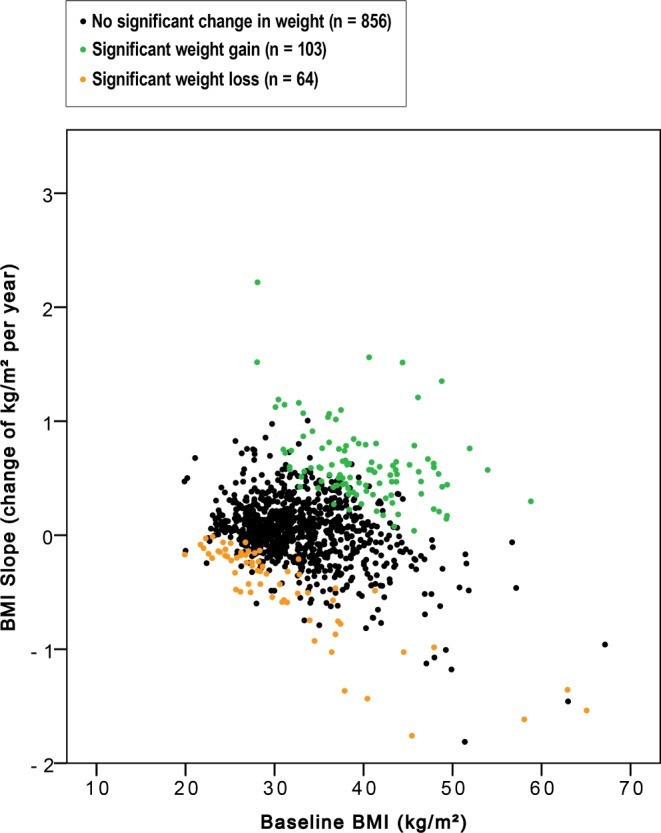
Individual BMI development during the 5-year follow-up period in relation to the BMI at the beginning of the CPAP treatment (n = 1,023 OSAS patients). Using the Bayesian hierarchical model, patients with significant change in weight were identified at the posterior probability level of > 90%.
The odds of having significant increase in CPAP adherence were greater among patients with higher baseline BMI (OR 1.06, 95% CI 1.0–1.1, p < 0.001) or ascending BMI slope (OR 2.63, 95% CI 1.6–4.5, p < 0.001). Other predictors for increased adherence were more severe disorder (OR 1.01, 95% CI 1.0–1.0, p = 0.012), greater CPAP pressure (OR 1.22, 95% CI 1.1–1.3, p < 0.001), and having psychiatric disorder (OR 2.87, 95% CI 1.8–4.5, p < 0.001). Having IFG or T2D (OR 1.47, 95% CI 1.0–2.2, p = 0.058) showed borderline association to increased CPAP adherence (Table 2). At the cohort level the mean CPAP usage slope did not correlate with the mean GHQ-12 slope (R2 = 0.03, p = 0.4), but showed a weak negative correlation with the mean ESS slope (R2 = −0.13, p < 0.001) indicating that daytime sleepiness increased slightly among the patients who diminished the use of CPAP.
Another clinically interesting group were the patients who used CPAP > 8 h/day on average (n = 115). They did not differ in terms of age (mean 57.0 ± 9.4 vs. 55.4 ± 9.9 years, p = 0.1) or gender (28.7% vs. 23.7% of them women, p = 0.2), but they were more obese (mean BMI 35.8 ± 6.9 vs. 33.2 ± 6.3 kg/m2, p < 0.001) and had a more severe disorder (mean AHI 41.8 ± 26.2 vs. 32.7 ± 22.5, p < 0.001) at the commencement of treatment compared to the rest of the cohort. Fifty-five of them (47.8%) had an increasing trend in CPAP adherence. Among these patients, a psychiatric disorder was found in 18 cases (32.7% vs. 14.8% in the rest of the patients, p < 0.001), IFG or T2D in 28 (50.9% vs. 39.8%, p = 0.1), and COPD in only 2 (3.6% vs. 4.5%, p = 0.8) cases. A majority of the patients (n = 34, 61.8% vs. 33.3%, p < 0.001) had severe obesity (baseline BMI 37.2 ± 6.6 kg/m2 on average).
Weight Changes during the CPAP Treatment
Based on the Bayesian hierarchical model, the mean annual weight gain in the OSAS cohort of 1,023 patients was 0.06 kg/m2 during CPAP treatment (Figure 5). For the majority of OSAS patients (n = 856, 83.7%), weight did not significantly change during the 5-year follow-up period (mean annual weight gain 0.04 kg/m2). Among these patients the baseline BMI was 32.3 ± 4.9 kg/m2 for men and 34.6 ± 7.3 kg/m2 for women. However, we identified 103 patients out of 1,023 (10.1%) who showed significant weight gain (mean annual weight gain 0.63 kg/m2). In this patient group, mean height was 1.76 meters and mean baseline BMI 40 kg/m2. Thus, weight for a patient with these characteristics would be 124 kg on average at the commencement of treatment. According to the mean annual weight gain of 0.63 kg/m2, weight would be 134 kg (+10 kg) after 5 years and 138 kg (+14 kg) after 7 years of CPAP treatment. Sixty-four patients (6.3%) showed significant weight loss (mean annual weight loss 0.47 kg/m2) during the long-term CPAP treatment. (Table 3, Figure 6).
Figure 5. Change in BMI.
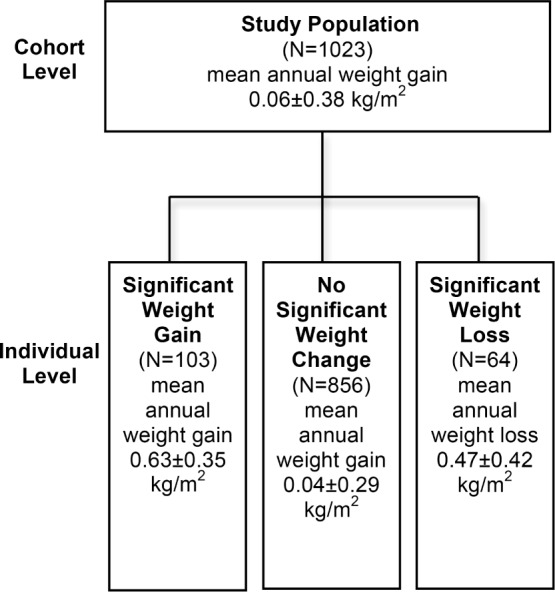
Flow diagram of change in BMI during 5-year treatment with CPAP at the cohort and at the individual level. Patients with significantly ascending (significant weight gain) or descending (significant weight loss) BMI trend were identified at the posterior probability level > 90%.
Table 3.
Clinical characteristics that associated with significant weight gain or significant weight loss during the 5-year follow-up period determined by univariate logistic regression analysis.
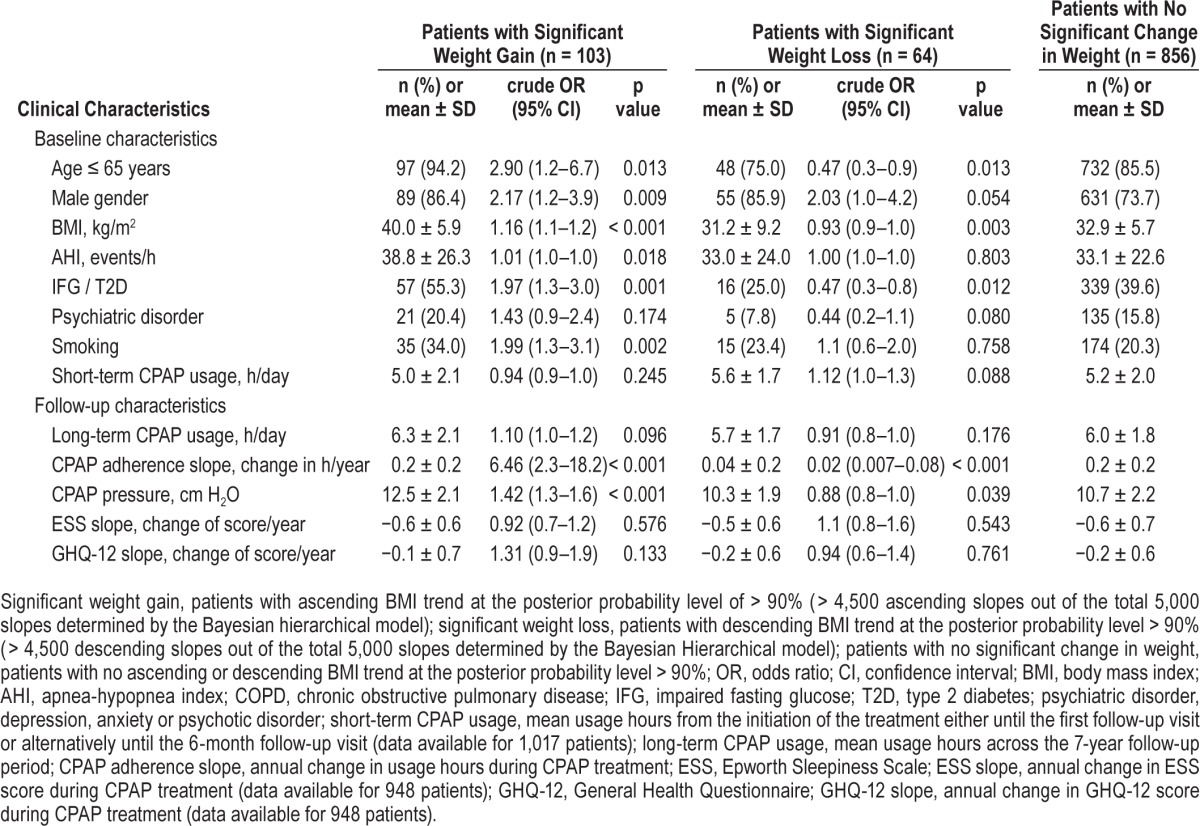
Figure 6. BMI slope.
Individual development of CPAP adherence during the 5-year follow-up period in relation to the CPAP usage at the beginning of the CPAP treatment (n = 1,017 OSAS patients). Using the Bayesian hierarchical model, patients with significant change in CPAP adherence were identified at the posterior probability level > 90%.
Women's weight showed more stable tendencies than men's. The odds of a male showing significant weight gain were 2.2 (95% CI 1.2–3.9, p = 0.009) times greater than for a female. Male gender also associated with weight loss (OR 2.03, 95% CI 1.0– 4.2, p = 0.054) (Table 3). Significant weight gainers were younger (50.9 ± 9.5 vs. 56.1 ± 9.7 years, p < 0.001), and they had higher baseline BMI (40.0 ± 5.9 vs. 32.8 ± 6.0 kg/m2, p < 0.001) and AHI (38.8 ± 26.3 vs. 33.1 ± 22.7, p = 0.018) compared to the rest of the patients. Aging diminished the risk of weight gain: 18.6% of patients aged < 40 years, 10.5% of patients aged 40–65 years, and 4.1% of patients aged > 65 years showed significant weight gain (p = 0.002 for the trend). The corresponding numbers for significant weight loss were 6.8% for patients aged < 40 years, 5.4% for patients aged 40–65 years, and 11.0% for patients aged > 65 years (p = 0.002 for the trend). The age of the patients with significant weight loss was 58.0 ± 10.5 years on average. The mean ESS and GHQ-12 slopes did not associate with weight changes.
Patients with AHI < 5
An AHI < 5 was observed among 138 patients of the total 2,356 patients (5.9%) with SDB. Due to symptoms and other clinical findings suggestive for OSAS, all these patients were initially directed to CPAP treatment. Forty-seven of them fulfilled the recruitment criteria, and were included in the statistical analyses. As the rest of the cohort, they were highly adherent to CPAP treatment (mean long-term usage time 6.1 ± 1.8 h/ day). However, they were more often women (38.3% vs. 23.6%, p = 0.021), and they had more frequently psychiatric disorders (34.0% vs. 14.9%, p < 0.001) compared to the patients with AHI ≥ 5. Differences in age (53.8 ± 8.9 years vs. 55.7 ± 9.9, p = 0.2), BMI (31.8 ± 5.2 vs. 33.6 ± 6.4 kg/m2, p = 0.061), IFG or T2D occurrence (27.7% vs. 40.9%, p = 0.071), or COPD occurrence (6.4% vs. 4.4%, p = 0.5) were not observed compared to the patients with AHI ≥ 5. The mean annual weight gain in the patients with AHI < 5 was 0.12 ± 0.3 kg/m2. At the individual level, majority of them had no significant changes in BMI (92%) or CPAP adherence (77%). Only 2 of the 103 patients (1.9%) with significant weight gain, and 2 of the 64 patients (3.1%) with significant weight loss had an AHI < 5. Correspondingly, the mean annual change in CPAP adherence was 10.2 ± 13.5 min and only 4 of the 111 patients (3.6%) with significant increase and 7 of the 137 patients (5.1%) with significant decrease in CPAP adherence had an AHI < 5.
DISCUSSION
We investigated individual weight control retrospectively in a large, single-center OSAS cohort who had been using CPAP treatment for on average of seven years. From the beginning the patients were highly committed to the treatment and the use of CPAP remained good throughout the follow-up period. Among the majority of patients, daytime sleepiness and psychological distress were reduced. Heterogeneity in weight control was observed. While more than 80% showed no significant change in their weight during the treatment, in 10% of patients significant weight gain was observed. In this group of patients, the weight gain was 16 times faster than in the patients with no significant change in weight. The most concerning observation was that these weight gainers were significantly younger (mean 51 years, the highest risk in those under 40 years) and had already more severe obesity (mean BMI 40 kg/m2) at the beginning of the treatment than the patients in the rest of the cohort. These patients are in great risk to develop obesity hypoventilation syndrome, and other obesity- and OSAS-related comorbidities later in life. The significant weight gainers were also more frequently men (86%), had more severe OSAS (mean AHI 39), higher prevalence of IFG or T2D (55%), and slightly higher long-term CPAP usage (mean 6.3 h/day). Six percent of the cohort lost weight significantly. These patients were older (mean 58 years) and leaner (mean BMI 31 kg/m2) already at baseline. An interesting finding was also a group of CPAP adherent patients whose clinical presentation suggested OSAS, but AHI was within normal range (< 5).
At the individual level most of the patients did not show significant weight change during the 5-year follow-up period but a slight mean annual weight gain of 0.04 kg/m2 was observed. In general, middle aged Finns are prone to gradually gain weight.20 A recent study among the Finnish employees (aged 40–60 years) showed that the annual weight gain was approximately 0.06 kg/m2 for men and 0.11 kg/m2 for women.21 Thus in our study, the majority of patients did not show significant weight change but gained at a slightly slower rate than the age-matched population in general. The mean baseline BMI among the OSAS patients was, however, remarkably higher (men: 33, women: 35 kg/m2) than in the reference population (26 and 25 kg/m2, respectively). The results suggest that despite the high baseline BMI, weight gain tends to decelerate or stabilize during the CPAP treatment in most OSAS patients. Men represented the majority among significant weight gainers. Male gender showed also borderline association to weight loss. Women tended to maintain their weight better, but however, these findings do not exclude short-term weight cycling.
Some of the previous epidemiological studies have reported similar results.9–11,22,23 Stenlöf et al.22 discovered that even though patients with untreated OSAS have increased energy expenditure (EE) levels during sleep, they often result in gaining weight. CPAP treatment was shown to normalize patients' nocturnal EE while their BMI remained at the same level, implying that CPAP treatment might have positive effects on weight control. On the other hand, it has been suggested that the decrease in nocturnal EE during CPAP treatment, thus resulting in positive energy balance, might lead to weight gain if concurrent favorable changes in daytime EE or energy in-take levels are not observed.23 Like in ours, other studies have reported an increase in BMI during CPAP therapy.10,11,23 The combination of CPAP treatment and diet intervention has been shown to be more effective than the diet or CPAP therapy alone.24 However, a 2-year study on weight reduction therapy among OSAS patients showed that adding CPAP treatment to the weight loss program did not contribute to greater results.9 In the present study a physician and a trained nurse gave the patients instructions for weight loss and maintenance at the commencement of treatment. According to the patient's motivation, further diet counseling at dietician was arranged, but was not systematically offered to all patients.
Previous studies have shown that long-term adherence to CPAP treatment can be predicted shortly after initiation.25 The same observation was also made in this cohort: the mean short-term use of the CPAP was already on recommended level (> 4 h/day). This caused a ceiling effect constraining the number of patients with significant increase in CPAP adherence and prevented us to estimate the dose-response. Interestingly however, impaired glycemic control as well as severe obesity and psychiatric disorders were associated with increasing CPAP adherence. The finding emphasizes that the existence of comorbidities may result in improved use of CPAP, which should be remembered, in addition to good motivation, among patients with constantly improving CPAP usage hours. Symptoms of psychiatric disorder may also interact with OSAS symptoms and this patient group should not be overlooked when considering patients for CPAP treatment. Only a weak association between AHI and CPAP adherence was confirmed.26 Baseline smoking, asthma, COPD, and the trend of subjective daytime sleepiness (ESS) or psychological distress (GHQ-12) did not associate with CPAP adherence.
In our cohort, 5% of the patients had an AHI < 5, even though clinical symptoms strongly suggested OSAS. They were more frequently women and had more frequently psychiatric disorders when compared to the rest of the cohort. It has been reported that women are twice as likely as men treated for depression prior diagnosing OSAS.27 In addition, AHI has been shown to be approximately 20% higher in patients investigated with polysomnography (PSG) than in those studied with cardiorespiratory polygraphy as we did.28,29 In cardiorespiratory polygraphy, the analysis time includes wake periods leading to a dilution of AHI. Night-to-night variability may result in false negative results.30 Especially in women, prolonged episodes of non-apneic partial upper airway obstruction can occur,31–34 which may also explicate the good adherence of this patient group in our study.33 However, in this patient group, only four patients showed change in weight and eleven in CPAP adherence. Therefore, their influence on the predictors of the outcomes was minimal.
Our study has several strengths despite its retrospective nature. First, our cohort is one of the largest. Second, the proportion of females is significant. Third, the mean follow-up period of 7 years is one of the longest in this field of research. Fourth, we investigated the changes in weight and CPAP adherence not only at the cohort but also at the individual level using the Bayesian hierarchical model for linear trend. Individual long-term changes in weight and CPAP adherence in OSAS patients have not been reported previously.
Our study has also some limitations. First, the retrospective design did not allow us to have a proper control group for long-term weight changes. However, in our study the majority of OSAS patients did not show significant weight change during the CPAP treatment but gained at a slightly slower rate than the age-matched Finnish population in general. To our knowledge, no controlled study has addressed the effect of long-term (> 6 months) CPAP treatment on weight changes in OSAS patients. On the other hand, a recent meta-analysis35 and a randomised controlled trial23 on short-term CPAP therapy are in agreement with our results. Second, diagnostic cardiorespiratory polygraphy was performed either at home or in hospital. This might affect AHI28 but will not affect weight changes during the follow-up period. Third, our clinical cohort had high short-and long-term usage hours of CPAP. This precluded accurate estimation of the impact of CPAP adherence on body weight changes.
In conclusion, we discovered that at our clinic almost half of the patients with SDB commencing CPAP therapy continued the treatment at least for 5 years. In this patient population the compliance to CPAP treatment did not decrease over time. Only a negligible proportion of the patients were able to achieve consistent weight loss. While the majority of study population gained weight at a slightly slower rate than the age-matched population at large, 10% of the patients continued significant weight gain during CPAP treatment. These patients were young and severely obese already at the beginning of the treatment and therefore the main focus of dietary interventions should be on this patient group.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was supported in part by grants from Turku University Hospital (the governmental EVO funding) and The Research Foundation of the Pulmonary Diseases. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank RN Heli Rajalin and NP Sirkka Hakko from their valuable help in identification of the patients who have had CPAP device treatment at our clinic.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ANOVA
one-way analysis of variance
- BMI
body mass index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CPAP
continuous positive airway pressure
- EE
energy expenditure
- ESS
Epworth Sleepiness Scale
- GHQ-12
General Health Questionnaire
- IFG
impaired fasting glucose
- OR
odds ratio
- OSAS
obstructive sleep apnea syndrome
- PSG
polysomnography
- SD
standard deviation
- SDB
sleep-disordered breathing
- T2D
type 2 diabetes
REFERENCES
- 1.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of sleep apnea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 2.Loube DI, Gay PC, Strohl KP, Pack AI, White DP, Collop NA. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statement. Chest. 1999;115:863–6. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- 3.Engleman HM, Martin SM, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnea-hypopnea syndrome. Lancet. 1994;343:572–5. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 4.Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep. 2006;4:564–71. doi: 10.1093/sleep/29.4.564. [DOI] [PubMed] [Google Scholar]
- 5.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176:1274–80. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 6.Fisher D, Pillar G, Malhotra A, Peled N, Lavie P. Long-term follow-up of untreated patients with sleep apnea syndrome. Respir Med. 2002;96:337–43. doi: 10.1053/rmed.2001.1277. [DOI] [PubMed] [Google Scholar]
- 7.Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight loss in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97:896–7. doi: 10.1016/s0002-8223(97)00220-4. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi KI, Chin K, Akamizu T, et al. Acylated ghrelin level in patients with OSA before and after nasal CPAP treatment. Respirology. 2008;13:810–6. doi: 10.1111/j.1440-1843.2008.01357.x. [DOI] [PubMed] [Google Scholar]
- 9.Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight reduction program in the treatment of obstructive sleep apnea syndrome with or without baseline nasal CPAP: a randomized study. Sleep Med. 2004;5:125–31. doi: 10.1016/j.sleep.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Redenius R, Murphy C, O'Neill E, Hamwi MA, Zallek SN. Does CPAP lead to change in BMI? J Clin Sleep Med. 2008;4:205–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia JM, Sharafkhaneh H, Hirshkowitz M, Elkhatib R, Sharafkhaneh A. Weight and metabolic effects of CPAP in obstructive sleep apnea patients with obesity. Respir Res. 2011;12:80. doi: 10.1186/1465-9921-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65:829–32. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 13.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 14.Engleman HM, Asgari-Jirhandeh N, McLeod AL, Ramsay CF, Deary IJ, Douglas NJ. Self-reported use of CPAP and benefits of CPAP therapy: a patient survey. Chest. 1996;109:1470–6. doi: 10.1378/chest.109.6.1470. [DOI] [PubMed] [Google Scholar]
- 15.Woehrle H, Graml A, Weinreich G. Age- and gender-dependent adherence with continuous positive airway pressure therapy. Sleep Med. 2011;12:1034–6. doi: 10.1016/j.sleep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population based study. Chest. 2002;121:430–5. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 17.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring subjective daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg DP, Gater R, Sartorius N, et al. The validity of two versions of the GHQ-12 in the WHO study of mental illness in general health care. Psychol Med. 1997;27:191–7. doi: 10.1017/s0033291796004242. [DOI] [PubMed] [Google Scholar]
- 20.Peltonen M, Harald K, Männistö S. Kansallinen Finriski 2007-terveystutkimus, Tutkimuksen toteutus ja tulokset. Kansanterveyslaitoksen julkaisuja B 34/2008. [Google Scholar]
- 21.Silventoinen K, Tatsuse T, Martikainen P, et al. Occupational class differences in body mass index and weight gain in Japan and Finland. J Epidemiol. 2013;23:443–50. doi: 10.2188/jea.JE20130023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenlöf K, Grunstein R, Hedner J, Sjöström L. Energy expenditure in obstructive sleep apnea: effects of treatment with continuous positive airway pressure. Am J Physiol. 1996;271:1036–43. doi: 10.1152/ajpendo.1996.271.6.E1036. [DOI] [PubMed] [Google Scholar]
- 23.Quan SF, Budhiraja R, Clarke DP, et al. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea. J Clin Sleep Med. 2013;9:989–93. doi: 10.5664/jcsm.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomasouli MA, Brady EM, Davies MJ, et al. The impact of diet and lifestyle management strategies for obstructive sleep apnea in adults: a systematic review and meta-analysis of randomised controlled trials. Sleep Breath. 2013;17:925–35. doi: 10.1007/s11325-013-0806-7. [DOI] [PubMed] [Google Scholar]
- 25.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 26.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 27.Smith R, Ronald J, Delaive K, Walld R, Manfreda J, Kryger MH. What are obstructive sleep apnea patients being treated for prior to this diagnosis? Chest. 2002;121:164–72. doi: 10.1378/chest.121.1.164. [DOI] [PubMed] [Google Scholar]
- 28.Hedner J, Grote L, Bonsignore M, et al. The European Sleep Apnoea Database (ESADA): report from 22 European sleep laboratories. Eur Respir J. 2011;38:635–42. doi: 10.1183/09031936.00046710. [DOI] [PubMed] [Google Scholar]
- 29.Escourrou P, Grote L, Penzel T, et al. The diagnostic method has a strong influence on classification of obstructive sleep apnea. J Sleep Res. 2015;24:730–8. doi: 10.1111/jsr.12318. [DOI] [PubMed] [Google Scholar]
- 30.Skiba V, Goldstein C, Schotland H. Night-to-night variability in sleep disordered breathing and the utility of esophageal pressure monitoring in suspected obstructive sleep apnea. J Clin Sleep Med. 2015;11:597–602. doi: 10.5664/jcsm.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104:781–7. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- 32.Polo-Kantola P, Rauhala E, Helenius H, Erkkola R, Irjala K, Polo O. Breathing during sleep in menopause: a randomized, controlled, crossover trial with estrogen therapy. Obstetr Gynecol. 2003;102:68–75. doi: 10.1016/s0029-7844(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 33.Anttalainen U, Saaresranta T, Kalleinen N, Aittokallio J, Vahlberg T, Polo O. CPAP adherence and partial upper airway obstruction during sleep. Sleep Breath. 2007;11:171–6. doi: 10.1007/s11325-007-0102-5. [DOI] [PubMed] [Google Scholar]
- 34.Mohsenin V. Gender differences in the expression of sleep-disordered breathing: role of upper airway dimensions. Chest. 2001;120:1442–7. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 35.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Benseñor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258–64. doi: 10.1136/thoraxjnl-2014-205361. [DOI] [PubMed] [Google Scholar]