Abstract
Study Objectives:
Obstructive sleep apnea (OSA) in stroke patients is associated with worse functional and cognitive status during inpatient rehabilitation. We hypothesized that a four-week period of continuous positive airway pressure (CPAP) treatment would improve cognitive and functional outcomes.
Methods:
We performed a randomized controlled trial in stroke patients admitted to a neurorehabilitation unit. Patients were assigned to rehabilitation treatment as usual (control group) or to CPAP treatment (CPAP group). Primary outcomes were cognitive status measured by neuropsychological examination, and functional status measured by two neurological scales and a measure of activities of daily living (ADL). Secondary measures included sleepiness, sleep quality, fatigue, and mood. Tests were performed at baseline and after the four-week intervention period.
Results:
We randomly assigned 20 patients to the CPAP group and 16 patients to the control group. The average CPAP compliance was 2.5 hours per night. Patients in the CPAP group showed significantly greater improvement in the cognitive domains of attention and executive functioning than the control group. CPAP compliance was associated with greater improvement in cognitive functioning. CPAP did not result in measurable improvement on measures of neurological status or ADL, or on any of the secondary measures.
Conclusions:
CPAP treatment improves cognitive functioning of stroke patients with OSA.
Commentary:
A commentary on this article appears in this issue on page 467.
Citation:
Aaronson JA, Hofman WF, van Bennekom CA, van Bezeij T, van den Aardweg JG, Groet E, Kylstra WA, Schmand B. Effects of continuous positive airway pressure on cognitive and functional outcome of stroke patients with obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med 2016;12(4):533–541.
Keywords: obstructive sleep apnea, stroke, CPAP, cognitive status, functional status
INTRODUCTION
The estimated prevalence of obstructive sleep apnea (OSA) in stroke patients is as high as 38% to 70%.1 Despite its high prevalence in the stroke population, OSA is often left undiagnosed and untreated.2,3 Main causes of underdiagnosis are lack of awareness of health care professionals, lack of complaints by patients, and difficult access to sleep laboratory-based testing.4 Untreated OSA is associated with a higher risk of recurrent stroke and mortality, and worse cognitive and functional status.5–8 More specifically, in an earlier study by our group, we found that stroke patients with OSA showed more impairments in (1) the cognitive domains of attention, executive functioning, visuoperception, psychomotor ability and intelligence, and in (2) neurological status and activities of daily living (ADL) than stroke patients without OSA.7 Treatment of choice for OSA is continuous positive airway pressure treatment (CPAP). In otherwise healthy OSA patients, CPAP effectively treats OSA by improving the breathing pattern during sleep, leading to improvement of daytime sleepiness, health status, and depressive symptoms.9 Consequently, it seems plausible that CPAP treatment could improve stroke outcome. The randomized controlled trials (RCTs) that have been published, however, show mixed results.10–15
BRIEF SUMMARY
Current Knowledge/Study Rationale: In the general population treatment of obstructive sleep apnea (OSA) with continuous positive airway pressure (CPAP) results in improvement of daily functioning, but the effect of CPAP treatment on stroke recovery is still inconclusive. Therefore, our primary aim was to evaluate the effect of CPAP treatment on the rehabilitation outcome of stroke patients with OSA.
Study Impact: This study indicates that CPAP treatment improves the cognitive outcome of stroke patients with OSA. These beneficial effects offer preliminary evidence for the use of CPAP treatment as part of a rehabilitation program for stroke patients.
In a recent review of CPAP treatment in stroke patients, Tomfohr and colleagues conclude that none of the studies have found improvements in activities of daily living (ADL) or cognitive functioning, and that studies on the beneficial effect of CPAP on neurological recovery, daytime sleepiness, and depressive symptoms are still inconclusive.16 Three studies found improvement after CPAP on neurological status (Canadian Neurological Scale or National Institute of Health Stroke scale),10–12 while two others did not.13,14 The two latter studies suffered from low compliance and insufficient power, which might have influenced the findings. Three studies investigated the effect on cognitive functioning. However, two of the studies used only a brief mental status examination to investigate cognition, and the other study only assessed specific neuropsychological measures of vigilance and executive functioning.12,14,15 Tomfohr et al. argue that the lack of findings on cognitive measures may be explained by the differing levels of sensitivity of the tests. They suggest that more nuanced neuropsychological tests may show specific cognitive domains that are affected by CPAP.16
The primary aim of our study was to evaluate the effectiveness of CPAP treatment in stroke patients during inpatient rehabilitation using a comprehensive battery of neuropsychological tests and neurological and ADL scales. We hypothesized that CPAP treatment would improve cognitive and functional outcomes over a four-week period. Furthermore, we hypothesized that CPAP would also positively affect sleepiness, sleep quality, fatigue, and mood during inpatient rehabilitation.
METHODS
Study Design
We performed a randomized controlled trial (RCT) of CPAP treatment in stroke patients with blind assessment of outcome measures. Patients were randomized to receive four weeks of CPAP treatment or treatment as usual (control group). The minimization method was used to balance the groups for age (< 50, 50–59, 60–69, ≥ 70 years), severity of OSA (apnea-hypopnea index [AHI, see below]: 15–29, 30–59, ≥ 60), stroke subtype (ischemic or hemorrhage), and severity of cognitive impairment (cognitive status; very severe ≤ −3, severe = −3 to −2, moderate = −2 to −1, mild ≥ −1 standard deviation below the norm; see below). After the four-week intervention period, patients in the control group were offered CPAP treatment. Assessments were performed at baseline (T0) and after the four-week intervention period (T1). In addition to the RCT, we included assessments at a two-month follow-up (T2). We also included a group of non-OSA patients to compare their recovery on the outcome measures with that of the OSA groups. This study is part of the prospective TOROS study (Dutch Trial Register NTR3412). The institutional review board of the Academic Medical Centre in Amsterdam approved the study. A detailed description of the trial design has been published elsewhere.17
Subjects
We recruited patients from the neurorehabilitation unit of Heliomare rehabilitation center. Subjects were eligible if they had a stroke confirmed by a neurologist, were admitted in Helio-mare between one and 16 weeks post-stroke, were between 18 and 85 years of age, and were able to participate in the sleep study and in a neuropsychological assessment in Dutch. Patients were excluded if they had prior diagnosis of sleep apnea, diagnosis of central sleep apnea, or severe OSA (AHI > 60 and oxygen desaturations < 70%), which could endanger the patient's health if treatment was not started immediately. Other exclusion criteria were severe, unstable medical conditions, respiratory failure, history of severe congestive heart failure, traumatic brain injury, severe aphasia, severe confusion, or severe psychiatric comorbidity. All subjects provided written informed consent before participation.
Sleep Studies
The overnight recordings consisted of standardized pulse oximetry (WristOx; Nonin Medical, Plymouth, USA) and ambulatory overnight cardiorespiratory polygraphy (Embletta; Embla, Ottawa, Canada). The oxygen desaturation index (ODI) was calculated from pulse oximetry data using automated analyses. The ODI was defined as the mean number of oxygen desatu-rations ≥ 3% per hour. Patients with an ODI ≥ 5 were further tested for OSA by polygraphy.
Polygraphy included recordings of airflow by oronasal thermistor, oxygen saturation and heart rate by pulse oximetry, and respiratory effort by abdominal wall and thoracic wall motion recording. The data were recorded with a multichannel digital polygraphic system. Trained staff manually scored the polygraph recordings for apnea and hypopnea events. Apnea was defined as a reduction of airflow of ≥ 90% for ≥ 10 sec, and hypopnea was defined as a reduction of airflow of ≥ 50% for ≥ 10 sec followed by an oxygen desaturation ≥ 3%. Apneas with thoracic motion, without thoracic motion or with initial lack of motion followed by respiratory effort, were classified as obstructive, central, or mixed, respectively. The apnea-hypopnea index (AHI) was defined as the mean number of apneas and hypopneas per hour in bed. Patients with AHI ≥ 15 on polygraphy were diagnosed with sleep apnea (moderate to severe). OSA was diagnosed when ≥ 50% of the respiratory events were of the obstructive type; central sleep apnea was diagnosed when > 50% of respiratory events were of the central type. Patients with a normal ODI (< 5) or AHI < 15 were classified as non-OSA patients.
Intervention
CPAP treatment was set up and monitored by a specialized “Respicare” team. This team consists of two rehabilitation physicians, two nurse practitioners, and four nurses working on the neurorehabilitation unit specialized in sleep and breathing disorders. Before CPAP treatment was initiated, personalized instructions were given and a written manual for the CPAP device was provided. If possible, the partner or a close relative was also provided with instructions on the use of the CPAP device. CPAP treatment was evaluated together with the patient, and CPAP titration was performed using pulse oximetry. The pressure was adjusted until the ODI was reduced to normal (ODI < 5). If titration by nocturnal oximetry failed to adequately reduce the ODI, CPAP was titrated by polygraphy to reduce the AHI to < 5 or to the highest pressure tolerated. The CPAP device was provided with a memory card to evaluate the effectiveness of CPAP therapy over time and to monitor CPAP compliance. In our study, patients were considered to be compliant if they used CPAP for a minimum of 1 h/night. Good compliance was, in accordance with general consensus, defined as > 4 h CPAP usage for ≥ 5 nights per week.18 The Respicare team had contact with the patients regularly during the intervention period to help troubleshoot problems and encourage compliance. Patients who were discharged during the four-week intervention period were followed up by telephone interview and invited for an outpatient consultation.
Outcome Measures
The primary outcome measures were cognitive and functional status. For cognitive status the following nine domains were assessed: vigilance, attention, memory, working memory, executive functioning, language, visuoperception, psychomotor ability, and intelligence. A trained psychological technician administered the battery of standardized neuropsychological tests. The assessment battery consisted of the following tests: (1) Psychomotor Vigilance Task to test vigilance and reaction time, (2) D-KEFS Trail Making Test for visual attention and mental flexibility (3) d2 Test of Attention, a measure of sustained and selective attention; (4) Rey's Auditory Verbal Learning Test for verbal memory; (5) WAIS-III Letter Number Sequencing to test working memory, (6) Tower of London for the assessment of executive functioning, (7) Category Fluency to assess verbal fluency, (8) Bells Test, a test for visuoperception and visual neglect, (9) Finger Tapping Test to assess psychomotor ability and motor speed, and (10) WAIS-III Matrix Reasoning, a measure for nonverbal abstract reasoning. For a number of cognitive domains, nonverbal alternative tests were administered to patients with aphasia: Color Trails Test for visual attention and mental flexibility, Location Learning Test to test visual-spatial memory and WMS-IV Symbol Span for visual working memory. Categorization of tests into cognitive domains was based on conventional classification as described in a standard textbook of neuropsychological assessment.19 The classification of neuropsychological tests per cognitive domain and a more elaborate description of the tests with references are given in the supplemental material (Table S1).
The obtained test scores were transformed into demographically corrected z-scores using reference data of healthy adults as published in the test manuals with exception of the Dutch version of the Rey's Auditory Verbal Learning, test for which up-to-date Dutch norms were used. References of the normative data are included in the supplemental material. All tests were corrected for age, and the Color Trails test, Location Learning Test and Rey's Auditory Verbal Learning Test were corrected for both age and education. For three tests (Psycho-motor Vigilance Task, Bells Test, and Finger Tapping Task), reference data were not available. For these tests age-adjusted z-scores were calculated using a linear regression based approach including age as independent variable. We calculated the regression weights for the non-OSA group and subsequently applied them to all patients. If patients were unable to complete a task, the overall lowest obtained z-score for that test was given. For most domains, multiple tests were used; the average z-score for each domain was calculated.
Functional status was assessed by measures of neurological status and functional independence. The rehabilitation physician administered two scales of neurological status, the Canadian Neurological Scale (CNS)20 and the National Institutes of Health Stroke Scale (NIHSS).21 The obtained scores were transformed into z-scores using the mean and standard deviation of the patient group at T0, and averaged into one score for neurological status.
A trained nurse- practitioner scored the level of functional independence on the physical functioning subscales (mobility and self-care) of the Utrecht Scale for Evaluation of Rehabilitation (USER).22 The obtained scale scores were transformed into z-scores and averaged into one score for functional independence in the same way as the neurological status.
Secondary outcome measures were sleepiness (Stanford Sleepiness Scale),23 fatigue (Checklist Individual Strength),24 anxiety and depression (Hospital Anxiety and Depression Scale),25 and subjective sleep quality (Sleep Quality Scale).26
Demographic, clinical, and neurological data (age, sex, education level, body mass index [BMI], stroke type, stroke localization, stroke classification, time since onset of stroke, single versus recurrent stroke) were obtained from the medical files. The level of education was classified into 7 categories according to the UNESCO International Standard Classification of Education.27 Stroke classification at the time of hospital presentation was scored according to the Oxfordshire Community Stroke Project criteria.28 We categorized lacunar stroke (LACS) as mild, partial anterior circulation stroke (PACS) and posterior circulation stroke (POCS) as moderate, and total anterior circulation stroke (TACS) as severe. A full description of the assessment procedures has been published previously.17
Statistical Analysis
Data analyses were performed using SPSS (version 19.0). We compared baseline data of the 2 groups using Student t-test, χ2 test, or Mann-Whitney U test, as appropriate.
To compare differences between the control and intervention group over time, we calculated difference scores for our outcome measures for T1-T0. The groups were compared on primary outcomes with a multivariate analysis of covariance (MANCOVA) with age, severity of OSA, and stroke severity as covariates. We included these variables because we expected that they would influence the recovery rate of stroke patients, with younger age and more severe OSA ameliorating recovery, and greater stroke severity impeding recovery. Two separate MANCOVAs for cognition and functional status were performed, followed by a Benjamini-Hochberg correction for multiple comparisons.29 In case of missing outcomes, the last observation carried forward method was used.
Secondary outcomes were compared with separate analyses of covariance (ANCOVA), again including age, severity of OSA, and stroke severity as covariates. Effect sizes were calculated with partial eta squared. An effect size of 0.01 was considered small, 0.06 moderate, and 0.14 large.
All analyses were conducted on an intention-to-treat (ITT) basis. Additionally, we performed per-protocol analyses for T1 on patients who completed the assessment and met our criteria for minimal CPAP compliance (> 1 h/night). For all statistical tests, significance was set at a p value ≤ 0.05. We hypothesized that the CPAP group would show greater improvement at T1 than the control group and thus tested this hypothesis one-tailed.
In addition to the RCT analyses, we compared differences in recovery between the CPAP and control group at T2 (T2-T1) applying the same statistical analyses as described for T1. The USER scores were only available for a small group (14 patients) and were therefore excluded from the T2 analyses. As treatment was offered in both groups, we did not have a hypothesis for the outcome at T2 and therefore used two-tailed p values. The results of the T2 analyses are presented in the supplemental material (Tables S2–S4) and are not further discussed in the paper.
Finally, we compared the recovery rate of the 2 groups of OSA patients (CPAP and control) to a group of non-OSA stroke patients. For this comparison we conducted 2 MANCOVAs for the primary outcome measures, with age and stroke severity as covariates. To adjust for multiple comparisons a Bonferroni correction was performed. We hypothesized that the OSA control group would show less improvement than the CPAP group and non-OSA group.
RESULTS
Subjects
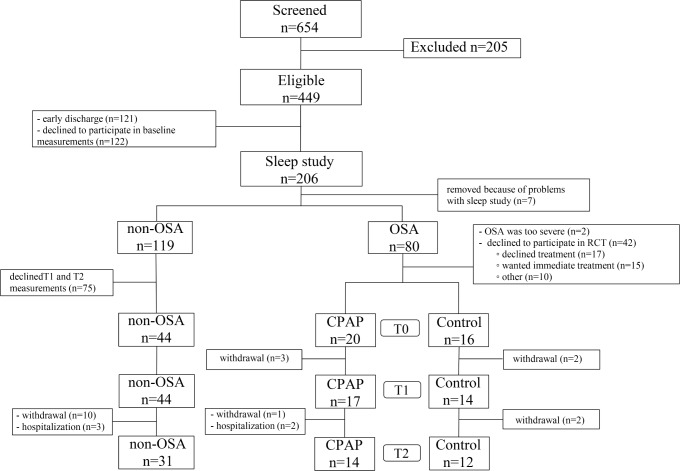
Between October 2011 and September 2014, we screened 654 patients, of whom 449 patients were eligible (Figure 1). Of these 449 patients, 206 agreed to participate in the sleep study. We diagnosed 80 patients (39%) with OSA (AHI ≥ 15). Two patients were excluded because immediate treatment was indicated and 42 patients declined to participate in the RCT. The remaining 36 patients were randomly assigned to the CPAP group (n = 20) or control group (n = 16). Three patients in the CPAP group and 2 in the control group withdrew from the study before the 4-week assessment. Five patients were lost to follow-up. We also included 44 non-OSA patients as a comparison group.
Figure 1. Patient flow chart.
Patients with OSA had a mean age of 59.1 years (± 8.6; range, 42–74) and average BMI of 27.1 (± 5.8; range, 18–46). The mean AHI was 34.2 (± 14.8; range, 15–83). The majority of patients had a first-ever ischemic stroke (56%) and were admitted to Heliomare, on average, 16.8 days (± 15.0; range, 3–71) after the stroke. The CPAP and control group showed similar baseline characteristics (Table 1), and did not differ significantly at baseline on measures of cognitive or functional status, or any of the secondary outcome measures (Tables 2–4).
Table 1.
Patient characteristics of the groups minimized for age, severity of OSA, stroke subtype, and severity of cognitive impairment.
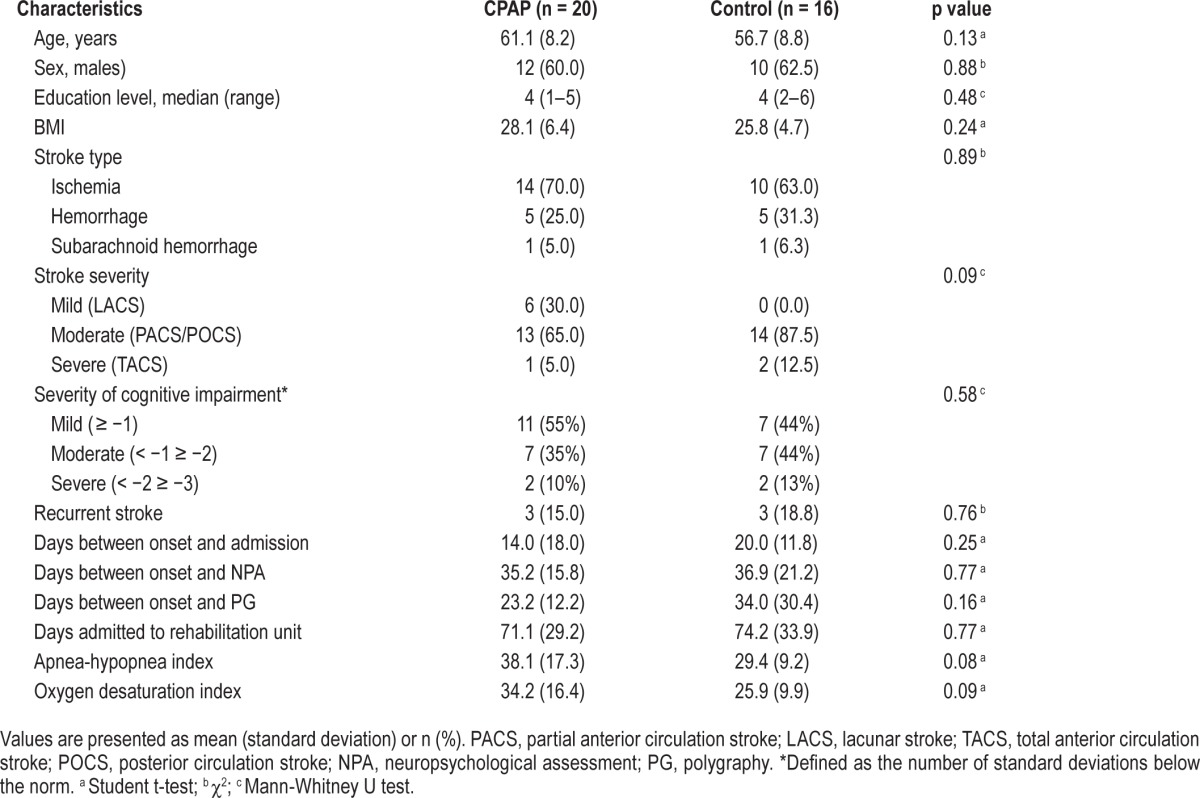
Table 2.
Cognitive outcomes.
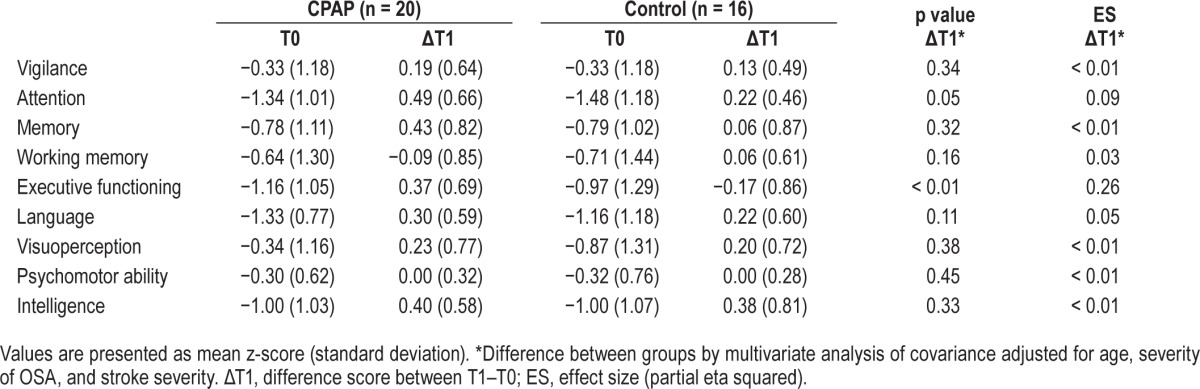
Table 3.
Functional outcomes.
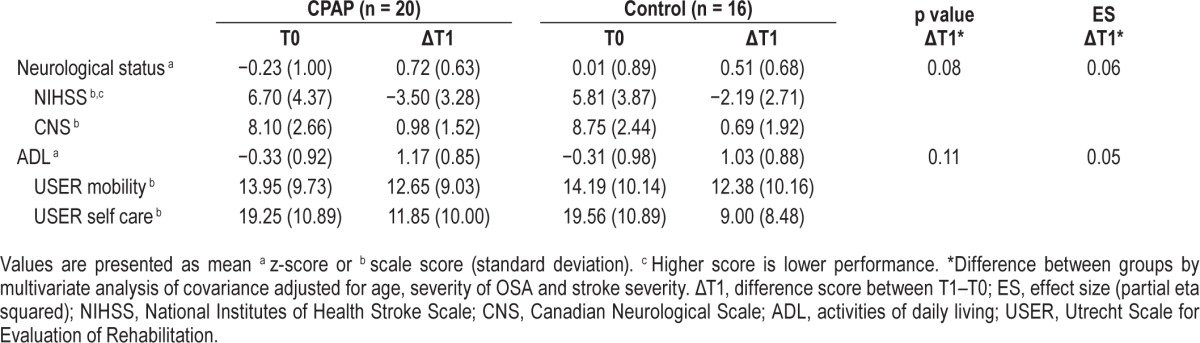
Table 4.
Secondary outcomes.
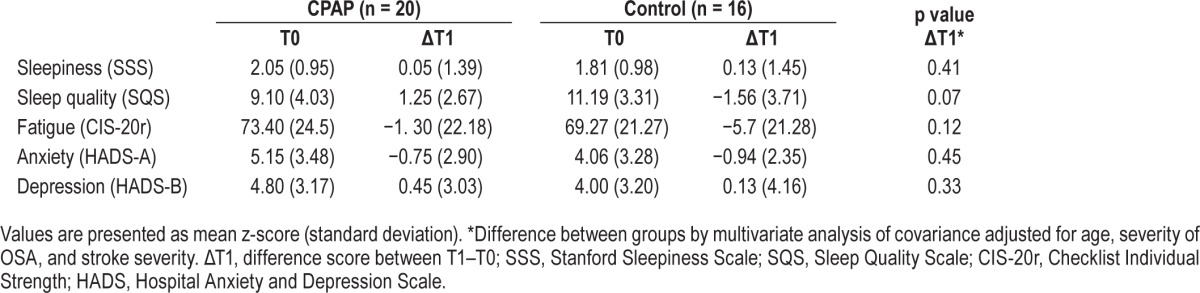
Sleep Measures
The mean compliance in the CPAP group was 2.5 h/night at T1 (± 2.8; range, 0–9). Nine patients used CPAP for < 1 h per night and were considered noncompliant to CPAP treatment. The mean CPAP use by the 11 compliant patients was 4.4 h/night (± 2.5; range, 1.3–9.0), with seven patients showing good CPAP compliance (> 4 h/day, ≥ 5 days a week). At two-month follow-up, eight of the 11 compliant patients at T1 were still using CPAP, with an average compliance of 4.9 (± 2.9) h/night. Ten of the 14 patients still participating in the control group at T1 started CPAP treatment after the four-week intervention period. The mean compliance during the follow-up period for this group was 3.2 h/night (± 2.5; range, 0.3–7.8).
After the four-week intervention period, 14 patients from the CPAP group and 12 from the control group agreed to polygraphy. Compared to the control group, the CPAP compliant patients (n = 10) showed a significant reduction in AHI (CPAP −25.9 versus control −7.6, p = 0.02). The mean AHI in the treatment group fell below the OSA cutoff of 15 (11.0 ± 11.0), while mean AHI in the control group was still above (21.9 ± 12.6). At follow-up, only 12 patients agreed to polygraphy. In the patients who were compliant to CPAP (n = 8), the AHI was 8.2 ± 6.2 compared to 32.8 ± 21.2 in the patients without CPAP use.
Primary Outcomes
The results of IIT analyses showed that the CPAP group experienced significantly greater improvements in cognitive status compared to the control group after the four-week intervention period (F9, 23 = 2.38, p = 0.02). Specifically, the CPAP group showed greater improvement in the domains of attention (p = 0.048) and executive functioning (p = 0.001), but not in the other cognitive domains (Table 2). The effects were moderate to large, partial η2 = 0.09 and 0.26, respectively. The profile of cognitive improvement of the CPAP and control group is visualized in Figure 2A. The CPAP group did not show significantly greater improvement in functional status after the 4-week intervention period (F2, 30 = 1.08, p = 0.18; Table 3).
Figure 2.
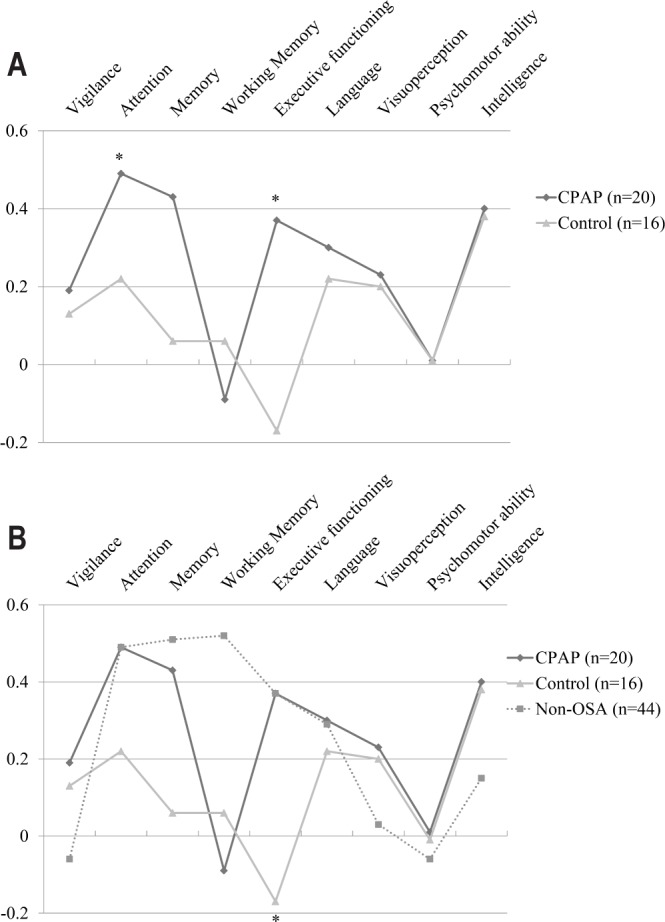
(A) Profile of mean z-scores of difference between T1 and T0 for the nine cognitive domains in CPAP group and control group. *Significant difference between the CPAP and control group. (B) Profile of mean z-scores of difference between T1 and T0 for the nine cognitive domains for the non-OSA group compared to the CPAP group and OSA control group. *Significant difference between the OSA control group and both the non-OSA group and CPAP group.
Secondary Outcomes
Based on the ITT analyses, no significant between-group differences were observed in improvement in sleep quality, levels of sleepiness and fatigue, or symptoms of depression and anxiety after the 4-week intervention period.
Per-Protocol Analyses
In the per-protocol analyses, we excluded two patients from the control group and three patients from the CPAP group, because they withdrew consent before T1 assessment. Another six patients were excluded because they were not compliant to CPAP (usage < 1 h/night).
In total, 25 patients (11 in the CPAP group and 14 in the control group) were included in the per protocol analyses. There were no significant differences between the groups on baseline characteristics. The results of the per-protocol analyses were in line with those of the ITT analyses, with the CPAP group showing significantly greater improvements in cognitive status (F9, 12 = 2.35, p = 0.04); specifically for the domains of attention (p = 0.044) and executive functioning (p = 0.006). The effect sizes were large, partial η2 = 0.14 and 0.33, respectively. As in the ITT analyses, no significant between-group differences were observed in the degree of improvement in functional status (F2, 19 = 1.04, p = 0.19) or any of the secondary outcome measures.
Comparison to Non-OSA Stroke Patients
Background characteristics of the non-OSA group compared to the OSA group are presented in the supplemental material (Table S5). In comparison to the OSA patients, the non-OSA patients were significantly younger (54 years ± 10.4), had a lower BMI (24.4 ± 3.6) and had a slightly higher level of education. To control for the background variability, we added education level as covariate to the analysis of cognitive functioning, and BMI to the analysis of functional status.
We found a significant difference in the improvement of cognitive functioning between the three groups (F18, 134 = 1.55, p = 0.042), with the CPAP and non-OSA group showing greater improvement in the domain of executive functioning than the OSA control group, respectively (p = 0.02 and p = 0.01). The effect size was moderate, partial η2 = 0.10. The cognitive profile of the three groups is visualized in Figure 2B. For functional status, we did not find a significant difference in improvement between the three groups (F4, 148 = 1.70, p = 0.08). Additional data on the non-OSA group in comparison to the OSA groups are presented in the supplemental material (Tables S6–S8).
DISCUSSION
In this randomized trial in stroke patients with OSA, we found that four weeks of CPAP treatment was associated with significant improvement in attention and executive functioning during inpatient rehabilitation. We did not find significant CPAP-associated improvements on measures of functional status, including neurological status and ADL, nor on secondary measures of sleepiness, sleep quality, fatigue, or mood. Even though the effect of CPAP on functional status was not significant, the trends for neurological status, ADL and sleep quality were all in the expected direction (p values 0.08–0.11). Our findings that CPAP has a beneficial effect of in the cognitive domains of attention and executive functioning are in contrast to earlier studies that showed no improvement after CPAP.12,14,15 This may be explained by the fact that we administered a full neuropsychological battery as opposed to a short cognitive assessment. Moreover, our results are in line with the effect of CPAP in the general OSA population in which beneficial effects of attention and executive functioning are often found.30,31
Other, more indirect support for our findings comes from baseline data of the TOROS study.7 In this TOROS substudy, we found that stroke patients with OSA showed greater impairments in a number of cognitive domains, including attention and executive functioning. These results suggest that OSA worsens the cognitive impairments of stroke patients, which might imply that these impairments are at least partially reversible with adequate CPAP treatment.
We did not observe a significant improvement in functional status (neurological functioning nor ADL) as a result of CPAP. Although the results on ADL are in accordance with previous studies,11,15 our findings on neurological functioning are not.11–13 There are a number of possible explanations for the lack of effect of CPAP on functional status in our study. First, inclusion of patients was independent of their functional status at admission. This resulted in inclusion of patients who already had a maximum neurological status score at baseline (up to 24% of patients depending on the applied scale); for these patients further improvement was not possible. Second, the functional outcome measures showed a strong ceiling effect at the assessment after the four-week intervention period (29% to 68% depending on the applied scale). Third, a number of more general limitations of our study, including the relatively small sample size and low CPAP compliance may have affected the results. These limitations will be discussed in more detail below.
In the stroke population, the effects of CPAP on sleepiness, quality of life and mood are not as clear-cut as in the general population.9 Some studies found small positive effects on measures of sleepiness and mood,12,15 while others did not.11,13,14 Our results correspond to the latter studies, as we did not find a significant effect of CPAP on any of our secondary measures. The lack of improvement may be explained by the fact that stroke patients with OSA do not report lower levels of sleep quality or higher levels of sleepiness, fatigue and depressed mood than stroke patients without OSA.7
In addition to the RCT, we compared the two OSA groups (CPAP and control) to a group of non-OSA stroke patients. We found that both the non-OSA patients and the OSA patients in the CPAP group showed larger improvement in the cognitive domain of executive functioning than the OSA control group, with no difference between the non-OSA and CPAP group. Although these results should be interpreted with caution as we were not able to match the groups, they do seem to support the hypothesis that untreated OSA negatively affects the recovery of cognitive functioning in stroke patients, and that adequate CPAP treatment of OSA in stroke patients can, at least partially, invert the OSA-associated cognitive impairments. These findings imply that the improvements are not only statistically significant, but also of clinical importance, given that we observed them in a small study sample.
The present study has a number of limitations that should be noted. First, the sample size of the study was small, despite screening of a large number of patients (n = 654; see Figure 1). Of the 206 patients who underwent a sleep study, 80 patients were diagnosed with OSA. Ultimately, just under half of these OSA patients agreed to participate in the RCT. Although these numbers seem very low, they are representative for research in this field. In a review of 17 studies on CPAP treatment in stroke patients, Tomfohr and colleagues reported that, of over 3,400 possible participants, only 4.8% were randomized to CPAP.16 In addition to the small included sample, a number of patients withdrew during the course of the study. Taken together, this limited the power of our study.
Second, we were not able to perform RCT analyses for the two-month follow-up, as the majority of patients in the control group received CPAP treatment after the four-week intervention period. Although, from a purely methodological perspective it would have been preferable to delay offering CPAP treatment to the control group until the two-month follow-up had been completed, this was not considered to be ethically acceptable.
Third, the compliance with CPAP in this study was poor, despite the support of our experienced Respicare team and involvement and education of primary caregivers. Given these compliance problems, the beneficial effects of CPAP on cognitive functioning obtained on the basis of the ITT analyses are all the more promising. Moreover, the results based on the per-protocol analysis suggest that, if CPAP compliance can be increased, even greater improvement may be expected. Further exploratory analysis showed that the seven patients with high compliance reported relatively large improvement in fatigue, as compared to the other patients (data available on request).
The results of our study once more underscore the need to improve CPAP compliance. In line with earlier research, we found that the pattern of adherence is established early in the treatment.32 Future research in this population should therefore specifically focus on the development of methods that augment the CPAP compliance of stroke patients in the first weeks of treatment. At present, the beneficial effects of CPAP on stroke outcome found in our study, as well as in a number of earlier studies, offer a preliminary evidence base for the use of this treatment as part of a rehabilitation program for stroke patients.16
In conclusion, this study indicates that CPAP treatment improves cognitive functioning of stroke patients with OSA during inpatient rehabilitation.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Brita Daniels, Irene Kos and Mario van Lieshout for their support with data collection.
ABBREVIATIONS
- ADL
activities of daily living
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- CNS
Canadian Neurological Scale
- LACS
lacunar stroke
- NIHSS
National Institutes of Health Stroke Scale
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PACS
partial anterior circulation stroke
- POCS
posterior circulation stroke
- TACS
total anterior circulation stroke
- USER
Utrecht Scale for Evaluation of Rehabilitation
REFERENCES
- 1.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 3.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in US communities. Sleep Breath. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 4.Colten H, Abboud F, Block G, et al. Washington, DC: National Academy of Sciences; 2006. Sleep disorders and sleep deprivation: an unmet public health problem. [PubMed] [Google Scholar]
- 5.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26:293–7. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 6.Turkington PM, Allgar V, Bamford J, Wanklyn P, Elliott MW. Effect of upper airway obstruction in acute stroke on functional outcome at 6 months. Thorax. 2004;59:367–71. doi: 10.1136/thx.2003.005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aaronson JA, van Bennekom CA, Hofman WF, et al. Obstructive sleep apnea is related to impaired cognitive and functional status after stroke. Sleep. 2015;38:1431–7. doi: 10.5665/sleep.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 9.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Rev. 2006:3. doi: 10.1002/14651858.CD001106.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Bravata DM, Concato J, Fried T, et al. Continuous positive airway pressure: evaluation of a novel therapy for patients with acute ischemic stroke. Sleep. 2011;34:1271–7. doi: 10.5665/SLEEP.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parra O, Sanchez-Armengol A, Bonnin M. Early treatment of obstructive sleep apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011;37:1128–36. doi: 10.1183/09031936.00034410. [DOI] [PubMed] [Google Scholar]
- 12.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42:1062–7. doi: 10.1161/STROKEAHA.110.597468. [DOI] [PubMed] [Google Scholar]
- 13.Hsu C, Vennelle M, Li H, Engleman HM, Dennis MS, Douglas NJ. Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure. J Neurol Neurosurg Psychiatry. 2006;77:1143–9. doi: 10.1136/jnnp.2005.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DL, Chervin RD, Kalbfleisch JD, et al. Sleep apnea treatment after stroke (SASTS) trial: is it feasible? J Stroke Cerebrovas Dis. 2013;22:1216–24. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandberg O, Franklin KA, Bucht G, Eriksson S, Gustafson Y. Nasal continuous positive airway pressure in stroke patients with sleep apnoea: a randomized treatment study. Eur Respir J. 2001;18:630–4. doi: 10.1183/09031936.01.00070301. [DOI] [PubMed] [Google Scholar]
- 16.Tomfohr LM, Hemmen T, Natarajan L, et al. Continuous positive airway pressure for treatment of obstructive sleep apnea in stroke survivors what do we really know? Stroke. 2012;43:3118–23. doi: 10.1161/STROKEAHA.112.666248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaronson JA, van Bennekom CA, Hofman WF, et al. The effect of obstructive sleep apnea and treatment with continuous positive airway pressure on stroke rehabilitation: rationale, design and methods of the TOROS study. BMC Neurol. 2004;14:e1471–2377. doi: 10.1186/1471-2377-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Resp Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 19.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. 4th ed. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 20.Cote R, Battista R, Wolfson C, Boucher J, Adam J, Hachinski V. The Canadian neurological scale validation and reliability assessment. Neurology. 1989;39:638. doi: 10.1212/wnl.39.5.638. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46:660–2. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 22.Post MW, van de Port IG, Kap B, Berdenis van Berlekom SH. Development and validation of the Utrecht scale for evaluation of clinical rehabilitation (USER) Clin Rehabil. 2009;23:909–17. doi: 10.1177/0269215509341524. [DOI] [PubMed] [Google Scholar]
- 23.Herscovitch J, Broughton R. Sensitivity of the Stanford sleepiness scale to the effects of cumulative partial sleep deprivation and recovery oversleeping. Sleep. 1981;4:83–91. doi: 10.1093/sleep/4.1.83. [DOI] [PubMed] [Google Scholar]
- 24.Vercoulen JH, Swanink C, Fennis JF, Galama J, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38:383–92. doi: 10.1016/0022-3999(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Visser P, Hofman WF, Kumar A, et al. Sleep and mood: measuring the sleep quality. In: Priest RG, Pletscher A, Ward J, editors. Sleep research. Baltimore, MD: University Park Press; 1979. pp. 135–45. [Google Scholar]
- 27.United Nations Educational Scientific and Cultural Organization. International standard classification of education: ISCED 1997. UNESCO. 1996. www.uis.unesco.org/Library/Documents/isced97-en.pdf.
- 28.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–6. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 30.Kylstra WA, Aaronson JA, Hofman WF, Schmand BA. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis. Sleep Med Rev. 2013;17:341–7. doi: 10.1016/j.smrv.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36:1297–305. doi: 10.5665/sleep.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5:229–40. doi: 10.1080/15402000701264005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.