Abstract
Background & objectives:
Malaria is a major public health problem in Tripura and focal disease outbreaks are of frequent occurrence. The State is co-endemic for both Plasmodium falciparum and P. vivax and transmission is perennial and persistent. The present study was aimed to review data on disease distribution to prioritize high-risk districts, and to study seasonal prevalence of disease vectors and their bionomical characteristics to help formulate vector species-specific interventions for malaria control.
Methods:
Data on malaria morbidity in the State were reviewed retrospectively (2008-2012) for understanding disease distribution and transmission dynamics. Cross-sectional mass blood surveys were conducted in malaria endemic villages of South Tripura district to ascertain the prevalence of malaria and proportions of parasite species. Mosquito collections were made in human dwellings of malaria endemic villages aiming at vector incrimination and to study relative abundance, resting and feeding preferences, and their present susceptibility status to DDT.
Results:
The study showed that malaria was widely prevalent and P. falciparum was the predominant infection (>90%), the remaining were P. vivax cases. The disease distribution, however, was uneven with large concentration of cases in districts of South Tripura and Dhalai coinciding with vast forest cover and tribal populations. Both Anopheles minimus s.s. and An. baimaii were recorded to be prevalent and observed to be highly anthropophagic and susceptible to DDT. Of these, An. minimus was incriminated (sporozoite infection rate 4.92%), and its bionomical characteristics revealed this species to be largely indoor resting and endophagic.
Interpretation & conclusions:
For effective control of malaria in the State, it is recommended that diseases surveillance should be robust, and vector control interventions including DDT spray coverage, mass distribution of insecticide-treated nets/long-lasting insecticidal nets should be intensified prioritizing population groups most at risk to avert impending disease outbreaks and spread of drug-resistant malaria.
Keywords: Anopheles baimaii, An. minimus, malaria transmission, northeast India, Plasmodium falciparum, Tripura, vector bionomics
India is a vast country with about a billion people living at risk of malaria. Disease transmission is complex due to varied ecology, multiplicity of disease vectors and bionomical characteristics1,2. The northeastern region of India, which accounts for about 4 per cent of the country's population, is malaria endemic and consistently contributes 10 per cent of cases (majority of which are Plasmodium falciparum), and 20 per cent of reported deaths annually3. Among northeastern States, the State of Tripura is strategically placed for sharing a vast international border with Bangladesh (84% of its total border length) in the north, south and west. It is a landlocked State with undulating terrain and homeland to different ethnic tribes rich in diversity and cultural practices. Malaria is a major public health concern in the State with history of focal disease outbreaks characterized by high rise in cases and attributable deaths with large concentration of cases in tribal population groups living along international border4,5,6. Disease transmission is persistent and P. falciparum is the predominant infection (>90%); the remaining are P. vivax cases7,8. Mosquito fauna is rich and among six dominant mosquito vector species in India, both Anopheles minimus and An. baimaii are implicated as major disease vectors9,10,11,12. Despite decades of attempted control interventions, malaria transmission remained uninterrupted in large tracts of the land. There exists very little information on seasonal abundance and infectivity of vector mosquito species, preferred breeding sources, and their present susceptibility status to residual insecticide in use for vector control. The present study was aimed to review retrospectively, data on disease transmission for the period 2008–2012 to help prioritize high-risk districts, to understand transmission dynamics, and to study seasonal prevalence of malaria vectors and their bionomical characteristics to help formulate vector species-specific interventions for containing impending disease outbreaks.
Material & Methods
This study was conducted by the National Institute of Malaria Research (NIMR) Field Station at Guwahati, Assam, India and the study protocol was approved by the Institutional Ethics Committee. The study was based in malaria endemic blocks of South Tripura district of Tripura State. Entomological and malaria prevalence surveys were conducted during June - December 2012.
Topography, study populations and climate: The landscape of the State can be divided into the three zones, i.e. high physiographic zone (hills), medium physiographic zone (undulating land) and low physiographic zone (plains), and altitude varies from 12-940 meters above sea level. Tripura State is endowed with vast evergreen forest reserve (~60% of land area) rich in fauna and flora. The State is split into four districts (recently divided into eight administrative units) namely North Tripura, West Tripura, Dhalai and South Tripura. There are about 19 different tribal communities including both aborigines (e.g. Tripuri, Reang) and those migrated population groups (e.g. Munda, Orang) which together constitute about 31 per cent of the total population of the State (3.6 million)13. Majority of ethnic tribal population groups live largely in hilly areas each characterized by linguistic chords and rich cultural heritage. The terrain is difficult and population is sparse living in poverty along the international border areas with little awareness on disease and prevention, and have poor access to healthcare services. The area is highly receptive for malaria transmission on account of tropical climatic conditions favouring mosquito breeding, longevity and active transmission of the malaria parasite. It rains heavily on account of extended monsoons during April - September, and total annual rainfall varies from ~2-2.5 metres. Most parts of the year (March/April - September/October) are hot and humid and temperatures range from 21-34°C, with relative humidity 70-80 per cent (Source: India Metrological Department, Agartala).
Disease surveillance and control interventions: Under National Vector Borne Disease Control Programme (NVBDCP) guidelines, the State has a structured surveillance system in place down to the village level based on fortnightly active case detection by domiciliary visits and passive agencies supplemented by recently introduced ASHA (accredited social health activist) worker providing diagnostic and treatment services at door step14. Also, there exists a provision for mass blood surveys in high-risk population groups for parasite screening and treatment to contain focal disease outbreaks. For each district there is appointed District Malaria Officer. In each district, block level Primary Healthcare Centre has a diagnostic facility to ensure early case detection and treatment for visiting patients and serves as reporting centre on malaria incidence. Mosquito vector control is based on two rounds of DDT indoor residual spraying (1g/m2) routinely conducted during March-May and then during June-August corresponding to peak transmission periods restricted to areas reporting high incidence of malaria and death cases. Vector control interventions are strengthened by impregnation of community-owned mosquito nets with synthetic pyrethroid and/or supply of long-lasting insecticidal net (LLIN) distributed gratis among high-risk groups.
Parasitological investigations: The data based on State surveillance on malaria morbidity were reviewed retrospectively for the period 2008-2012 to ascertain disease transmission trends and identify high-risk districts for contributing most cases and deaths (Source: State Health Directorate of Tripura). Cross-sectional mass blood surveys were conducted in villages of Hrishyamukh (Belonia subdivision), Manubankul (Sabroom subdivision) and Silachari (Karbook subdivision) blocks of South Tripura district during June-December, 2012 to ascertain the prevalence of malaria and proportions of parasite species. In these three blocks, 28 different villages were surveyed (population, 10420) for malaria prevalence. In each study village every fourth household was reached to meet the target of about 25 per cent population coverage for blood-smear collection. A finger-prick blood sample was taken from all volunteers for screening of malaria parasite. Both thick and thin blood smears were stained with Jaswant Singh Bhattacherji (JSB) rapid stain and examined microscopically for malaria positivity and species identification, respectively. Malaria positive cases were administered radical treatment as per national drug policy in force15. Data for relative prevalence of malaria in different study locations were analysed by chi-square test.
Entomological survey techniques: Entomological investigations were undertaken during June-December 2012 in the same three study blocks, i.e. Hrishyamukh (Belonia subdivision), Manubankul (Sabroom subdivision) and Silachari (Karbook subdivision) to have representative data. To ascertain the prevalent anopheline mosquito species and their relative abundance, day-resting catches were made in 38 malaria endemic villages both in human dwellings indoor during morning hours (0600-0800h) and cattle sheds during evening hours (1800-2000h) by hand catch method aided by battery torch light. The relative abundance of mosquito species was expressed as number of mosquito adults collected per person hour. These mosquito collections were supplemented by early morning total catch by pyrethrum spray inside human dwellings and CDC miniature light traps (both indoor and outdoor) between 1900-0500 h. In addition, all night dusk-to-dawn (1900-0500 h) mosquito-landing catches were made in human dwellings (both indoor and outdoor) in villages reporting high incidence of malaria using human volunteers as bait. Mosquito adults landing on exposed body parts were collected on hourly basis to study the mosquito behaviour and identify the probable vectors for having predilection for human host. All mosquitoes thus collected were identified to the species level using regional pictorial keys16,17. The entomological inoculation rates (EIR) were determined as product of the human mosquito landing rate and proportions of mosquitoes carrying sporozoites in the salivary glands for estimation of transmission intensities. Among mosquito species collected, An. minimus, An. baimaii and An. jeyporiensis were dissected for salivary glands in 0.9 per cent saline for detection of sporozoites. These data were supplemented by host blood meal analysis using human and bovine antiserum with counter current immuno-electrophoresis technique to ascertain anthropophilic index18. The mosquito vector species incriminated were subject to molecular characterization based on ITS2- rDNA for correct identification of species/sibling species within the taxon as per procedures detailed by Singh et al19. To determine the species-specific breeding sites of anopheline mosquito vector species, larval collections were made from different habitats including paddy fields, seepage streams, ponds, ditches and river bed-pools. Immature stages were reared in the laboratory till emergence for confirmation of identification of the mosquito species. To ascertain present susceptibility status against diagnostic concentration of the candidate insecticide used in vector control programme, field collected mixed age female mosquito adults of An. minimus and An. baimaii were exposed to DDT (4%) using WHO's standard insecticide susceptibility test kit procedures20.
Results
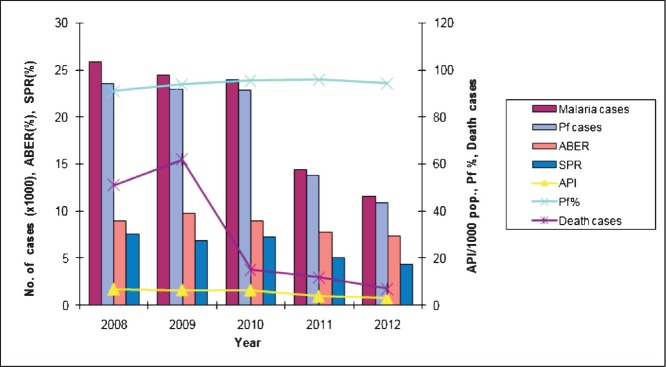
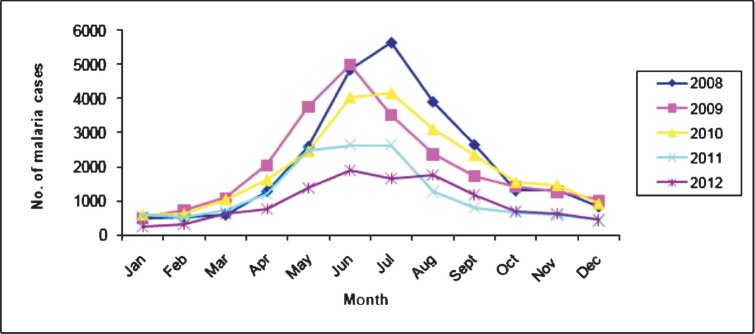
Malaria morbidity and seasonal prevalence: The data based on State disease surveillance on malaria-attributable morbidity for the years 2008-2012 are presented in Table I. During this period, total cases registered decreased from 25894 in 2008 to 11565 in 2012 inclusive of both P. falcipraum and P. vivax. Declining transmission trends were clearly discernable beginning 2011evidenced by parasite rate and annual case incidence (Fig. 1). Number of death cases recorded each year declined in parallel with reducing trends of disease transmission and every single death case was confirmed microscopically to be due to P. falciparum infection. For each year, the annual blood examination rate (% of population screened for malaria parasite) cumulative for the State remained <10 per cent (the expected rate of fever prevalence in the communities). The overall smear positivity rate (SPR) decreased from 7.6 per cent in 2008 to 4.3 per cent in 2012. Similar trends were seen for annual parasite incidences that decreased from 6.9 to 3.1 confirmed cases per thousand population/year. Among the four districts of the State, South Tripura and Dhalai were adversely affected for consistently reporting highest number of cases compared to West Tripura and North Tripura. Malaria cases were recorded in all months, however, with the commencement of the rainy season in April, there was a sudden rise in cases beginning May that peaked during June-August (Table II, Fig. 2). These were also the months of recorded high rainfall. Beginning September, there was a steady decline in cases but numbers varied with record low during December-February corresponding to dry months/low rainfall/winter season. The transmission profile was quite consistent for the years reported (2008-2012) but disease transmission trends were observed to be steadily declining.
Table I.
Distribution of malaria cases in different districts of Tripura during 2008-2012, Northeast India
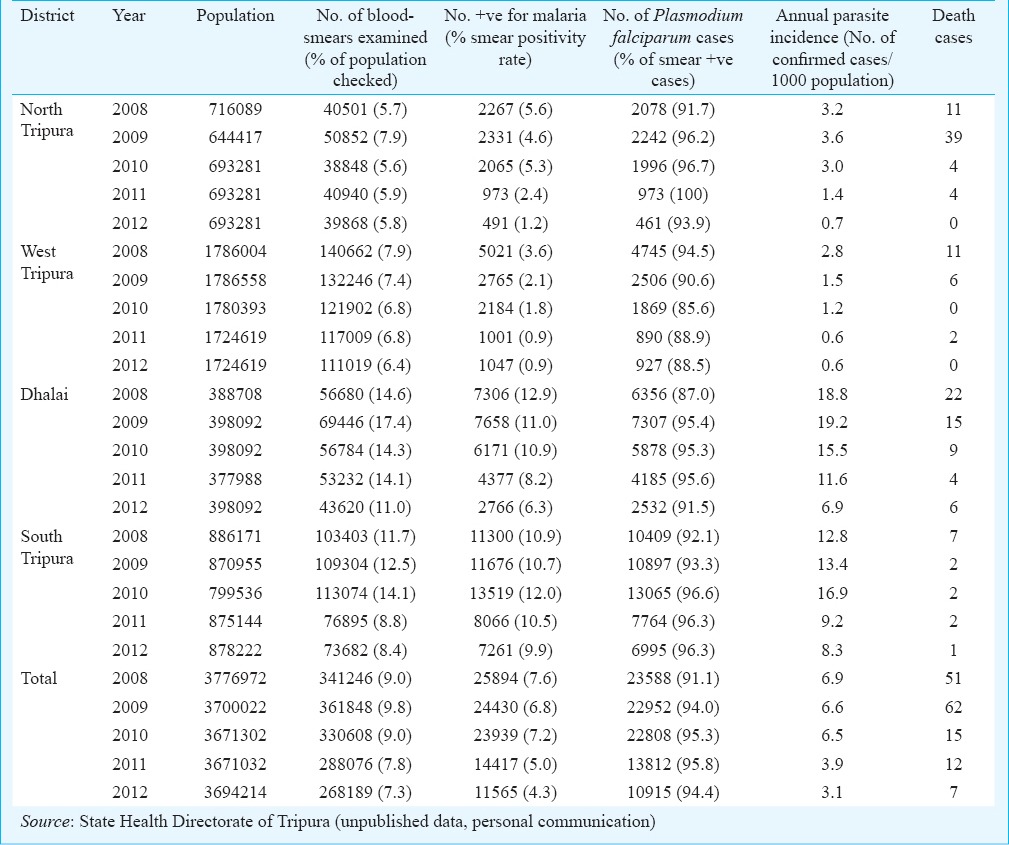
Fig. 1.
Malaria-attributable morbidity and transmission trends during 2008-2012 in Tripura, Northeast India. Pf, Plasmodium falciparum; ABER, Annual blood-smear examination rate; SPR, % smears positive for malaria parasite; API (annual parasite incidence), number of malaria cases per thousand population (Source: State Health Directorate of Tripura (unpublished data, personal communication).
Table II.
Meteorological data and monthly distribution of malaria cases for data based on 2012 in Tripura, Northeast India
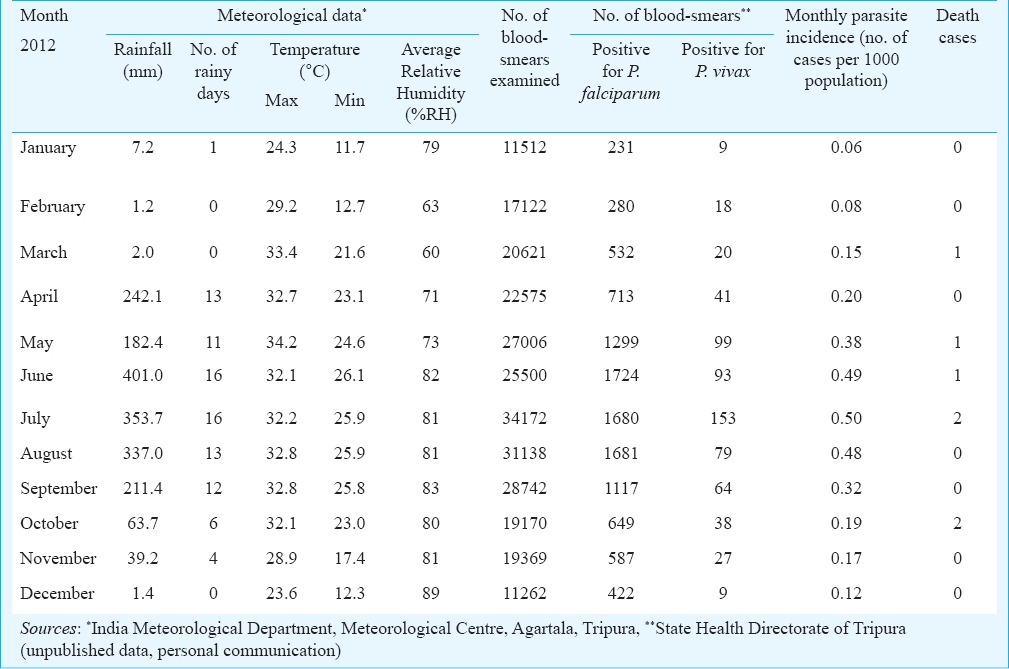
Fig. 2.
Monthly distribution of malaria cases in Tripura during 2008-2012. (Source: State Health Directorate of Tripura (unpublished data, personal communication).
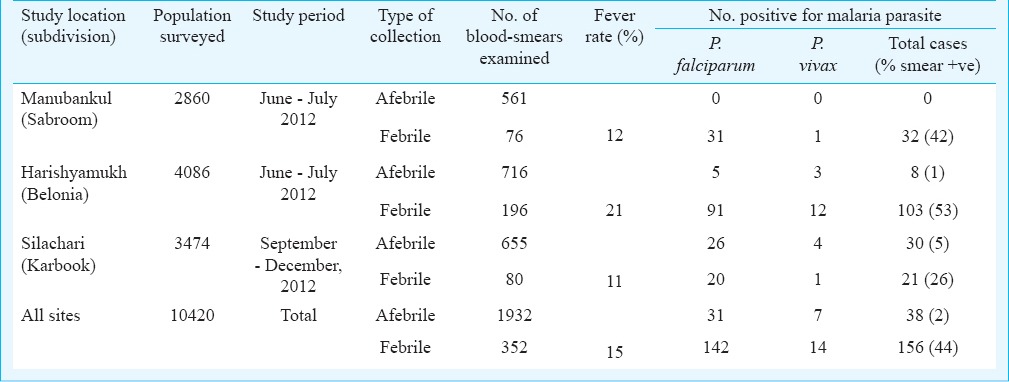
The point prevalence study in different villages of all three study locations of South Tripura district revealed that overall fever rate was 15 per cent (352/2284) but varied from 11-21 per cent between locations (Table III). Malaria cases were recorded in all age groups and differences in parasite positivity was not significant. Malaria positivity in afebrile cases ranged from 0-5 per cent and overall two per cent (38/1932) of afebrile cases were positive for malaria, majority of whom were P. falciparum cases (82%). In febrile cases, malaria positivity varied from 26-53 percent, and of total blood smears examined 44 per cent (156/352) were positive for malaria, of whom P. falciparum was the predominant infection (91%); the remaining were P. vivax cases. Given the heterogeneity in malaria transmission among study villages the variation in parasite prevalence between the study sites was significant (P <0.001).
Table III.
Results of cross-sectional malaria prevalence surveys in malaria endemic blocks of South Tripura district, Tripura, Northeast India
Entomological observations
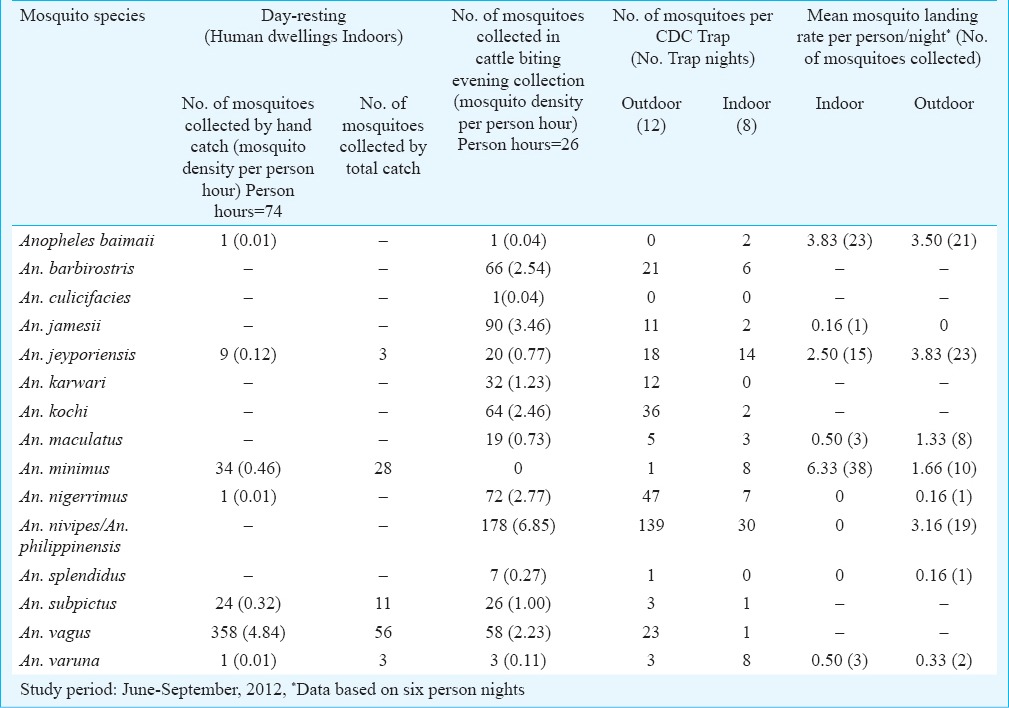
Mosquito abundance and resting behaviour: By various sampling techniques including day-resting catches from human dwellings indoors, evening collections from cattle sheds and CDC miniature traps (both indoor and outdoor) during wet season (June-September), 15 different anopheline mosquito species were identified (Table IV). Among these, An. vagus, An. minimus and An. subpictus were most abundant in human dwellings indoor and recorded density was 4.84, 0.46 and 0.32 per person hour respectively. These mosquito species were captured resting on walls, hanging clothes and other articles within the house typically made of split bamboo with thatched roofing. These observations were corroborated by total catch method in human dwellings for relative abundance of indoor resting mosquito populations. An. minimus mosquito adults collected indoors either were unfed, fully fed, semi-gravid or gravid whereas An. baimaii were unfed or fully fed (data not shown). In cattle biting evening catches, An. nivipes/An. philippinensis were most predominant; other major species included An. jamesii, An. nigerrimus, An. barbirostris, An. kochi and An. vagus in order of their relative abundance. In dry season (November-December), however, density for respective mosquito species was comparatively low in all ecotypes; An. minimus as well as An. baimaii were not recorded to occur (data not shown).
Table IV.
Relative abundance of anopheline mosquito species in South Tripura district, Tripura, Northeast India
Sibling species composition of mosquito vectors: Both An. minimus and An. baimaii were subjected to molecular identification for existence of their siblings within the taxon by DNA sequencing. Of the total 44 mosquito adults of An. minimus and eight specimens of An. baimaii sequenced for ITS2- rDNA, all were characterized to be An. minimus s.s. and An. baimaii (the member species of An. dirus species complex), respectively, confirmed to be prevalent in Tripura.
Feeding behaviour and host preferences: An. minimus and An. baimaii mosquito species were observed to be highly anthropophilic. Of the 24 mosquito blood meals of An. minimus, 22 (92%), and all of An. baimaii (10/10) collected during July-September 2012 were positive for human blood. These observations were further substantiated by dusk-to-dawn human landing catches in which both An. minimus and An. baimaii exhibited strong predilection for human blood meal. An. minimus, however, was mostly endophagic (mean mosquito landing rate 6.33 per person night), and biting activity occurred during 2100-0300 h but it was more pronounced midnight onwards till 0300 h. In contrast, An. baimaii searched human host equally both indoor and outdoor and mean mosquito landing rate was 3.83 and 3.50 per person, respectively, and peak biting activity occurred during 2100 h till midnight. An. jeyporiensis was observed active throughout the night both indoor and outdoor and mean mosquito landing rate varied from 2.50-3.83 per person. An. nivipes/An. philippinensis were exclusively exophagic and biting activity occurred all through the night, and mean mosquito landing rate was 3.16 per person night. An. maculatus was also mostly exophagic and mosquito landing rate varied from 0.50 to 1.33 per person night.
Vector incrimination: The salivary glands of mosquito adults collected from human dwellings indoors and dusk-to-dawn human landing catches were dissected for detection of sporozoites. Of the total 61 An. minimus mosquitoes dissected, three (4.92%) were sporozoite positive. All other mosquito species including An. baimaii (0/24) and An. jeyporiensis (0/18) were sporozoite negative.
Breeding habitats: Mosquito breeding was recorded in a variety of habitats including ponds, streams, ditches, paddy field and river beds. Of various prevalent Anopheles species, An. barbirostris, An. jamesii, An. maculatus and An. varuna were observed breeding in almost all habitats; however, An. minimus were recorded exclusively in slow flowing seepage water streams with grassy banks. Paddy fields were the most preferred habitat for most prevalent Anopheles species except for An. minimus, An, karwari, and An. subpictus. Instead An. nivipes/An. philippinensis, An. splendidus and An. tessellatus were recorded exclusively in paddy fields.
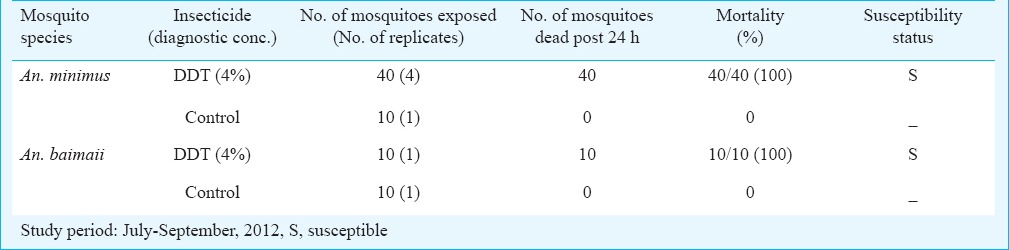
Insecticide susceptibility status: The field collected mixed aged mosquito adults of An. minimus and An. baimaii were exposed to DDT (4%), the currently used insecticide in vector control programme in the State. For both these mosquito species (even though number of mosquitoes exposed remained inadequate), 100 per cent mortality was observed and both An. minimus and An. baimaii were assessed to be fully susceptible (Table V).
Table V.
Insecticide susceptibility status of adult mosquito vector populations in malaria endemic villages of South Tripura district, Tripura, Northeast India
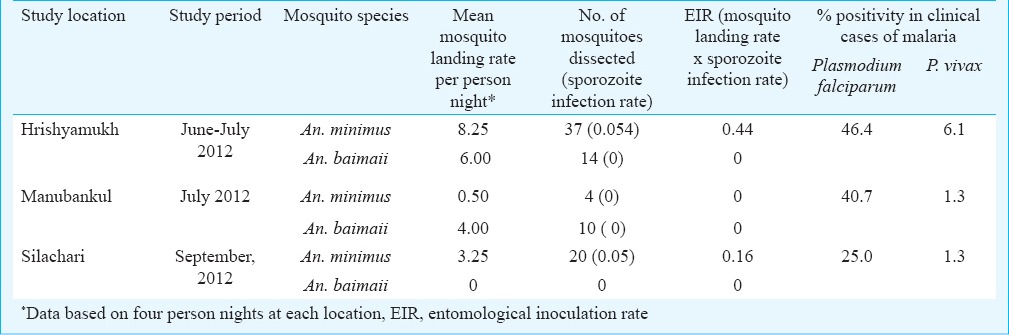
Mosquito vectors, infectivity and relative risk: Though both An. minimus and An. baimaii were abundant and actively fed on human host, but only An. minimus was incriminated by detection of motile sporozoites in salivary glands; all An. baimaii mosquitoes dissected were sporozoite negative. However, EIRs (product of mosquito landing rate x sporozoite infection rate) for An. minimus varied between locations investigated (Table VI).
Table VI.
Mosquito landing rates, sporozoite infectivity and transmission intensities of malaria in different blocks of South Tripura district, Tripura, Northeast India
Discussion
The northeast region of India is the major contributor for drug-resistant varieties of P. falciparum malaria but the relative proportions of P. falciparum and P. vivax varies across its landscape21. Among the northeastern States, Tripura consistently contributed majority P. falciparum (>90%) cases22. Our study results were in conformity with the State surveillance data for P. falciparum being the predominant infection. P. vivax malaria constituted <10 per cent of cases each year and little is known of its present susceptibility status to chloroquine and relapsing pattern. Given the contextual determinants there exists every possibility of total replacement of P. vivax with that of drug-resistant malaria similar to reports in central India23. For treatment of P. falciparum malaria, there are already reports of drug resistance to artemisinin based combination therapy in Tripura24. The problem of malaria control continues to be complex along international borders due to poor inter-country coordinated vector control interventions, illiteracy, and large concentrations of ethnic tribes living with poor access to healthcare services resulting in hidden parasite reservoir and continued transmission25,26.
Malaria receptivity, varied between districts evidenced by number of reported cases, a pattern that was consistent with districts of South Tripura and Dhalai reporting highest number of cases in comparison to West Tripura and North Tripura corresponding to proportional concentration of tribal populations and forest cover. The transmission was typically perennial and persistent with seasonal peak corresponding to months of heavy rainfall evidenced by monthly distribution of cases, but disease trends were clearly and steadily declining. This phenomenon could be ascribed to induction of newer interventions including artemisinin-based combination therapy for treatment of P. falciparum malaria and mass impregnation of community-owned mosquito nests/induction of long-lasting insecticidal nets for personal protection against mosquito bites. Similarly reducing number of malaria-attributable death cases could be due to induction of rapid diagnostic test kits for on-the-spot diagnosis and treatment of P. falciparum malaria. Cross-sectional mass blood surveys, however, revealed that malaria was widely prevalent both in febrile and in afebrile cases but transmission was heterogeneous.
The present study revealed that both An. minimus and An. baimaii were prevalent during monsoon season (June-September) corresponding to high transmission period. The population abundance of both these mosquito species varied across ecotypes evidenced by low prevalence of An. minimus in human dwellings indoors compared to mosquito landing rate per person/night. Among these, An. minimus were incriminated and proven efficient mosquito vectors in the State but role of An. baimaii could not be substantiated clearly for lack of sporozoite infectivity. Furthermore, data on mosquito blood meal analysis for An. baimaii remained inadequate for want of sufficient number of mosquitoes other than human bait catch to substantiate host preferences. Though the sample size remained inadequate, but both An. minimus and An. baimaii mosquito species were reaffirmed to be fully susceptible to DDT. The present data on seasonal abundance and infectivity suggested that the spray coverage for years together remained patchy and inadequate for control of vector populations. Molecular assays reaffirmed that of the An. minimus and An. dirus species complex, An. minimus s.s. and An. baimaii were the only member species prevalent in Tripura similar to reports from other northeastern States19,27. However, bionomical characteristics of both these species varied. An. minimus was largely indoor resting species (endophilic) and endophagic, and slow flowing seepage water streams were the species-specific breeding habitats. In contrast, An. baimaii mosquitoes were not recorded resting indoors (largely exophilic) but actively searched human host both indoors and outdoors. An. jeyporiensis might as well be probable vector in this region for having indoor resting characteristics like that of An. minimus and biting preferences similar to An. baimaii calling for additional investigations.
It is hypothesized that population densities of both An. minimus and An. baimaii are rapidly depleting in the face of ecological changes and interventions in force as evidenced by disease transmission reduction. But the challenge persists and strategic reforms are needed for continuing battle against malaria28,29. Given the bionomical characteristics of mosquito vector species in Tripura, it is proposed that DDT spray coverage, mass distribution of insecticide-treated nets/long-lasting insecticidal nets coupled with increased awareness for disease prevention should be intensified prioritizing high-risk districts.
Acknowledgment
The authors thank the State Programme Officer, Tripura; the Medical Officer Incharge of Silachari, Hrishyamukh and Manubankul Block Primary Health Centers; the District Malaria Officer, South Tripura, and Shrimati Swapna Sinha, Medical Officer, Udaipur for logistics support and consultations; and Dr. P.K. Mohapatra, Regional Medical research Centre, Dibrugarh, for statistical analyses. The study was financially supported by the Indian Council of Medical Research under North-East Fund assistance initiative (document number HIV/18/08/36/2010-ECD-II). Meteorological data were provided by the India Meteorological Department, Meteorological Centre, Agartala.
Footnotes
Conflicts of Interest: None.
References
- 1.Das A, Anvikar AR, Cator LJ, Dhiman RC, Eapen A, Mishra N, et al. Malaria in India: the centre for the study of complex malaria in India. Acta Trop. 2012;121:267–73. doi: 10.1016/j.actatropica.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh V, Mishra N, Awasthi G, Dash AP, Das A. Why is it important to study malaria epidemiology in India? Trends Parasitol. 2009;25:452–7. doi: 10.1016/j.pt.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2007;77(Suppl 6):69–78. [PubMed] [Google Scholar]
- 4.Misra BG, Dhar SK. Malaria in Tripura state. Indian J Malariol. 1955;9:111–23. [PubMed] [Google Scholar]
- 5.Dhiman S, Goswami D, Rabha B, Gopalakrishnan R, Baruah, I, Singh L. Malaria epidemiology along Indo-Bangladesh border in Tripura state, India. Southeast Asian J Trop Med Public Health. 2010;41:1279–89. [PubMed] [Google Scholar]
- 6.Dhiman S, Gopalakrishnan R, Goswami D, Rabha B, Baruah I, Singh L. Malaria incidence among paramilitary personnel in an endemic area of Tripura. Indian J Med Res. 2011;133:665–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Shah NK, Dhillon GP, Dash AP, Arora U, Meshnick SR, Valecha N. Antimalarial drug resistance of Plasmodium falciparum in India: changes over time and space. Lancet Infect Dis. 2011;11:57–64. doi: 10.1016/S1473-3099(10)70214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goswami D, Baruah I, Dhiman S, Rabha B, Veer V, Singh L, et al. Chemotherapy and drug resistance status of malaria parasite in northeast India. Asian Pac J Trop Med. 2013;6:583–8. doi: 10.1016/S1995-7645(13)60101-7. [DOI] [PubMed] [Google Scholar]
- 9.Das SC, Bhuyan M, Baruah I, Talukdar PK. Mosquito survey in Tripura. Indian J Malariol. 1991;28:129–34. [PubMed] [Google Scholar]
- 10.Prakash A, Bhattacharyya DR, Mohapatra PK, Mahanta J. Investigation on malaria vectors and mosquito fauna in south Tripura district, Tripura state. Indian J Malariol. 1998;35:151–9. [PubMed] [Google Scholar]
- 11.Dhiman S, Gopalakrishnan R, Goswami D, Das NG, Baruah I, Rabha B, et al. Diversity, spatio-temporal distribution and biting activity of mosquitoes in Tripura state, India. Entomon. 2009;34:223–32. [Google Scholar]
- 12.Dev V, Sharma VP. The dominant mosquito vectors of human malaria in India. In: Manguin S, editor. Anopheles mosquitoes - new insights into malaria vectors. Croatia: INTECH Publications; 2013. pp. 239–71. [Google Scholar]
- 13.Tribal Research Institute, Government of Tripura, India. [accessed on April 29, 2015]. Available from: http://www.tritripura.in/tri/Tribes/Index.aspx .
- 14.Malaria control strategies. National Vector Borne Disease Control Programme. Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India. [accessed on May 20, 2012]. Available from: http://www.nvbdcp.gov.in/malaria11.html .
- 15.National Drug Policy on Malaria 2012. National Vector Borne Diseases Control Programme, Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India. [accessed on May 20, 2012]. Available from: http://www.nvbdcp.gov.in .
- 16.Wattal BL, Kalra NL. Region-wise pictorial keys to the female Indian Anopheles. Bull Natl Soc India Malar Mosq Borne Dis. 1961;9:85–138. [Google Scholar]
- 17.Das BP, Rajagopal R, Akiyama J. Pictorial keys to the species of Indian Anopheline mosquitoes. J Pure Appl Zool. 1990;2:131–62. [Google Scholar]
- 18.Bray RS, Gill GS, Killick-Kendrick R. Current and possible future techniques for the identification of blood meals of vector haematophagus arthropods. Geneva: World Health Organization; 1984. WHO/MAL/84.1013. [accessed on June 20, 2012]. Available from: http://apps.who.int/iris/bitstream/10665/65918/1/WHO_MAL_84.1013. pdf? ua=1 .
- 19.Singh OP, Nanda N, Dev V, Bali P, Sohail M, Mehrunnisa A, et al. Molecular evidence of misidentification of Anopheles minimus as Anopheles fluviatilis in Assam (India) Acta Trop. 2010;113:241–4. doi: 10.1016/j.actatropica.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Instructions for determining the susceptibility or resistance of adult mosquitoes to organochlorine, organophosphate and carbamate insecticides - diagnostic test. WHO/VBC/81.806. [accessed on June 19, 2012]. Available from: whqlibdoc.who.int/temp/WHO_VBC_81.806_cor.1.pdf .
- 21.Joshi H, Prajapati SK, Verma A, Kang’a S, Carlton JM. Plasmodium vivax in India. Trends Parasitol. 2008;24:228–35. doi: 10.1016/j.pt.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Magnitude of the problem. National Vector Borne Disease Control Programme. Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India. [accessed on May 20, 2014]. Available from: http://www.nvbdcp.gov.in/malaria3.html .
- 23.Singh N, Nagpal AC, Saxena A, Singh MP. Changing scenario of malaria in central India: the replacement of Plasmodium vivax by P. falciparum (1986-2000) Trop Med Int Health. 2004;9:364–71. doi: 10.1046/j.1365-3156.2003.01181.x. [DOI] [PubMed] [Google Scholar]
- 24.Mishra N, Kaitholia K, Srivastava B, Shah NK, Narayan JP, Dev V, et al. Declining efficacy of Artesunate plus sulphadoxine-pyrimethamine in Northeastern India. Malar J. 2014;13:284. doi: 10.1186/1475-2875-13-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dev V, Phookan S, Sharma VP, Dash AP, Anand SP. Malaria parasite burden and treatment seeking behavior in ethnic communities of Assam, northeastern India. J Infect. 2006;52:131–9. doi: 10.1016/j.jinf.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Dev V, Sangma BM, Dash AP. Persistent transmission of malaria in Garo hills of Meghalaya bordering Bangladesh, north-east India. Malar J. 2010;9:263. doi: 10.1186/1475-2875-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prakash A, Sarma DK, Bhattacharyya DR, Mohapatra PK, Bhattacharjee K, Das K, et al. Spatial distribution and r-DNA second internal transcribed spacer characterization of Anopheles dirus (Diptera: Culicidae) complex species in north-east India. Acta Trop. 2010;114:49–54. doi: 10.1016/j.actatropica.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 28.John TJ, Dandona L, Sharma VP, Kakkar M. Continuing challenge of infectious diseases in India. Lancet. 2011;377:252–69. doi: 10.1016/S0140-6736(10)61265-2. [DOI] [PubMed] [Google Scholar]
- 29.Sharma VP. Battling malaria iceberg incorporating strategic reforms in achieving Millennium Development Goals & malaria elimination in India. Indian J Med Res. 2012;136:907–25. [PMC free article] [PubMed] [Google Scholar]