Abstract
Study Objectives:
To determine if treatment of obstructive sleep apnea (OSA) and obesity hypoventilation syndrome (OHS) among patients with chronic hypoxemia is associated with reduced healthcare utilization.
Methods:
We performed a retrospective cohort study of 129 obese, hypoxemic patients who underwent polysomnography and were prescribed positive airway pressure (PAP) therapy. During a 2-year follow-up period we examined the associations between adherence to PAP therapy and rates of hospitalization, emergency room (ER) visits, and outpatient visits.
Results:
Severe OSA and OHS were common, as were hypertension, cardiovascular, and pulmonary disease. Forty-nine percent of patients were adherent with PAP therapy. Compared to patients who were not adherent to PAP therapy, adherent patients had significantly lower rates of all-cause hospitalization (incident rate ratio [IRR]:0.55, 95% CI 0.33, 0.93) after adjustment for age, sex and hospitalisation rates prior to treatment. Adjustment for additional comorbidities attenuated this association (IRR: 0.61, 95% CI 0.35, 1.06). Adherence with PAP therapy was associated with lower odds of frequent hospitalization (odds ratio 0.23, 95% CI 0.07, 0.73). There were no significant differences in the rates of ER or outpatient visits between adherent and non-adherent patients.
Conclusions:
Adherence with PAP treatment in patients with chronic hypoxemia and chronic medical disorders is associated with reduced rates of hospitalization, which has significant benefit both for patients and the healthcare system.
Citation:
Povitz M, Tsai WH, Pendharkar SR, Hanly PJ, James MT. Healthcare use in individuals with obesity and chronic hypoxemia treated for sleep disordered breathing. J Clin Sleep Med 2016;12(4):543–548.
Keywords: hypoxemia, obstructive sleep apnea, hypoventilation, obesity
INTRODUCTION
The prevalence of obstructive sleep apnea (OSA) in the general population ranges from 9% to 24%; however, it may affect up to 80% of obese hypoxemic individuals.1,2 OSA is associated with high rates of healthcare utilization, which burden patients and contribute to substantial costs of care.3–6
Treatment of OSA or obesity hypoventilation syndrome (OHS) may reduce healthcare utilization,4,7 suggesting real world benefits, both for patients and the healthcare system. However, previous studies that have shown an association between OSA treatment and reduced healthcare utilization were performed in cohorts where the burden of comorbidity was low.4,8 A similar reduction in healthcare utilization in individuals with OSA and comorbid conditions that cause of chronic hypoxemia has not been investigated.
Since 2009, evaluation and treatment of sleep disordered breathing (SDB) in obese hypoxemic individuals has been a requirement for funding of supplemental oxygen in Alberta, Canada.9 Considerable healthcare resources have been devoted to implement diagnostic sleep testing and optimize continuous positive airway pressure (CPAP) or noninvasive ventilation (NIV) adherence in this setting. The objective of this study was to determine if adherence with positive airway pressure (PAP) therapy, CPAP or NIV, was associated with changes in healthcare utilization. We also sought to determine whether adherence was associated with a lower probability of being a high healthcare user since these individuals use disproportionately more healthcare resources.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Treatment of both OSA and OHS has been shown to decrease healthcare utilization; however previous studies have studied patients without hypoxemia or significant comorbidities. This study assessed healthcare utilization rates in a cohort of obese hypoxemic individuals.
Study Impact: This study demonstrates the association of treatment adherence with positive airway pressure therapy with reduced hospitalization rates. This reduction may offset the cost of treatment and increase in outpatient visits seen.
METHODS
Study Design and Cohort Formation
We performed a retrospective cohort study of all obese (body mass index [BMI] ≥ 30 kg/m2) and hypoxemic (arterial partial pressure of oxygen [PaO2] < 60 mm Hg) individuals referred to a single sleep centre in Calgary, Alberta between January 1, 2009, and October 30, 2013. During this time, obese individuals in Alberta were required to undergo polysomnography (PSG) to obtain public funding for home oxygen therapy. The Foothills Medical Centre (FMC) Sleep Centre is one of 3 publically funded PSG facilities evaluating hypoxemic patients in the province, and the only facility of this kind in Calgary, (population ∼1.1 million). Oxygen funding criteria during the study required that patients be offered PAP therapy if SDB was diagnosed.
Patients were selected from the FMC Sleep Centre database. We identified all patients who had undergone PSG and arterial blood gas (ABG) testing on the same date. Arterial blood gasses are routinely performed at our facility during PSG hookup. This study included only individuals with follow-up data for at least one year after PSG, in order to provide sufficient time to optimize adherence and determine outcomes following prescription of PAP therapy. Non-Alberta residents were excluded to ensure complete ascertainment of healthcare utilization for the cohort. This study was approved by the conjoint research ethics board of the University of Calgary (REB13-0032).
Exposure
The exposure of interest was adherence to either CPAP or NIV. Satisfactory adherence was defined as a download from CPAP or NIV units showing > 4 h of use on 70% of nights in the first 6 months following PSG, or as a clinical note of adherence with PAP therapy by an experienced clinician (clinical adherence). Respiratory therapists with additional training in PAP therapy and sleep physicians documented self-reported PAP adherence based on 4 h of use on 70% of nights. Adherence to supplemental oxygen was not measured. Patients with significant SDB were offered follow up at the FMC Sleep Centre to optimize treatment adherence. The FMC Sleep Centre comprises physicians who are certified in respiratory and sleep medicine and experienced respiratory therapists. Patients were evaluated and treated for co-morbid sleep disorders. Ventilatory equipment (CPAP, oxygen concentrators, NIV) was provided by private home care companies under contract with the Alberta government. The home care company configured and maintained the equipment which was verified by the Sleep Centre staff. Funding for ventilatory equipment was available through a combination of private insurance and government programs. Patients who did not follow up at the sleep center or with a respiratory home care company, or whose downloads showed < 4 h of use on 70% of nights10 were considered non-adherent.
Outcomes
Healthcare utilization rates were determined from provincial administrative data records collected by the Alberta Government Department of Health and Wellness. Hospital admissions were determined from the hospital discharge abstract database, while emergency room (ER), urgent care visits, as well out outpatient visits to Alberta Health Services facilities were determined from the provincial ambulatory care reporting system. Alberta Health Services is the sole provider of healthcare in Alberta, Canada. Visits to the sleep centre clinic and PSG laboratory were included as outpatient visits. Patients were followed for health system contacts from 2 years prior to the date of PSG until 2 years after PSG or the date of patient death. The variable follow-up period was chosen to allow inclusion of patients with more recent PSG dates.
Covariates
Comorbidities were identified by chart review, and supplemented with administrative data. For each patient up to 3 years of discharge abstract data containing up to 25 diagnoses and procedure codes were analyzed for the presence of the comorbidities in the Charlson Index.11 The presence of SDB was determined from baseline PSG performed in all patients. Chronic obstructive pulmonary disease was identified with spirometry, which was available for 110 individuals.
Polysomnography was performed with continuous monitoring of 3-channel electroencephalogram, 2-channel electroculogram, submental electromyogram, single-lead electrocardiogram, oro-nasal thermistor, nasal airflow, abdominal and thoracic respiratory inductance plethysmography bands, pulse oximetry, and transcutaneous partial pressure of carbon dioxide. Data were collected and analyzed using Sandman software (Natus Medical, USA). The PSGs were scored using Canadian Thoracic Society Criteria.12 An obstructive apnea was defined as ≥ 90% reduction in airflow for ≥ 10 sec with persistent respiratory effort. A central apnea was defined as a reduction in airflow ≥ 90% for ≥ 10 sec without respiratory effort. A hypopnea was defined as ≥ 50% reduction in airflow lasting ≥ 10 sec or any reduction in airflow for ≥ 10 sec accompanied by > 3% drop in oxygen saturation or an arousal.
Obstructive sleep apnea was defined as an apnea hypopnea index (AHI) > 5 events per hour. Mild OSA was defined as an AHI > 5 to < 15, moderate OSA ≥ 15 to < 30, and severe OSA as ≥ 30. The obesity hypoventilation syndrome was defined as an arterial partial pressure of carbon dioxide (PaCO2) during wakefulness ≥ 45 mm Hg in the presence of obesity (BMI ≥ 30 kg/m2) and absence of other causes of hypoventilation such as severe chronic lung disease.13
Statistical Analyses
Means and standard deviations were determined for normally distributed variables, and medians and interquartile ranges were determined for non-normally distributed variables. Proportions and their 95% confidence intervals (95% CI) were calculated using the Clopper-Pearson method. Differences between groups were tested using the χ2 test for count data, ANOVA for normally distributed data or the Kruskal-Wallis test for skewed data. Healthcare utilization rates were calculated for each type of utilization (hospital, ER, or outpatient) as the sum of all healthcare contacts divided by the time at risk for each individual. The time at risk for each individual was calculated in years as the time from the index PSG to the end of available follow-up data. Unadjusted healthcare utilization rates were compared between adherent and non-adherent participants using the Wilcoxon signed rank test. The Wilcoxon rank-sum test was used to compare the crude rates of the 2 subgroups of adherent patients (download data and clinical adherence) prior to combining them for further analysis.
Separate negative binomial regression models were fit to model associations between PAP treatment adherence and rates of healthcare utilization for hospitalization, ER visits, and outpatient visits after the index PSG. In order to reduce treatment selection bias, we fit a series of models to determine incidence rate ratios of healthcare utilization for PAP treatment adherent compared to non-adherent participants, after excluding those not offered therapy. Two sets of adjusted models were fit; the first modeled the rate of health-care utilization following PSG, adjusted for, age, sex, BMI, and the rate of each respective healthcare utilization event (hospitalization, ER visit, or outpatient visits) prior to PSG. The second set of models further adjusted for potentially confounding comorbidities: hypertension, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and diabetes.6 Interactions between PAP adherence and a diagnosis of OSA, COPD, and OHS were tested but these terms were excluded from final models as they were not statistically significant.
We also examined the odds of high healthcare utilization (based on rates of hospitalization, emergency/urgent care visits, or outpatient visits) after the index PSG. We defined high healthcare utilizers as those with rates above the 75% percentile for the entire cohort. We then performed logistic regression to assess the association between adherence to PAP therapy and odds of high healthcare use (hospitalizations, emergency visits, and outpatient visits), adjusting for high healthcare use in the period prior to the index PSG (for the same type of healthcare use), age, sex, BMI, and presence of the following diagnoses COPD, CHF, OSA, OHS, hyper-tension, and diabetes. Interactions between PAP adherence and a diagnosis of OSA, OHS and COPD were tested, but excluded from final models as they were not statistically significant. The assumptions for negative binomial regression and logistic regression models were tested and met. All analyses were performed using Stata version IC 13 (College Station, TX).
RESULTS
Patients
One hundred eighty-four patients underwent PSG and ABG during the study period (Figure 1). Thirteen patients were excluded due to death in the first year of follow-up (range 0.2 to 11.5 months), 5 were excluded due to insufficient follow up time (range 10.3 to 11.8 months), and 5 were excluded because information on healthcare contact was not available prior to the index PSG. Ten of those who died were not treated, and 2 were non-adherent. Three of those with insufficient follow-up were treated, but only one had achieved satisfactory adherence. Twenty-two of 161 patients had full-night diagnostic PSG versus 139 who had split-night studies. Thirty-two of the 161 remaining patients did not have significant SDB and were not treated.
Figure 1. Study flow diagram.
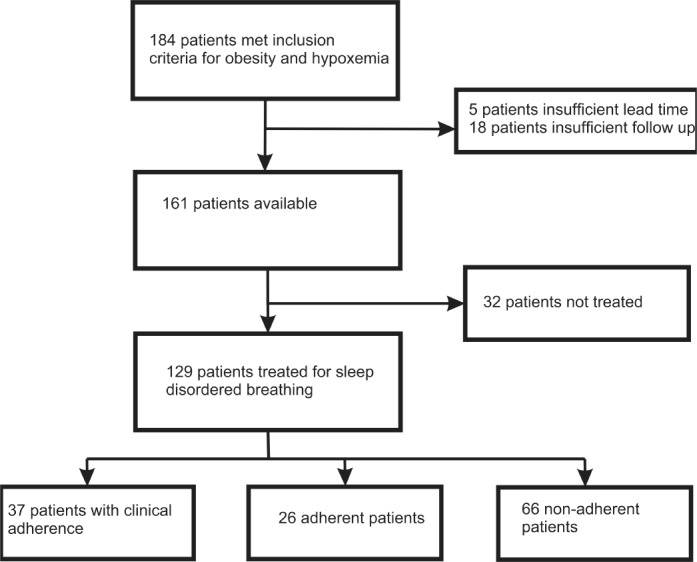
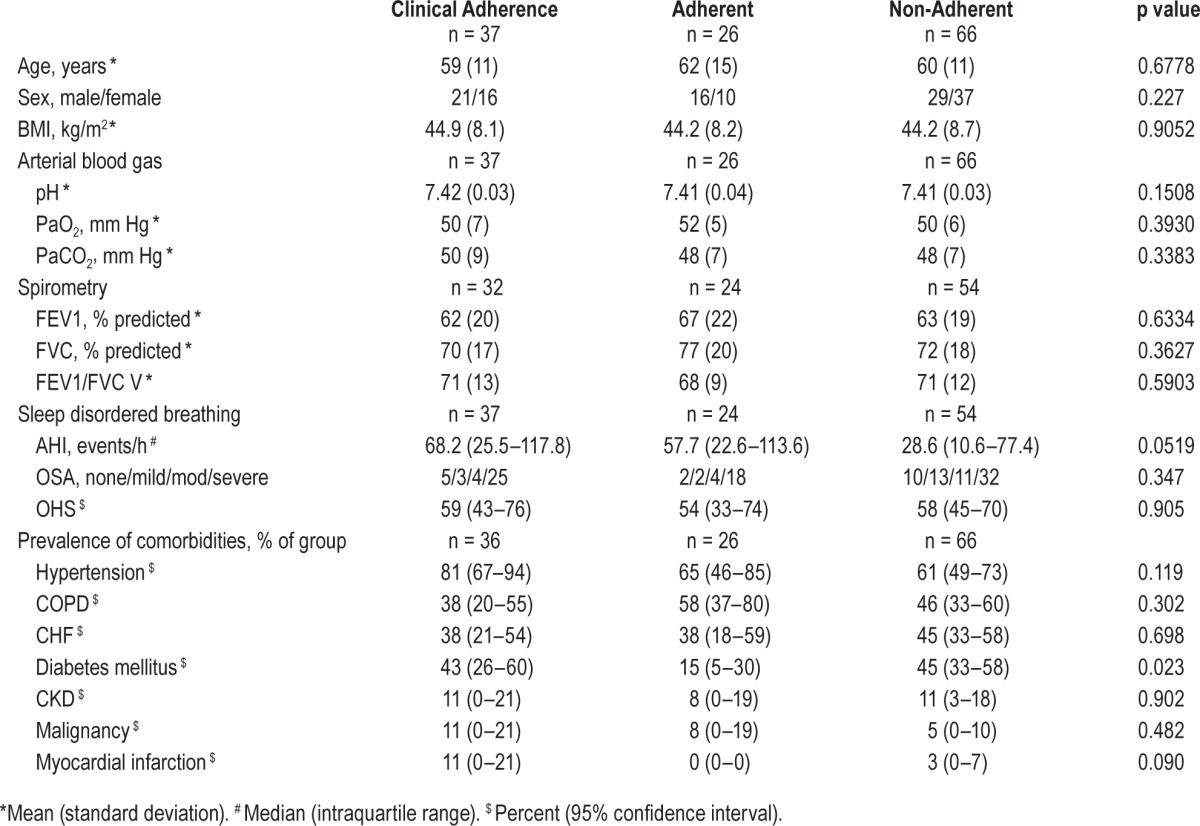
Twenty-six patients were adherent with PAP therapy based on equipment downloads and 37 were adherent based on clinician report. Sixty-six patients were documented by clinicians to be non-adherent or had downloads from PAP equipment that did not meet the prespecified definition of satisfactory adherence. Only the prevalence of diabetes differed significantly between patients who were and were not adherent (Table 1).
Table 1.
Baseline characteristics of all patients (n = 129).
Healthcare Utilization Rates Pre and Post Polysomnography
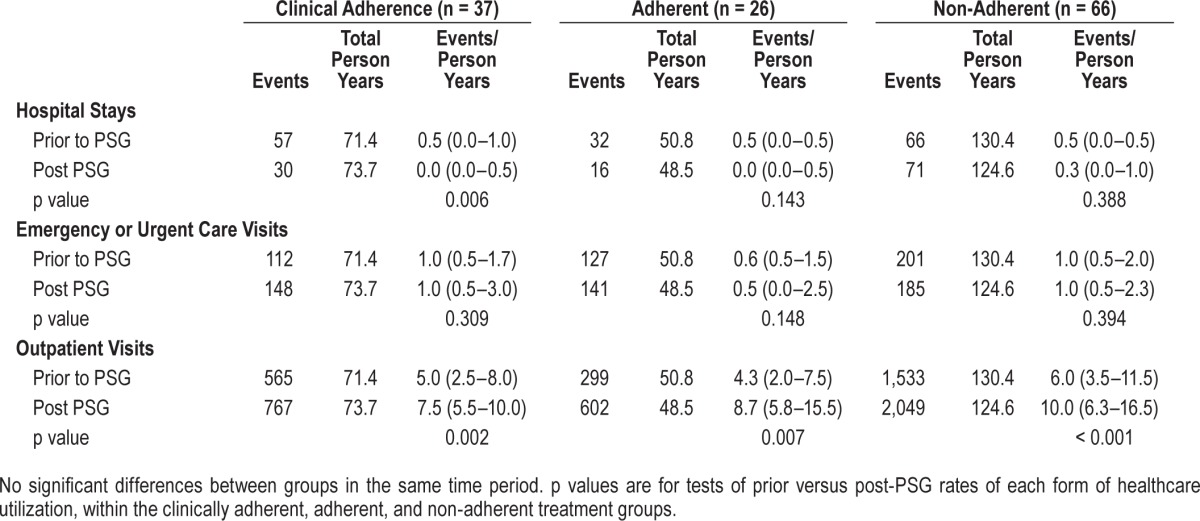
In both the period before and after PSG, hospital admissions were less common (0–0.3/year) than ER (0.5–1/year) or ambulatory visits (5.24–10/year) for all groups (Table 2). The crude rates of hospitalization fell in the clinically adherent group during the period following PSG testing (p = 0.006). There were statistically significant increases in the rate of outpatient visits following PSG for all groups. There was no statistically significant difference in the crude healthcare utilization rates between patients whose adherence was defined clinically versus by download data.
Table 2.
Crude healthcare utilization rates per year.
Relative Rates of Healthcare Utilization between Adherent and Non-Adherent Groups
Compared to non-adherent participants, those who were adherent to PAP therapy had a lower rate of hospitalization when adjusted for age, sex, BMI and prior healthcare utilization rate, (IRR: 0.55 [0.33, 0.93]); this association was attenuated once adjusted for comorbidities (IRR 0.61, 95% CI 0.35, 1.06) (Figure 2). Although there were more ER visits and fewer outpatient visits associated with adherence in the partially adjusted model, neither of these changes were statistically significant (ER visits: IRR: 1.22 [95% CI 0.84, 1.75]; outpatient visits: IRR: 0.85 [95% CI 0.67, 1.12]). Results from subgroup analyses of patients with electronic downloads of PAP use showed similar findings (Table S1, supplemental material).
Figure 2. Healthcare utilization during the follow-up period.
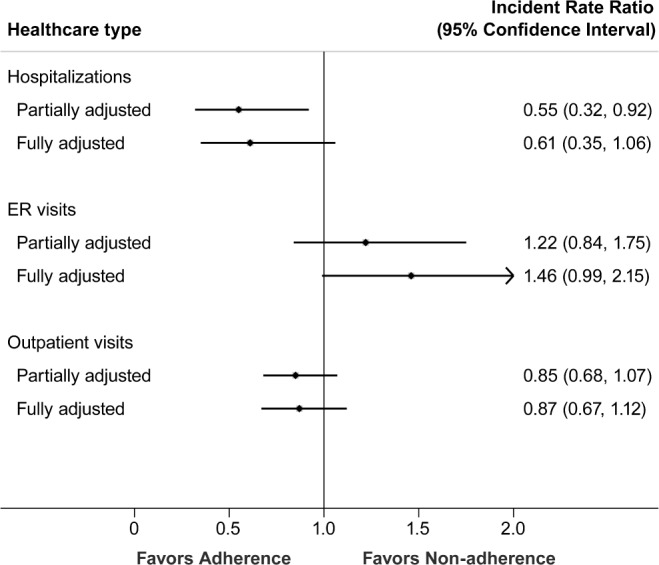
This plot shows the incidence rate ratio between those adherent and non-adherent with PAP therapy. The solid dot represents the point estimate while the bars represent the 95% confidence intervals. The solid vertical line is the line of no difference. Partially adjusted models adjusted for pre-treatment healthcare use age, sex, and BMI. Fully adjusted models were additionally adjust for comorbidity (obesity hypoventilation syndrome, congestive heart failure, hypertension, and diabetes)
Odds of High Healthcare Use
The cutoffs for the upper quartiles for health utilization rates in the cohort were 94 per 100 person years for hospitalization, 250 per 100 person years for ER visits, and 1,526 per 100 person years for outpatient visits. After adjusting for age, sex, BMI, high healthcare utilization prior to the PSG, and comorbidity, adherence to PAP therapy was associated with lower odds of frequent hospitalization (odds ratio [OR] = 0.23 95% CI [0.07, 0.73]). There were no significant associations between adherence and the odds of frequent ER visits (OR = 2.73 95% CI (0.85, 8.78) or frequent outpatient visits (OR = 0.37, 95% CI (0.13, 1.02).
DISCUSSION
In this cohort of obese, hypoxemic patients with SDB and a high prevalence of comorbid disease, approximately 50% were adherent with PAP therapy. Adherence with PAP therapy was associated with reduced healthcare utilization reflected by (1) a fall in the crude hospitalization rate compared to that observed prior to starting PAP, and (2) a lower rate of hospitalization and a smaller likelihood of frequent hospitalization compared to non-adherent patients. In contrast, ER visits did not change after PAP was started, nor was there any difference in the relative rate of ER visits between adherent and non-adherent groups. Although the crude rate of outpatient visits increased after PAP was started, the relative rates of out-patient visits between adherent and non-adherent groups did not significantly differ.
There appeared to be a trade-off between lower hospitalization rates and an increase in outpatient visits following PSG. The increasing number of outpatient visits following PSG may reflect the SDB therapy, which was not present prior to PSG, and increasing chronic disease management following access to healthcare providers who initiated referrals or testing to diagnose SDB. Because outpatient visits are less costly and preferable to more frequent hospitalizations, the rise in out-patient healthcare contacts seen in concert with a reduction in hospitalization rates could be interpreted as favorable for the healthcare system.
Previous studies of change in health care utilization associated with treatment of OSA or OHS have reported PAP adherence of 72% to 82%.4,8 The lower level of adherence to PAP therapy in our study may be due to specific characteristics of the cohort. For example, patients were mandated to attend the sleep center in order to qualify for supplemental oxygen funding rather than having a specific concern about SDB or troubling sleep symptoms. In addition, many patients had been treated with supplemental oxygen for years and were skeptical about alternative therapies. This may have impacted their enthusiasm for PAP therapy, which is a significant determinant of long-term adherence.14 Finally, the high prevalence of comorbid disease may have contributed to some patients' difficulty adhering to PAP therapy.
The lower hospitalization rate seen following PAP treatment in our cohort is consistent with previous research. Bahammam et al. showed that there were lower healthcare costs and number of days spent in hospital associated with treatment of SDB. These investigators did not include the number of hospitalizations as an outcome measurement and patients with high healthcare utilization, defined as greater than 70 days of hospitalization over 7 years, were excluded from the study.4 Banno et al. showed a similar finding in women but did not provide information about comorbidities.8 Berg et al. showed that untreated OHS was associated with higher rates of healthcare utilization compared to healthy or obese individuals without OHS. This study also showed that treatment of OHS was associated with lower healthcare utilization. However, the study did not include non-adherent patients to control for temporal trends in healthcare utilization or other non-NIV interventions which might reduce utilization.7 In contrast more recently a population study of OSA or OHS patients in Denmark showed that they had high healthcare costs that continued to increase despite treatment of their SDB.15
This study has limitations. First, the cohort was drawn from a single centre, which may limit the generalizability of our findings. However, studying a cohort in the province of Alberta may have minimized referral bias due to the government policy for funding of supplemental oxygen therapy, which is not the case in many other jurisdictions. Mandatory testing should have decreased bias from patients or clinicians who do not believe that treatment of SDB is beneficial. Secondly, we included a clinical definition of adherence with PAP therapy. This may have led to misclassification of patients as adherent when, in fact, they were non-adherent or vice versa. If this occurred, we anticipate that it would have biased our results toward the null hypothesis, which is not what we found. Furthermore, the crude healthcare utilization rate was not different between those whose adherence was determined by electronic download versus clinical assessment, and a sensitivity analysis excluding patients with only clinical adherence showed similar results. Thirdly, adherence to one therapy may also be a marker of better overall treatment adherence, which could be associated with lower healthcare utilization. Non-adherence with other medical therapies would likely be associated with increased healthcare utilization, which would confound the outcome of interest. However, this may be is disputed by the findings of Villar et al.,16 who showed that non-adherence to CPAP was not associated with non-adherence to 3 cardiovascular medications in a cohort with severe OSA and cardiovascular disease. Finally, there may be residual confounding due to variation in adherence with supplemental oxygen therapy, which was not measured, and socioeconomic status. Copayments and ongoing replacement of perishable equipment components (e.g., masks) may have posed a barrier to care for patients with limited finances. Our sleep clinic staff tried diligently to facilitate ongoing adherence by accessing lower cost providers or supplying the perishable equipment to such patients without cost.
In conclusion, we found that obese hypoxemic patients with SDB and a high prevalence of comorbid disease who are adherent with PAP therapy experienced significantly lower rates of hospitalization following initiation of PAP therapy, and lower hospitalization rates utilization than patients who were non-adherent to PAP. Our findings suggest that the clinical benefits of PAP therapy can also be observed in patients with obesity and hypoxemia, and that there may be value to strategies to identify and treat SDB in these patients.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding for this project was received from the Faculty of Medicine Sleep Research Program, University of Calgary. Marcus Povitz received a scholarship from the Western Regional Training Centre for health services research. Neither funding source contributed to the design or conduct of the study. Dr. Pendharkar received payment for interpretation of ambulatory sleep tests from VitalAire Canada Inc. Dr. Tsai received payment from Respiratory Homecare Solutions for interpretation of level III testing and participated in speaking engagements for Boehringer-Ingelheim. Dr. Hanley has consulted for Dream Sleep Respiratory Services and is on the speakers bureau of Philips Respironics. Dr. James has indicated no financial conflicts of interest. No off label use of pharmaceutical or medical devices is described in this paper. This work was performed at the University of Calgary.
ABBREVIATIONS
- ABG
arterial blood gas
- AHI
apnea hypopnea index
- BMI
body mass index
- CHF
congestive heart failure
- COPD
chronic obstructive pulmonary disease
- CPAP
continuous positive airway pressure
- ER
emergency room
- FMC
Foothills Medical Centre
- IRR
incident rate ratio
- NIV
noninvasive ventilation
- OHS
obesity hypoventilation syndrome
- OR
odds ratio
- OSA
obstructive sleep apnea
- PaCO2
arterial partial pressure of carbon dioxide
- PaO2
arterial partial pressure of oxygen
- PAP
positive airway pressure
- PSG
polysomnography
- SDB
sleep disordered breathing
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Povitz M, James MT, Pendharkar SR, Raneri J, Hanly PJ, Tsai WH. Prevalence of Sleep-disordered breathing in obese patients with chronic hypoxemia. a cross-sectional study. Ann Am Thorac Soc. 2015;12:921–7. doi: 10.1513/AnnalsATS.201412-551OC. [DOI] [PubMed] [Google Scholar]
- 3.Ronksley PE, Hemmelgarn BR, Heitman SJ, et al. Excessive daytime sleepiness is associated with increased health care utilization among patients referred for assessment of OSA. Sleep. 2011;34:363–70. doi: 10.1093/sleep/34.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahammam A, Delaive K, Ronald J, Manfreda J, Roos L, Kryger MH. Health care utilization in males with obstructive sleep apnea syndrome two years after diagnosis and treatment. Sleep. 1999;22:740–7. doi: 10.1093/sleep/22.6.740. [DOI] [PubMed] [Google Scholar]
- 5.Ronald J, Delaive K, Roos L, Manfreda J, Bahammam A, Kryger MH. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22:225–9. doi: 10.1093/sleep/22.2.225. [DOI] [PubMed] [Google Scholar]
- 6.Broemeling AM, Watson DE, Prebtani F. Population patterns of chronic health conditions, co-morbidity and healthcare use in Canada: implications for policy and practice. Healthc Q. 2008;11:70–6. doi: 10.12927/hcq.2008.19859. [DOI] [PubMed] [Google Scholar]
- 7.Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest. 2001;120:377–83. doi: 10.1378/chest.120.2.377. [DOI] [PubMed] [Google Scholar]
- 8.Banno K, Manfreda J, Walld R, Delaive K, Kryger MH. Healthcare utilization in women with obstructive sleep apnea syndrome 2 years after diagnosis and treatment. Sleep. 2006;29:1307–11. doi: 10.1093/sleep/29.10.1307. [DOI] [PubMed] [Google Scholar]
- 9.Respiratory Policies and Procedures: Alberta Aids to Daily Living. Government of Alberta; 2011. Respiratory Policies and Procedures: Alberta Aids to Daily Living; pp. 1–32. [Google Scholar]
- 10.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 11.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 12.Fleetham J, Ayas N, Bradley D, et al. Canadian Thoracic Society guidelines: diagnosis and treatment of sleep disordered breathing in adults. Can Respir J. 2006;13:387–92. doi: 10.1155/2006/627096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med. 2011;183:292–8. doi: 10.1164/rccm.201008-1280CI. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett D, Wong K, Richards D, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep. 2013;36:1647–54. doi: 10.5665/sleep.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennum P, Kjellberg J. Health, social and economical consequences of sleep-disordered breathing: a controlled national study. Thorax. 2011;66:560–6. doi: 10.1136/thx.2010.143958. [DOI] [PubMed] [Google Scholar]
- 16.Villar I, Izuel M, Carrizo S, Vicente E, Marin JM. Medication adherence and persistence in severe obstructive sleep apnea. Sleep. 2009;32:623–8. doi: 10.1093/sleep/32.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


