Abstract
Study Objectives:
The primary objective of this study was to describe characteristics of sleep across the three domains of sleep quality, daytime sleepiness, and behavioral alertness in community-dwelling adults with heart failure. The secondary objective was to identify modifiable factors associated with behavioral alertness.
Methods:
A sample of 280 adults with chronic heart failure was enrolled. Widely used, validated, and sensitive measures of sleep quality (Pittsburgh Sleep Quality Index), daytime sleepiness (Epworth Sleepiness Scale, Stanford Sleepiness Scale), and behavioral alertness (Psychomotor Vigilance Test [PVT]) were collected at baseline, 3 and 6 months. Sociodemographic and clinical characteristics, including exercise, were measured at baseline.
Results:
Participants were primarily male and functionally compromised with a mean left ventricular ejection fraction of 35 percent. The majority of the sample (73%) reported poor sleep quality. The mean (± SD) Epworth Sleepiness Scale score was low (7.0 ± 4.6), indicating they did not perceive daytime sleepiness. In contrast, behavioral alertness was relatively poor as evidenced by a slow PVT mean response time (3.09 ± 0.76). Participants who reported exercising at least one hour in the past week were more alert and had faster response times than those reporting no exercise.
Conclusions:
Although sleep quality was poor and behavioral alertness was compromised, these heart failure patients did not feel sleepy. Exercise may help to promote behavioral alertness and reduce daytime sleepiness in adults with heart failure.
Citation:
Masterson Creber R, Pak VM, Varrasse M, Dinges DF, Wald J, Riegel B. Determinants of behavioral alertness in adults with heart failure. J Clin Sleep Med 2016;12(4):589–596.
Keywords: heart failure, sleep, psychomotor performance, reaction time, exercise
INTRODUCTION
In the United States heart failure (HF) is the fastest growing cardiovascular syndrome, affecting at least 5.7 million adult Americans.1,2 The costs of managing HF are estimated to be over $39 billion annually in the United States.1 Given the prevalence and cost of this syndrome, it is particularly relevant to address factors that interfere with patients' abilities to care for themselves. Poor sleep quality is known to influence the ability to remember to take medications, eat a healthy diet, and respond appropriately to symptoms.3
There is a growing awareness that sleep disturbances are common in HF and these symptoms are not explained by sleep disordered breathing.4–7 Over half of HF patients report insomnia symptoms.4 The most commonly reported problems are initiating and maintaining sleep; objective sleep assessments have shown shorter sleep duration, frequent arousals, and sleep stage changes in HF patients.8 Yet, in spite of these known sleep problems, surprisingly few patients with HF complain of daytime sleepiness,6 an observation that has been explained by central adrenergic alerting mechanisms operant in HF.5 This mismatch between sleep problems and self-reported complaints of daytime sleepiness makes the study of sleep disturbances challenging in this population.
Identifying such problems is important because sleep disturbances are associated with adverse consequences including insulin resistance, neurobehavioral and performance deficits, and motor vehicle accidents.9 HF patients with chronic sleep problems had an almost 2-fold increased risk for all-cause hospitalization at one year follow-up.10 Identifying sleep problems in people who do not report daytime symptoms of poor sleep can assist in the identification of those who will benefit from interventions to promote better sleep.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Poor sleep quality is known to be associated with poor self-care in adults with heart failure. Although self-reported daytime sleepiness is known to be a poor indicator of sleep quality, little is known about the usefulness of objectively measured behavioral alertness as an indicator of sleep quality in heart failure.
Study Impact: This study demonstrated that patients with heart failure demonstrate poor objective behavioral alertness despite reporting not feeling sleepy. In the context of incongruence with self-reported sleep measures, objective measures of behavioral alertness can improve the clinical assessment. Future research should include objective measures of exercise to understand the mechanisms by which exercise improves sleep quality, daytime sleepiness, and behavioral alertness in heart failure.
The primary aim of this study was to describe characteristics of sleep across the three domains of sleep quality, daytime sleepiness, and behavioral alertness in community-dwelling adults with HF. The objective measurement of behavioral alertness is a sensitive approach to identifying sleep disturbances in those who are unaware of the problem. The secondary aim was to identify modifiable factors associated with behavioral alertness measured over time.
METHODS
This was a secondary analysis of data obtained from a prospective cohort study of the relationship between excessive daytime sleepiness and self-care in adults with chronic HF. A detailed description of the study methods has been published previously and are summarized briefly here.3 Clinically stable patients were enrolled from outpatient settings and followed for 6 months. Data on sleep quality, daytime sleepiness, and behavioral alertness were collected in person at baseline, 3 months, and 6 months, usually during home visits. Clinical information was abstracted from the medical record by nurses.
Sample
Study participants were enrolled from a university affiliated referral center, a veteran's medical center, and a large regional medical center in the Northeastern United States. Only adults with chronic, symptomatic HF were enrolled. Chronic HF had to have been confirmed by the physician provider based on echocardiographic and clinical evidence before enrollment. Potential participants were screened to assure that they had visual acuity sufficient to read study materials, hearing sufficient to engage in a dialogue, and the ability to read and understand English adequately. Patients were excluded if they worked nights or rotating shifts because of our interest in sleep, were receiving renal dialysis, or had a history of serious drug or alcohol abuse within the past year or major depression. Specifically, anyone with a score > 10 on the Patient Health Questionnaire-9 (PHQ-9),11 a measure of depression, was excluded if positive responses included the classic indicators of depression such as anhedonia. The rationale for this exclusion was the known relationship between depression and self-care. Anyone with significant cognitive impairment with a score < 24 on the Telephone Interview of Cognitive Status was excluded.
All study procedures complied with the Declaration of Helsinki and institutional review board approval was obtained from the University of Pennsylvania and all participating institutions. Written informed consent was obtained from all participants.
Measurements
Sleep Quality
Sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI), a self-report measure of the perception of habitual sleep quality.12 The PSQI measures 7 sleep domains. A global score (0–21 points) is obtained by summing scale domain scores. Higher global scores (≥ 5) indicate poorer overall sleep quality. Internal consistency reliability of the PSQI is typically in the range of 0.77 to 0.83.13 Concurrent and discriminative validity of the PSQI has been demonstrated with comparison to multiple sleep questionnaires, polysomnography, and clinical evaluation.
Daytime Sleepiness
The Epworth Sleepiness Scale (ESS) quantifies daytime sleepiness by asking questions about the tendency to fall asleep in eight routine, daily situations rated on a 4-point Likert scale.14 Possible scores range from 0 to 24, with higher scores indicating increased daytime sleepiness. The ESS has shown good test-retest reliability (r = 0.82) and internal consistency (α = 0.88).15
The Stanford Sleepiness Scale (SSS) is a self-administered questionnaire that was used to measure self-reported patterns of sleepiness during the day.16 Scores range from 1 to 7, with a score ≥ 4 indicating sleepiness at a particular moment of time. The SSS is sensitive to both sleep deprivation and time of day.17
Behavioral Alertness
Behavioral alertness was measured with the PVT. Over the last two decades, the PVT has become the most widely used measure of behavioral alertness.18,19 A single 10-min test, was administered to subjects during the normal diurnal period. Participants were told to respond as rapidly as possible to the appearance of a simple cue of light occurring at random intervals (between 2–10 sec); reaction time (RT) was measured. PVT mean response time was measured as the reciprocal transform of the reaction time (1/RT). For calculating mean 1/ RT and slow est 10% 1/RT, each RT (ms) was divided by 1,000 and then reciprocally transformed to normalize the variance and reduce the influence of outlier values. Responses were considered valid if reaction times were ≥ 100 milliseconds (ms) and ≤ 500 ms. The reciprocal response is sensitive to total and partial sleep loss.19 Generalized response slowing is reflected by worsening of the fastest 10% of RTs.9
Measurement of Covariates
Sociodemographic characteristics were self-reported and included gender, race, financial status, education, perceived health and social support (measured with the Multidimensional Scale of Perceived Social Support). Responses on the Multidimensional Scale of Perceived Social Support range from 1 (very strongly disagree) to 7 (very strongly agree), with higher scores indicating higher levels of perceived support. Comorbidities were recorded directly from the medical record and scored with the Charlson Comorbidity Index. New York Heart Association (NYHA) functional class was scored Class I-IV by a single cardiologist using data obtained by research staff from a standardized interview. Body mass index (BMI) was calculated based on patient weight measured during the data collection visit and self-reported height. Detailed information on participants' medications, including number of medications and those associated with daytime sleepiness, were collected. As noted earlier, the PHQ-9 was used to exclude patients with major depression, but in those without major depression we continued using the PHQ-9 for descriptive purposes. Exercise was measured by self-report with the question, “How much did you exercise in the last week?” measured in categories (none, minimal (< 1 h/week) or adequate (1–3 h/week). Sleep apnea was noted in the medical record review. If a recent polysomnograph was identified, the total number of minutes assessed, oxygen saturation, pulse, and apnea hypopnea parameters were extracted. If no sleep study had been performed in the past year, one night of sleep was assessed in the home using Embletta (Medcare, Buffalo, NY), a highly sensitive and specific screening device useful in quantifying the apnea hypopnea index (AHI) in persons with suspected sleep apnea.20 Data from the Embletta were scored by specially trained sleep technicians at the Penn Medicine Sleep Center and scored according to the standard American Academy of Sleep Medicine classification.21
Statistical Analyses
Descriptive statistics were used to calculate frequencies with percentages and means with standard deviations for the total sample for demographic and clinical characteristics, and self-reported and objective sleep measures. The primary outcome in the study was the PVT mean response times at baseline, 3 months, and 6 months. Data were inspected visually using multiple plots to check for linearity assumptions. Linear mixed effect models were used to examine predictors of PVT performance at over time. Confidence intervals of the predictors are reported, and p < 0.05 was considered statistically significant. Estimates from the linear mixed effect models were confirmed with generalized estimating equation models with robust standard errors.
Covariate selection started with a priori factors assumed to be associated with behavioral alertness. In addition, all variables that had a statistically significant (p < 0.05) association with the PVT mean 1/RT were included in the model building process. Covariate selection entailed a robust model comparison approach,22 starting with a basic model and comparing it with a model in which one or more variables were added. A priori factors that were considered in the models but were not significant were removed, including depression and left ventricular ejection fraction. Consistent with previous publications from the parent study, race, gender, site and NYHA class were retained as a priori covariates in these analyses.3 Sleep disordered breathing was considered, even though prior studies indicate that daytime sleepiness is not explained by sleep apnea in these patients.4–7 As this variable had missing data, it was analyzed both with available data and again after multiple imputations based on age, gender and BMI at baseline. Data were imputed using the method of chained equations as implemented in the Stata program ice command.23 After imputation, data were checked to confirm reasonable values. Confidence intervals were estimated by calculating within and between components of variance using the method of Rubin.24
As a final check for additional covariates that may have been overlooked, data-driven selection procedures were performed using forward selection and backward elimination with selection criteria set to p < 0.05. The exploratory analyses confirmed the results of the previous analyses and no additional variables were included. The final model included the following fourteen variables: gender, age, race, education, social support, perceived health (recoded into 2 categories: good/very good/excellent versus fair/poor as recommended in previous publications),25 kidney disease, BMI, medications causing daytime sleepiness, NYHA functional class, site, ESS, PSQI, and exercise.
Bivariate correlations were calculated among self-reported sleep measures (PSQI, ESS, and SSS) and PVT mean response time. Cohen's d effect sizes were interpreted according to conventional criteria (0.2–0.3 is considered small, > 0.3–0.8 is medium, and > 0.8 is large).26 Data were analyzed using StataSE version 13.1.
RESULTS
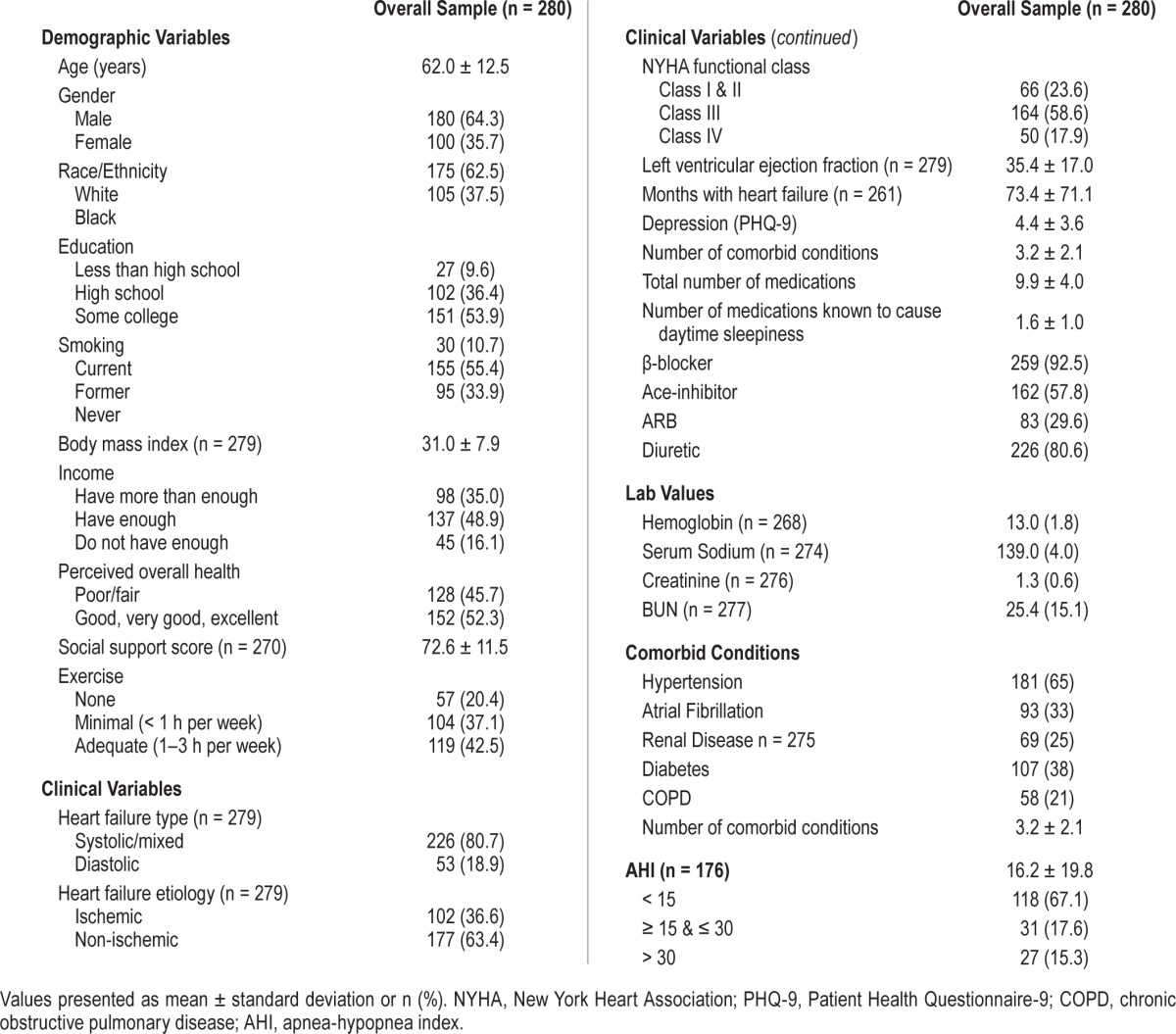
A total of 280 adults with chronic HF were enrolled into the study and attrition was low (13.6%, n = 38) over 6 months of follow-up. Participants were 62.5% male, had a mean age of 62 years and were functionally compromised (77% in NYHA class III or IV) with a mean reduced left ventricular ejection fraction of 35 (SD ± 17) (Table 1). On average, participants had 3 comorbid conditions other than HF, 25% had kidney disease, and on average participants were taking 10 medications per day, of which almost 20% were known to cause daytime sleepiness (e.g., narcotics, antihistamines). At baseline the mean AHI was 16.2 ± 19.8/h (n = 176). In total 33% of the 176 participants with data on this variable had sleep disordered breathing. Financially, almost 50% of the participants reported having “enough income to live on,” but 16% reported “not enough to make ends meet.” Over half of the participants had completed at least some college. Less than half reported adequate exercise (defined as 1–3 h in the past week) and about half rated themselves as having good/very good or excellent perceived health.
Table 1.
Baseline demographic and clinical characteristics of the sample.
Sleep Quality and Daytime Sleepiness
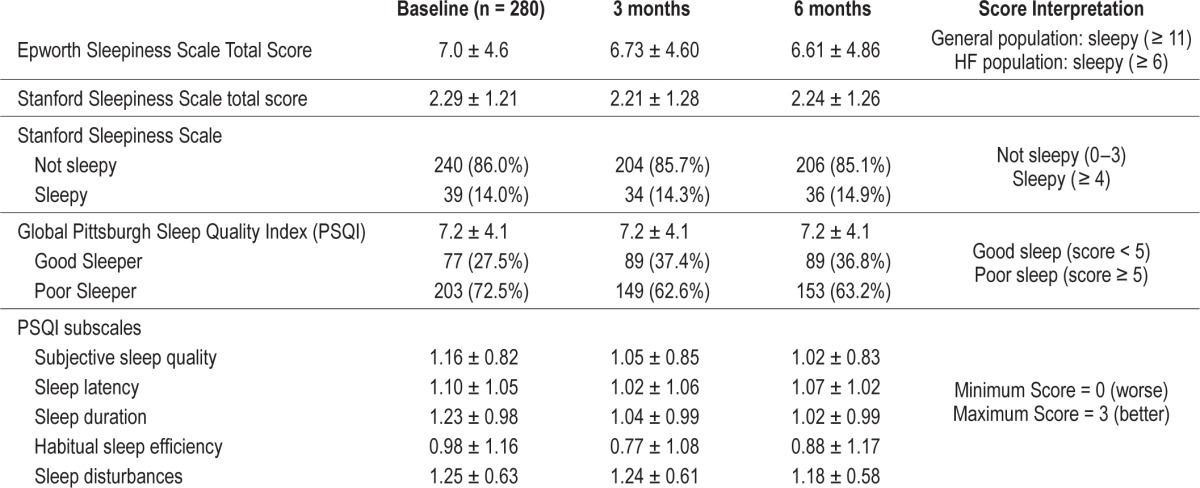
The majority (73%) of participants reported being “poor sleepers.” The mean PSQI was 7.2 ± 4.1 across the 3 time points with poor scores across all of the PSQI sub-scales of sleep quality, latency, duration, habitual sleep efficiency, and sleep disturbances Table 2. Only 14% of the sample reported feeling sleepy on the SSS. Overall, the average hours of nocturnal sleep reported was 6.3 (± 1.6) h/night. The mean ESS total score was 7, which compared to the general population is not considered sleepy. Subjective sleep variables at baseline, 3 months, and 6 months are reported in Table 2.
Table 2.
Self-reported and polysomnographic sleep characteristics at baseline, 3 months, and 6 months.
Behavioral Alertness
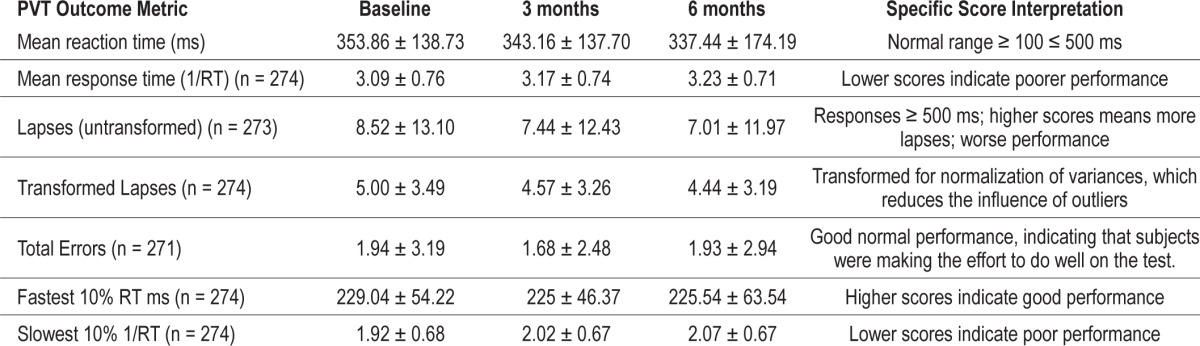
The distributions of PVT mean response times at baseline, 3 months, and 6 months are shown in Figure 1. Baseline, 3-month, and 6-month PVT outcome metrics and score interpretations are reported in Table 3. Behavioral alertness was poor as evidenced by the slow PVT raw average mean RT (345.28 ± 150.56 ms) and 1/RT (3.16 ± 0.74) over the 3 time points. The 2 measures of errors averaged over time, total errors (1.85 ± 2.90), and fastest 10% RT (226.68 ± 55.09 ms) indicated that participants were making the effort to perform well on the test. The correlations between PVT mean 1/RT and each subjective sleep measure were modest and consistent over time (PSQI: −0.273, −0.130, −0.164; ESS: −0.204, −0.340, −0.265 and SSS: −0.273, −0.130, −0.164).
Figure 1. Psychomotor vigilance test (PVT) response times measured at baseline, 3 months, and 6 months.
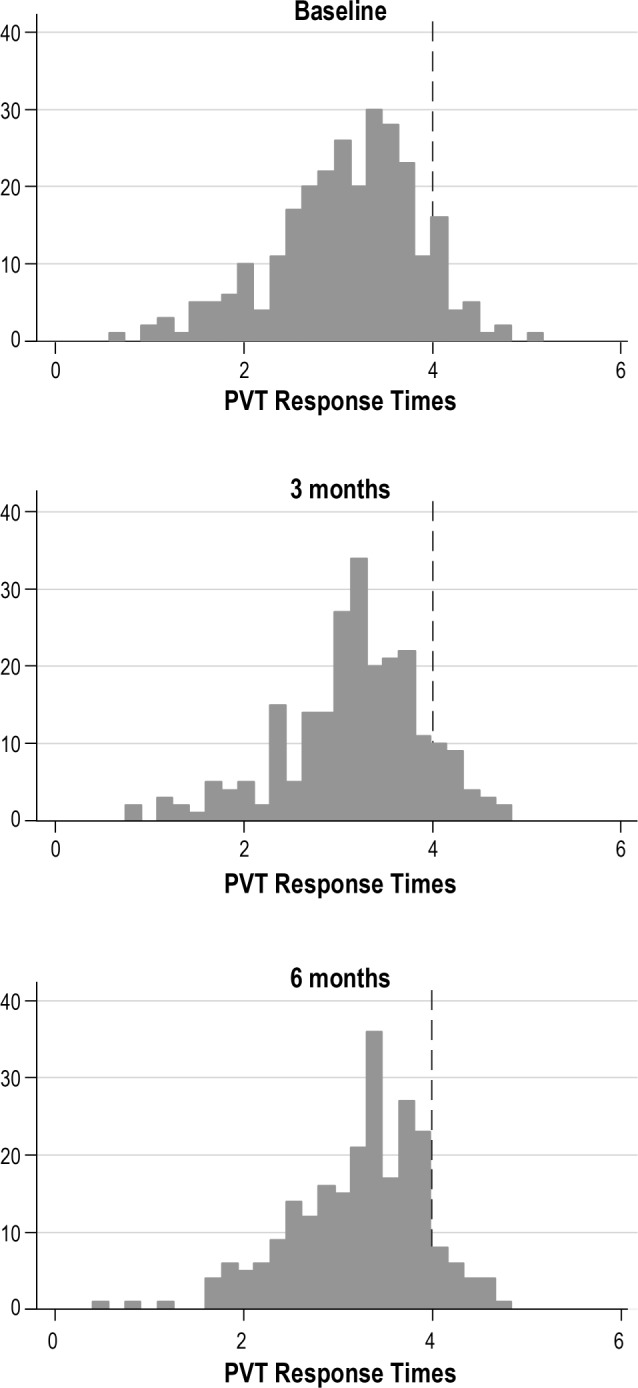
Response times < 4 are slower than average and indicate poorer psychomotor performance. The majority of the sample had poorer behavioral alertness than expected at all three points in time over 6 months.
Table 3.
Psychomotor vigilance test variables (PVT) at baseline.
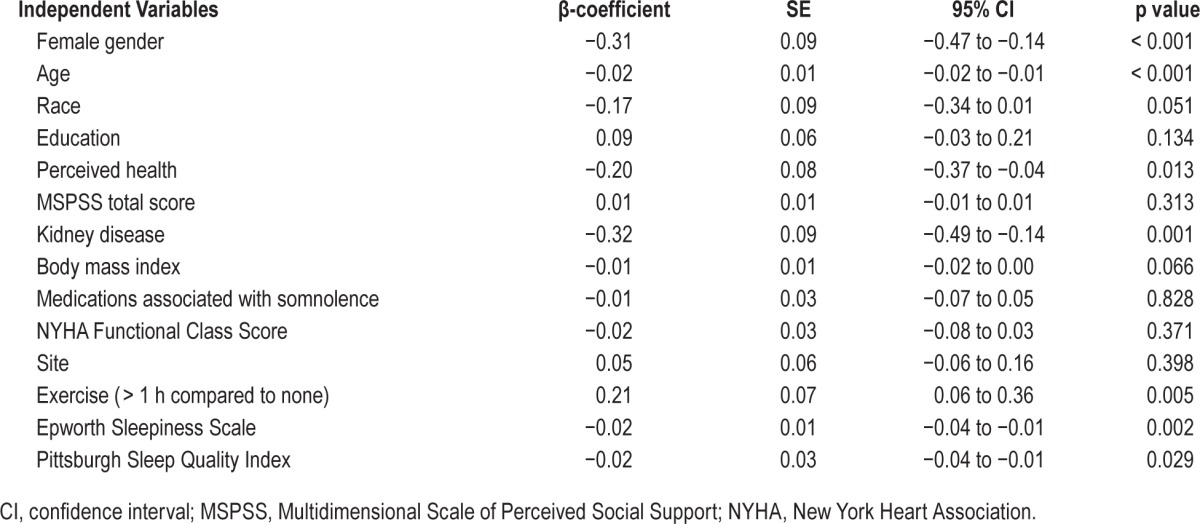
Characteristics associated with behavioral alertness were identified using mixed-effect linear modeling (Table 4). Demographic characteristics associated with slower response time included: older age and female gender (coefficient of one-year change in age (β = −0.02, p < 0.001; coefficient of female compared to male (β = −0.31, p < 0.001). Kidney disease was strongly associated with slower response time (β = −0.32, p = 0.001). Self-reported fair/poor perceived health was also associated with slower response time (coefficient of good/ very good/excellent compared to poor/fair health (β) = −0.20, p = 0.013). Higher ESS values (more sleepiness) and PSQI (worse sleep) were associated with slower response times (coefficient for a one-unit increase in ESS (β) = −0.02, p = 0.002 and PSQI (β) = −0.02, p = 0.029). Reporting more than one hour of exercise each week was associated with a faster response time (β = 0.21, p = 0.005).
Table 4.
Mixed-effect linear regression model for the mean psychomotor vigilance test response times over time (n = 268).
Data are not shown for the generalized estimating equation model because there were no significant differences with the mixed effect linear model results. Data also are not shown for the model that included imputed AHI data because AHI was not a significant predictor of PVT and there were no significant differences between the models with and without imputed AHI data. There were no differences in the results by site.
DISCUSSION
The aims of this study were to describe characteristics of sleep across three outcome domains and identify modifiable factors associated with behavioral alertness in community-dwelling adults with HF. The subjective sleep measures illustrated an interesting picture. Although the majority of participants reported being “poor sleepers,” only 14% of participants reported being “sleepy,” according to the SSS and daytime sleepiness scores were relatively low. Behavioral alertness was poor although participants reported not feeling very sleepy; however, their error scores were low indicating that they were trying to perform well on the test despite experiencing poor behavioral alertness. Overall, these study results indicate a distinct mismatch between daytime sleepiness and behavioral alertness.
The results of this study support those of others who have found that HF patients do not report daytime sleepiness.6,7 The proposed mechanism for the lack of daytime sleepiness is that these patients may have elevated suprabulbar subcortical noradrenergic activity that stimulates alertness and neutralizes perceptions of sleepiness. These effects may cause chronic sleep fragmentation and sleep deprivation27 or a state of heightened arousal, which then increases adrenergic drive. This arousal system has important adrenergic inputs that are integrated into the fight-or-flight response.28 It is possible that activation of the adrenergic system in HF may counteract the effects of both chronic sleep disruption and the sleep fragmentation, subsequently leading to a lack of daytime sleepiness.
We also identified specific factors contributing to behavioral alertness, focusing on those that can be modified in future interventions. Specifically, patients who exercised at least one hour per week had better alertness than those who were sedentary. One explanation is that exercise causes an increase in core body temperature, which facilitates the initiation of sleep due to the activation of heat dissipation mechanisms controlled by the hypothalamus.29 On the other hand, this finding could be due to reverse causality in that patients who feel dulled and inattentive may be less inclined to exercise. In reality the relationship between exercise and alertness is probably bi-directional; however, in this study we only evaluated the alertness as an outcome. This finding does suggest that exercise may be a useful approach for promoting behavioral alertness.
Another predictor of poor behavioral alertness was poor perceived health, a measure of self-reported health status. Perceptions of poor health have been shown to be a strong predictor of adverse health outcomes, particularly among HF patients.30 Those with poor perceived health are less likely to adhere to prescribed medical therapy and participate in physical activity, leading to decreased sleep quality and impaired behavioral alertness.31
The finding that kidney disease was strongly associated with slower response time can be explained by the known association between kidney disease and drowsiness.32 Sleep disorders are common in patients with kidney failure on dialysis,32,33 but we specifically excluded these patients. As impaired kidney function is so common in HF, further study is needed explore the association between kidney function and sleep parameters.
Two factors, gender and age, confirm the results of prior studies. Women are known to have slower response times compared to men independent of age and sleep pressure.34 This sex difference is likely due to response bias, as women tend to bias towards accuracy and men tend to bias toward speed.34 Older participants also had slower response times, consistent with prior studies35 and the known impact of age on vigilance related tasks.36 Notably, however, compared to relatively healthy older adults of roughly the same age, our population of HF patients performed much worse on PVT measures. In comparison, our cohort had lower PVT mean response times (3.09 ± 0.76 versus 3.85 ± 0.50), lower slowest 10% 1/RT (1.92 ± 0.68 versus 2.55 ± 0.5), and more lapses in attention (8.5 ± 13.1 versus 2.45 ± 1.50) compared to age- and gender-specific norms, all indicating poorer performance.37 Further research is warranted to understand if factors other than poor sleep contribute to the poor performance of these older, physically compromised individuals on the PVT compared to their healthy elderly counterparts.
We found no relationship between sleep disordered breathing and behavioral alertness consistent with a recent study by Bitter and colleagues.38 They reported that patients with systolic HF and sleep apnea (central or obstructive) reported no differences in sleep symptoms compared to HF patients without sleep apnea. All of the HF patients reported occasionally feeling chronically fatigued, experiencing daily sleepiness and napping. Conversely, a study by Gieb and colleagues reports that patients with a higher AHI had worse daytime fatigue, unintentional sleep, and impaired vigilance.39 The differences in our findings may be explained by differences in the prevalence of moderate and severe sleep apnea in the two samples. In the cross-sectional Gieb study, 67% and 26% of participants reported severe and moderate sleep apnea respectively, compared to 15% and 18% of severe and moderate sleep apnea in our sample. Another important difference was in the measurement of alertness. Gieb et al. measured alertness objectively using Quatember Maly, rather than the PVT, but it was completed on fewer than half (92/222 or 41%) of participants. Gieb and colleagues were able to more fully characterize sleep apnea in their sample due to recent polysomnography on each participant, while we were able to thoroughly compare objective and subjective measures of sleepiness at three points in time over 6 months. Given differences in the clinical characteristics of the sample, measurement, and the length of follow-up, both studies contribute unique but different information about sleepiness in the HF patient population.
Limitations
Limitations of this study include the use of data from a prospective cohort study, which, by design, excluded some chronic HF patients. The parent study excluded patients with depression, specifically a PHQ-9 > 10. Depression is associated with sleepiness so excluding patients with moderate depression may have led to an underestimate of sleep-related symptoms. Another limitation is that the AHI variable was obtained in two different ways (prior polysomnograph in a laboratory, Embletta study in the home). There was a significant amount of missing data for this variable, which was handled with robust methods for imputation in the sensitivity analysis models. In addition, exercise was measured by self-report rather than objectively, and the time of day in which exercise was performed was not reported. This sample was younger and more likely to be male than some community samples with HF, and thus these results may not be generalizable to all patients with HF. These limitations are partially offset by strengths, including measurement of sleep quality, daytime sleepiness, and behavioral alertness with subjective and objective measures over three points in time in a large sample of adults with chronic HF. We enrolled participants from three separate and distinctly different sites, which provided a robust and diverse sample. The population was well characterized, which allowed us to assess multiple confounders.
Implications and Future Research
We demonstrated that the typical match between daytime sleepiness and behavioral alertness was not found in patients with HF; instead, these patients demonstrated poor behavioral alertness even though they did not report feeling sleepy. Poor sleep quality has been shown to contribute to poor self-care and rehospitalization in these patients, underscoring the need to study sleep in adults with HF.40,41 The results of this study highlight the importance of objective measures of neurobehavioral performance especially in the context of incongruence with self-reported measures. Further research should include objective measures of exercise to understand the mechanisms by which exercise improves behavioral alertness in HF. If sleep can be improved through exercise it may be a useful method of improving self-care in HF patients.
DISCLOSURE STATEMENT
This was not an industry supported study. The parent study for this work was funded by a grant from the National Heart, Lung & Blood Institute [RO1HL084394] and by the Philadelphia Veterans Affairs Medical Center, VISN 4 Mental Illness Research, Education, and Clinical Center. The authors gratefully acknowledge the pre-doctoral funding for Ruth Masterson Creber provided by NIH/NINR [F31NR014086] and the National Hartford Centers of Geriatric Nursing Excellence Patricia G. Archbold Scholarship program from 2012–2014. We also acknowledge the post-doctoral funding for Ruth Masterson Creber by NIH/NINR [T32NR007969] at Columbia University School of Nursing. Dr. Wald has consulted for Medtronic. The other authors have indicated no financial conflicts of interest. The authors also gratefully acknowledge NIH funding for Dr. Victoria Pak [K99NR014675].
ACKNOWLEDGMENTS
The authors acknowledge Thomas A. Gillespie, MD, FACC, for scoring the NYHA interviews as well as the patients who participated in the study.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- ESS
Epworth Sleepiness Scale
- HF
heart failure
- NYHA
New York Heart Association
- PHQ-9
Patient Health Questionnaire-9
- PVT
Psychomotor Vigilance Test
- PSQI
Pittsburgh Sleep Quality Index
- RT
reaction time
- SSS
Stanford Sleepiness Scale
REFERENCES
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Riegel B, Moelter ST, Ratcliffe SJ, et al. Excessive daytime sleepiness is associated with poor medication adherence in adults with heart failure. J Card Fail. 2011;17:340–8. doi: 10.1016/j.cardfail.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redeker NS, Muench U, Zucker MJ, et al. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep. 2010;33:551–60. doi: 10.1093/sleep/33.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taranto Montemurro L, Floras JS, Millar PJ, et al. Inverse relationship of subjective daytime sleepiness to sympathetic activity in patients with heart failure and obstructive sleep apnea. Chest. 2012;142:1222–8. doi: 10.1378/chest.11-2963. [DOI] [PubMed] [Google Scholar]
- 6.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–22. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 7.Rao A, Georgiadou P, Francis DP, et al. Sleep-disordered breathing in a general heart failure population: relationships to neurohumoral activation and subjective symptoms. J Sleep Res. 2006;15:81–8. doi: 10.1111/j.1365-2869.2006.00494.x. [DOI] [PubMed] [Google Scholar]
- 8.Brostrom A, Stromberg A, Dahlstrom U, Fridlund B. Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs. 2004;19:234–42. doi: 10.1097/00005082-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 10.Johansson P, Brostrom A, Sanderman R, Jaarsma T. The course of sleep problems in patients with heart failure and associations to rehospitalizations. J Cardiovasc Nurs. 2015;30:403–10. doi: 10.1097/JCN.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 11.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–40. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 14.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 15.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17:703–10. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 16.Hoddes E, Dement W, Zarcone V. The development and use of the Stanford sleepiness scale (SSS) Psychophysiology. 1972;9:150. [Google Scholar]
- 17.Babkoff H, Caspy T, Mikulincer M. Subjective sleepiness ratings: the effects of sleep deprivation, circadian rhythmicity and cognitive performance. Sleep. 1991;14:534–9. doi: 10.1093/sleep/14.6.534. [DOI] [PubMed] [Google Scholar]
- 18.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 19.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng SSS, Chan T, To K, et al. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS) Respirology. 2010;15:336–42. doi: 10.1111/j.1440-1843.2009.01697.x. [DOI] [PubMed] [Google Scholar]
- 21.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell SE, Delancey HD. Mahwah, NJ: Erlbaum; 2004. Designing experiments and analyzing data: a model comparison perspective. [Google Scholar]
- 23.Royston P. Multiple imputation of missing values: update of ice. Stata J. 2005;5:527–36. [Google Scholar]
- 24.Rubin DB. New York, NY: John Wiley & Sons; 1987. Multiple imputation for nonresponse in surveys. [Google Scholar]
- 25.Johansson P, Brostrom A, Dahlstrom U, Alehagen U. Global perceived health and ten-year cardiovascular mortality in elderly primary care patients with possible heart failure. Eur J Heart Fail. 2008;10:1040–7. doi: 10.1016/j.ejheart.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 27.Aggarwal A, Esler MD, Lambert GW, Hastings J, Johnston L, Kaye DM. Norepinephrine turnover is increased in suprabulbar subcortical brain regions and is related to whole-body sympathetic activity in human heart failure. Circulation. 2002;105:1031–3. doi: 10.1161/hc0902.105724. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 29.Passos GS, Poyares DL, Santana MG, Tufik S, Mello MT. Is exercise an alternative treatment for chronic insomnia? Clinics (Sao Paulo, Brazil) 2012;67:653–60. doi: 10.6061/clinics/2012(06)17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson B, Pozehl B, Hertzog M, Zimmerman L, Riegel B. Predictors of overall perceived health in patients with heart failure. J Cardiovsc Nurs. 2013;28:206–15. doi: 10.1097/JCN.0b013e31824987a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care. 2007;45:521–8. doi: 10.1097/MLR.0b013e318032937e. [DOI] [PubMed] [Google Scholar]
- 32.Roumelioti ME, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol. 2011;6:986–94. doi: 10.2215/CJN.05720710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabbatini M, Crispo A, Pisani A, et al. Sleep quality in renal transplant patients: a never investigated problem. Nephrol Dial Transplant. 2005;20:194–8. doi: 10.1093/ndt/gfh604. [DOI] [PubMed] [Google Scholar]
- 34.Blatter K, Graw P, Munch M, Knoblauch V, Wirz-Justice A, Cajochen C. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res. 2006;168:312–7. doi: 10.1016/j.bbr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson RT, Allison S. Age and simple reaction time: decade differences for 5,325 subjects. J Gerontol. 1989;44:P29–35. doi: 10.1093/geronj/44.2.p29. [DOI] [PubMed] [Google Scholar]
- 36.Parasuraman R, Nestor P, Greenwood P. Sustained-attention capacity in young and older adults. Psychol Aging. 1989;4:339–45. doi: 10.1037//0882-7974.4.3.339. [DOI] [PubMed] [Google Scholar]
- 37.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30:1309–16. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bitter T, Westerheide N, Hossain SM, Prinz C, Horstkotte D, Oldenburg O. Symptoms of sleep apnoea in chronic heart failure--results from a prospective cohort study in 1,500 patients. Sleep Breath. 2012;16:781–91. doi: 10.1007/s11325-011-0575-0. [DOI] [PubMed] [Google Scholar]
- 39.Geib T, Plappert N, Roth T, et al. Prevalence of sleep-disordered breathing-related symptoms in patients with chronic heart failure and reduced ejection fraction. Can J Cardiol. 2015;31:839–45. doi: 10.1016/j.cjca.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Riegel B, Knafl GJ. Electronically monitored medication adherence predicts hospitalization in heart failure patients. Patient Prefer Adherence. 2013;8:1–13. doi: 10.2147/PPA.S54520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson P, Nieuwenhuis M, Lesman-Leegte I, van Veldhuisen DJ, Jaarsma T. Depression and the delay between symptom onset and hospitalization in heart failure patients. Eur J Heart Fail. 2011;13:214–9. doi: 10.1093/eurjhf/hfq200. [DOI] [PubMed] [Google Scholar]


