Abstract
Sudden deaths in children due to acute encephalitis syndrome (AES) from a tribal dominated district of Malkangiri in Odisha, India, was reported during September-November, 2012. The investigation was carried out to search for the possible viral aetiology that caused this outbreak. Clinico-epidemiological survey and seromolecular investigation were carried out to confirm the viral aetiology. Two hundred seventy two suspected cases with 24 deaths were observed. The patients presented with low to moderate grade fever (87%), headache (43%), vomiting (27%), cold (18%), cough (17%), body ache (15%), joint pain (15%), rash (15%), abdomen pain (9%), lethargy (5%), altered sensorium (8%), convulsion (2%), diarrhoea (3%), and haematemesis (3%). Laboratory investigation showed Japanese encephalitis virus (JEV) IgM in 13.8 per cent (13/94) in blood samples and JEV RNA in one of two cerebrospinal fluid (CSF) samples. Paddy fields close to the houses, high pig to cattle ratio, high density (33 per man hour density) of Culex vishnui mosquitoes, low socio-economic status and low health awareness in the tribal population were observed. This report confirmed the outbreak of JEV infection in Odisha after two decades.
Keywords: Acute encephalitis syndrome (AES), Culex vishnui, Japanese encephalitis virus (JEV), Malkangiri, Odisha
Japanese encephalitis (JE) is an important public health problem in South East Asian region and India as most of the outbreaks and sporadic encephalitis cases have been attributed to it1. In the last few years States like Uttar Pradesh (UP), West Bengal, Bihar, Andhra Pradesh (AP) and North Eastern States have been reporting regular cases of JE infection in India and it is also spreading to naive non endemic regions of the country2,3. Over three billion individuals are living in JE epidemic and/or endemic countries and it is estimated that approximately 67,900 JE cases occur annually in 24 countries4. From the State of Odisha in eastern India only one outbreak of JE was reported from Rourkela city of Sundergarh district in 19895. Sporadic JE cases have been diagnosed from hospitalized children between 1992 and 19956,7. Since then, there is no record of JE infection in the State. During September-November, 2012 children with acute encephalitis syndrome (AES) followed by deaths were from Malkangiri district of Odisha (as reported by State Health Department, Odisha).
Epidemiological investigation was carried out by the Regional Medical Research centre (ICMR), Bhubaneswar, during September-November 2012, to support public health measures taken by the State Health Department. The investigation covered four affected villages, i.e. Potrel and Uskapalli of Chitrakonda tehsil and Pradhaniguda and Charkiguda of Malkangiri tehsil. Average rainfall in the area in 2012 was 1700 mm and the temperature ranged between 13-47°C. The outbreak period was post-monsoon and average temperature was 35°C. Population in the affected villages belonged to tribal communities with low socio-economic status, who lived on cultivation and daily wages. House-to-house survey was undertaken to record the suspected cases, and information on clinical presentations, deaths, ecological conditions, domestic animals and birds, crops and vegetation, vectors, social events and food habits that might have possible association with neurological manifestation/involvement. Day-wise onset of cases and deaths was recorded up to the last case. A case of AES was defined as “acute onset of fever, change in mental status (such as confusion, disorientation, delirium or coma) and/or new onset of seizures (excluding simple febrile seizures) in a person of any age presenting at any time of the year”8. Blood samples were collected from all cases and asymptomatic contacts from the family or neighbouring household. CSF samples were collected only in hospitalized patients. Individual patients of the area who were under treatment at the district hospital for suspected AES were also enrolled.
Indoor (human dwelling and cattle shed) and outdoor resting mosquito collections were done using sucking tube and mosquito species were identified. Blood and CSF samples were tested by ELISA for dengue virus (DV) and Japanese encephalitis virus (JEV) IgM (ELISA kit, NIV, Pune), dengue NS1 antigen (Pan Bio, Australia) and IgM antibodies against enterovirus (EV) (Serion ElisAkit, Germany). Chandipura virus (CHPV) IgM was tested at the National Institute of Virology (NIV), Pune, using in-house protocol. All these samples were subjected to one step reverse transcription (RT)-PCR (Qiagen kit, Germany) to amplify viral RNA. Primers used for JEV and CHPV detection were as per those reported by Pujhari et al9 and Chadha et al10, respectively. Real time PCR (ABI, 7500, USA) was conducted to detect genus specific enterovirus (Fast Track Diagnostic kit, Luxemborg). Mosquitoes were pooled from indoor and outdoor collections and tested for JEV RNA by RT-PCR as described above9. The study was conducted after approval of the Human ethical committee of the Institute.
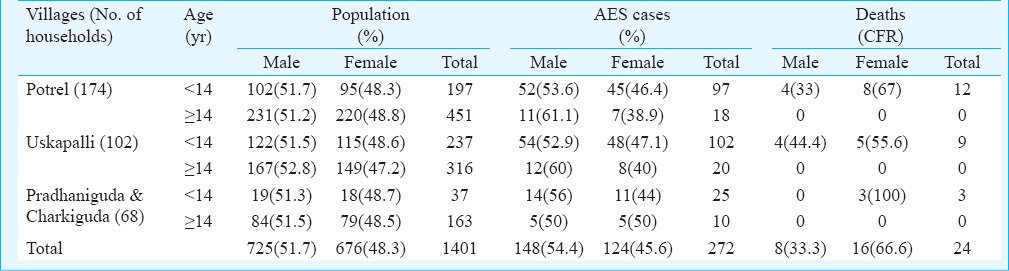
The outbreak appeared with sudden death of a girl child aged three who presented with fever and altered sensorium on September 16, 2012, from Potrel village. Cases and deaths continued over seven weeks with peaks during 3rd and 5th weeks. The last case was recorded on November 2, 2012. The population demography of the affected villages is shown in the Table. The affected four villages were under two tehsils, within a distance of around 18 km. A total of 272 AES cases and 24 deaths were recorded. The median age of the AES cases was five years (2 months 53 yr) and that of deaths was three years (2-10 yr). These 24 deaths covered 15 households, six families reporting multiple (two-three) deaths. Samples (serum-94, CSF-2) collected from 94 subjects (cases-55, contacts-39) were investigated. JEV specific IgM antibody was present in 11 (20%, 11/55) cases and two (5%, 2/39) contacts. One CSF sample had JEV IgM and the other revealed JEV RNA. One symptomatic case showed dengue RNA of serotype II. All these samples were negative for EV or CHPV. Result of duplicate testing at NIV, Pune was consistent with the laboratory result.
Table.
Age and sex distribution of total population, suspected cases and deaths in studied villages
The presenting symptoms in these 94 subjects were low to moderate grade fever (58.5%, n=55), headache (43%, n=41), vomiting (27%, n=26), cold (18%, n=17), cough (17%, n=16), body ache (15%, n=14), joint pain (15%, n=14), rash (15%, n=14), abdomen pain (9%, n=9), lethargy (5%, n=5), altered sensorium (89%, n=84), convulsion (12.7%, n=12), diarrhoea (3%, n=3) and haematemesis (3%, n=3). Mean recovery period was seven days. There was no neurological deficit following recovery. Case fatality rate (CFR) was 8.8 per cent (24/272) and mean period of survival was 54 h (24 h to five days) after onset of illness. All deaths were in children below 10 yr, affecting mostly females (66 %, 16/24). Other environmental factors indicated poor housing condition and growing paddy fields close to the living houses (<2 feet). Domestic animals like pigs, cattle, poultry were reared by 66.5, 41 and 22 per cent households, respectively and the animal/bird sheds were close to the housing habitat. Culex vishnui group, Cx. quinquefaciatus, Anopheles vagus, An. culicifacies, An subpictus were the mosquito vectors present with per man hour density (PMHD) of 33.0, 5.2, 7.1, 3.2 and 14.8, respectively. Mosquito samples (n=125) were tested for JEV RNA but none was positive.
The investigation showed appearance of JEV infection in the State of Odisha after two decades of last report. The presented outbreak of AES persisted for seven weeks with two peaks. This was probably due to initial indoor fogging and after resurgence added outdoor fogging which might have contained the outbreak. The clinical presentation was sudden onset of fever with a few bouts of vomiting followed by altered sensorium with or without convulsion. This was similar to the usual presentations recorded in previous reports that showed fever (80-97%), headache (61-74%), altered sensorium or convulsion (78-98%), and vomiting (45-61%)11,12. None of the cases had focal neurological deficits that was reported in JE endemic area of UP11. Haematemesis was observed in two cases, both of whom died. A report of JE outbreak from UP had also revealed evidence of gastric haemorrhage (54.5%)11. The CFR observed was 8.8 per cent and death mostly occurred in children below 10 yr with a short survival period (54 h). It might be due to the delay in hospitalization because of mild symptoms before sensorium was affected and rapid deterioration to coma and death within 2-6 h of hospitalization. As reported in previous studies, the affected area was observed to have poor living conditions13,14 with favourable environmental factors for breeding of Culex (post monsoon season, paddy fields)4,10, transmission of virus to pig during this season12, high pig to cattle ratio15 and low awareness of tribal population for present prophylaxis12. Successful isolation rate of JEV RNA from mosquito pool is also less common which may have been one of the reasons for absence of JEV in vectors during this outbreak16.
This report of Japanese encephalitis from this non-endemic area indicated towards a need for public health vigilance in areas having environmental risk for acquiring JE infection. This can prevent morbidity and mortality by early suspicion and investigation.
Footnotes
Conflicts of Interest: None.
References
- 1.Solomon T. Viral encephalitis in Southeast Asia. Neurol Infect Epidemiol. 1997;2:191–9. [Google Scholar]
- 2.Joshi R, Kalantri SP, Reingold A, Colford JM., Jr Changing landscape of acute encephalitis syndrome in India: a systematic review. Natl Med J India. 2012;25:212–20. [PubMed] [Google Scholar]
- 3.Japanese encephalitis. Ministry of Health & Family Welfare. Government of India. [accessed on December 27, 2015]. Available from: http://www.nvbdcp.gov.in/je-action-taken.html .
- 4.Wang H, Liang G. Epidemiology of Japanese encephalitis: Past, present, and future prospects. Therapeutics and Clinical Risk Management. 2015;11:435–48. doi: 10.2147/TCRM.S51168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vajpayee A, Mukherjee MK, Chakraborty AK, Chakraborty MS. Investigation of a outbreak of Japanese encephalitis in Rourkela city (Orissa) during 1989. J Commun Dis. 1991;23:18–21. [PubMed] [Google Scholar]
- 6.Devi PS, Behera PL, Swain A. Japanese encephalitis in Orissa. Indian Pediatr. 1996;33:702–3. [PubMed] [Google Scholar]
- 7.Dash AP, Chhotray GP, Mahapatra N, Hazra RK. Retrospective analysis of epidemiological investigation of Japanese encephalitis outbreak occurred in Rourkela, Orissa, india. Southeast Asian J Trop Med Public Health. 2001;32:137–9. [PubMed] [Google Scholar]
- 8.Solomon T, Thao TT, Lewthwaite P, Ooi MH, Kneen R, Dung NM, et al. A cohort study to assess the new WHO japanese encephalitis surveillance standards. Bull World Health Organ. 2008;86:178–86. doi: 10.2471/BLT.07.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pujhari SK, Prabhakar S, Ratho RK, Modi M, Sharma M, Mishra B. A novel mutation (S227T) in domain II of the envelope gene of Japanese encephalitis virus circulating in North India. Epidemiol Infect. 2011;139:849–56. doi: 10.1017/S0950268810001937. [DOI] [PubMed] [Google Scholar]
- 10.Chadha MS, Arankalle VA, Jadi RS, Joshi MV, Thakare JP, Mahadev PV, et al. An outbreak of Chandipura virus encephalitis in the Eastern districts of Gujarat state. India Am J Trop Med Hyg. 2005;73:566–70. [PubMed] [Google Scholar]
- 11.Kumar R, Tripathi P, Singh S, Bannerji G. Clinical features in children hospitalized during the 2005 epidemic of Japanese encephalitis in Uttar Pradesh, India. Clin Infect Dis. 2006;43:123–31. doi: 10.1086/505121. [DOI] [PubMed] [Google Scholar]
- 12.Anuradha SK, Surekha YA, Sathyanarayan MS, Suresh S, Satish P, Mariraj J, et al. Epidemiological aspects of Japanese encephalitis in Bellary, Karnataka, India. Int J Biol Med Res. 2011;2:691–5. [Google Scholar]
- 13.Loach TR, Narayan KG, Choudhary SP. Sero-epidemiologic studies on the 1980-epidemic of human encephalitis in East and West Champaran, Bihar, India. J Commun Dis. 1983;15:151–6. [PubMed] [Google Scholar]
- 14.Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. J Neurol Neurosurg Psychiatry. 2000;68:405–15. doi: 10.1136/jnnp.68.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijayarani H, Gajanana A. Low rate of Japanese encephalitis infection in rural children in Thanjavur district (Tamil Nadu), an area with extensive paddy cultivation. Indian J Med Res. 2000;111:212–4. [PubMed] [Google Scholar]
- 16.Lindahl JF, Ståhl K, Chirico J, Boqvist S, Thu HT, Magnusson U. Circulation of Japanese encephalitis virus in pigs and mosquito vectors within Can Tho City, Vietnam. PLoS Negl Trop Dis. 2013;7:e2153. doi: 10.1371/journal.pntd.0002153. [DOI] [PMC free article] [PubMed] [Google Scholar]


