Abstract
Background & objectives:
Studies have shown that certain flaviviruses influence susceptibility of mosquitoes by inhibiting/enhancing replication of important flaviviruses. Hence, a study was designed to determine whether Bagaza virus (BAGV), a flavivirus isolated from Culex tritaeniorhynchus mosquitoes in India, alters susceptibility of Cx. tritaeniorhynchus and Cx. quinquefasciatus mosquitoes to Japanese encephalitis (JEV) and West Nile viruses (WNV).
Methods:
JEV and WNV infection in Cx. tritaeniorhynchus and Cx. quinquefasciatus mosquitoes in the presence of BAGV was carried out by intrathoracic (IT) inoculation and oral feeding methods. Mosquitoes were infected with BAGV and WNV/JEV either simultaneously or in a phased manner, in which mosquitoes were infected with BAGV by IT inoculation followed by super-infection with JEV/WNV after eight days post-infection (PI). JEV and WNV yield on 7th and 14th day PI after super-infection was determined by 50 per cent tissue culture infective dose (TCID50) method.
Results:
In Cx. tritaeniorhynchus mosquitoes, prior infection with BAGV significantly reduced JEV and WNV replication while in Cx. quinquefasciatus, BAGV influence was only seen with WNV. Reduction in virus titre was observed in IT inoculated and oral fed mosquitoes irrespective of the infection mode. JEV replication was also found reduced in Cx. tritaeniorhynchus mosquitoes persistently infected with BAGV at passage four.
Interpretation & conclusions:
BAGV infection in Cx. tritaeniorhynchus and Cx. quinquefasciatus mosquitoes altered their susceptibility to JEV and WNV producing low virus yield. However, the role of BAGV in inhibiting JEV/WNV replication in field mosquitoes needs further investigations.
Keywords: Bagaza virus, intra thoracic inoculation, Japanese encephalitis virus, super infection, West Nile virus
Bagaza virus (BAGV), a mosquito borne arbovirus of genus Flavivirus, family Flaviviridae has been isolated repeatedly from different species of mosquitoes in Africa since its discovery from Bagaza district of Central African Republic in 19661. Initial studies have shown that BAGV is antigenically close to Israel turkey meningoencephalitis virus, an important viral pathogen of poultry in Middle East and South Africa2. The virus had a wide geographic distribution in West Africa as isolations were made from Central African Republic, Senegal, Cameroon and Mauritania involving more than seven mosquito species3,4,5. BAGV has never been associated with any outbreak of either human or veterinary importance despite its presence in the African continent for the last five decades until the detection of its role in the unusually high bird deaths (Partridges and pheasants) reported from Spain in 20106.
In India, BAGV was isolated from a pool of Culex tritaeniorhynchus mosquitoes collected during an outbreak of Japanese encephalitis virus (JEV) in Kerala7. Subsequent serological studies have demonstrated the presence of BAGV antibodies in 15 per cent of the human population in the area demonstrating subclinical infections in man1,7. Culex tritaeniorhynchus is the purported vector of JEV and is also a potential vector for West Nile virus (WNV). Some novel flaviviruses have been found to interact with pathogenic flaviviruses in mosquitoes. This study was, therefore, undertaken to determine the role of BAGV in replication of JEV and WNV in two important vector mosquitoes, i.e. Cx. tritaeniorhynchus and Cx. quinquefasciatus.
Material & Methods
The study was conducted at the Microbial Containment Complex, National Institute of Virology (NIV), Pashan, Pune, India. Infected mosquitoes were kept in plastic jars inside mosquito cages and all these experiments were carried out inside a bio-safety level-2 laboratory. Normal as well as infected mosquitoes were maintained at 28±2°C with 80±5 per cent relative humidity and 12:12 h light: dark cycle.
Viruses:
-
(i)
BAGV - BAGV strain No. BAG96363, isolated from a pool of Cx. tritaeniorhynchus mosquitoes in 19961 was used in the study. This isolate has undergone six passages in Baby hamster kidney (BHK-21) cell line. The virus at P-7 (stock) had a titre of 5.0 log10 50 per cent tissue culture infective dose (TCID50)/ml.
-
(ii)
JEV - Strain No. 057434, a human isolate obtained from Gorakhpur, India, in 2005 (NIV, unpublished data) has undergone several passages in mice and two passages in Vero E6 cell line. The stock virus prepared in Vero E6 cell line had a titre of 7 log10 TCID50/ml.
-
(iii)
WNV - The prototype strain of WNV (Eg101) procured from NIV virus repository was used in the study. The strain has undergone several passages in mouse brain and passaged once in Vero E6 cell line for stock preparation. The virus stock had a titre of 8.3 log10 TCID50/ml.
Virus stock preparation: BHK-21 cell line was preferred for stock preparation and virus titration based on the high virus yield in the cell line (unpublished data). To prepare virus stock, BHK-21 cells grown in 225 cm2 bottles were infected with 1MOI (multiplicity of infection) of BAGV as described earlier1. The cultures were observed daily for appearance of cytopathic effect (CPE) and when 70-80 per cent cells showed CPE, cultures were harvested. Virus was extracted from cells by freeze-thawing thrice and collecting the supernatant after centrifugation at 2790× g in a refrigerated (+4°C) centrifuge (Hettich, Germany) for 20 min. One ml aliquots of the suspension was made in sterile vials and stored at -86°C. One of the aliquots was titrated in the same cell line and virus titre was determined as mentioned earlier. BHK-21 cell line was maintained in minimum essential medium (MEM) supplemented with 10 per cent foetal bovine serum (FBS) and was passaged at every 3-4 days. Both MEM and FBS used for cell line maintenance were procured from Invitrogen, USA.
Insect flavivirus free mosquitoes were procured from the NIV insectary maintained at 28±2°C with 80±5 per cent relative humidity and 12:12 h light: dark cycle. Mosquito larvae were fed on a mixture of yeast powder and dog biscuit (3:1 w/w) while adults were maintained on a diet of 10 per cent glucose. Female mosquitoes were provided with 5-6 wk old chickens for blood meal on alternate days.
Infection of mosquitoes with virus: Infection of mosquitoes was carried out by intrathoracic inoculation (IT) and oral feeding. For IT inoculation, 50 mosquitoes for each experiment were immobilized by keeping them in ice for 5-10 min and inoculated intrathoracically with 100 plaque forming units (pfu) of BAGV inside a BSL-2 laboratory following the method described by Rosen and Gubler8. For oral feeding, mosquitoes (n=50 for each experiment) were starved for 12 h and allowed to feed on blood virus mixture through a chicken membrane as previously described1. Fully engorged mosquitoes were separated and used for the study. Infected mosquitoes (both IT inoculated and oral fed) were secured in plastic mosquito holding jars inside double walled mosquito cages and incubated at 28±2°C with 70-80 per cent humidity. Five mosquitoes were harvested at scheduled intervals and stored at -80°C. After completion of the experiment, mosquitoes of each day post-infection (PI) were triturated in 1 ml MEM containing 2 per cent FBS using a chilled mortar and pestle. The mosquito suspension was centrifuged; Millipore filtered (pore size=0.22 μm), diluted serially (ten-fold) and titrated in BHK-21 cells in quadruplicate. The cultures were observed daily, readings (cells with cytopathic effects) were scored, stained with amido black and virus titre of each day PI was determined9. All experiments were carried out in triplicate.
Determination of JEV/WNV replication in BAGV infected mosquitoes: The influence of BAGV in replication of JEV and WNV was studied using two methods. In the first method, mosquitoes were infected simultaneously with a mixture of BAGV and JEV or WNV by IT inoculation. In the second method, mosquitoes were infected with BAGV, incubated for eight days and superinfected with JEV/WNV. The infections were made by IT inoculation. In the oral infection method, mosquitoes were fed blood-virus mixture as described earlier. In all the cases, mosquitoes were incubated further, harvested on days 7 and 14 of infection with JEV/WNV, titrated and determined virus titre as described earlier. JEV/WNV yield in BAGV infected mosquitoes was compared with virus yield of mosquitoes infected with JEV/WNV alone and determined the influence of BAGV in replication of JEV/WNV.
Determination of effect of BAGV in oviposition and life cycle of mosquitoes: Culex quinquefasciatus and Cx. tritaeniorhynchus mosquitoes (n=50 each) were infected orally with BAGV and allowed to oviposit. The number of egg rafts obtained, duration of oviposition, hatching rate and duration to complete the larval and pupal stages, duration of emergence and adult survival were recorded. Similar data from an equal number of uninfected adults were also recorded and analyzed to determine the effect of BAGV in mosquito survival and oviposition.
Establishment of persistent infection of BAGV in Cx. tritaeniorhynchus mosquitoes: Culex tritaeniorhynchus mosquitoes were inoculated with BAGV as described earlier1, incubated for seven days and fed on normal infant mice. Fully engorged mosquitoes were collected, placed in double walled mosquito holding cages and allowed to lay eggs. Eggs were allowed to hatch and grow to adults. The eggs, larvae and adults were screened for presence of BAGV by tissue culture assay1. The mosquitoes were reared continuously upto 4th generation and used for experimental studies.
Results
BAGV yield in BHK-21 cell line: BAGV growth kinetics in BHK-21 cell line showed maximum yield of 5.5 and 5 log10 TCID50/ml in cells and tissue culture fluid (TCF), respectively at 36 h PI. However, virus titre in TCF was maintained upto 84th h PI while a sharp decline in titre was detected in cells (Fig. 1). During stock preparation, cells were harvested at 60 h PI and the titre was determined as 5 log10 TCID50/ml.
Fig. 1.
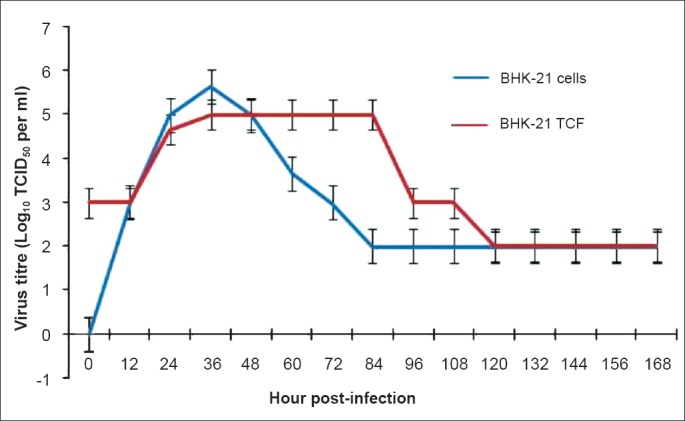
Growth kinetics of BAGV in BHK-21 cell line and tissue culture fluid (TCF). Values are shown as mean ± SD (n=3).
BAGV infection alters the replication of JEV/WNV during simultaneous infection: In the pilot study carried out by intrathoracic inoculation of Cx. tritaeniorhynchus mosquitoes, BAGV was found to play a significant role in inhibition of replication of both JEV and WNV (Fig. 2). In presence of BAGV, JEV replication was found affected both by simultaneous infection as well as in mosquitoes previously infected with BAGV. However, the influence was more prominent in the latter case as approximately 4 log reduction in virus titre was obtained both at 7th and 14th day PI (Fig. 2b) while the reduction in the simultaneous infection was only 2 log10 TCID50/ml (Fig. 2a). JEV titre in mosquitoes on ‘0’ day was approximately 2 log virus (100 pfu) in all the experiments. In the case of WNV replication in Cx. tritaeniorhynchus, simultaneous infection or super-infection with WNV yielded almost identical results (Fig. 2c, d) despite the initial titre of 2 log TCID50/ml on ‘0’day in all the experiments. At 7th day PI, reduction in WNV yield was prominent (4 log) while on 14th day PI, the difference in WNV yield was approximately 2 log10 TCID50/ml in either mode of infection.
Fig. 2.
Influence of BAGV infection in replication of JEV and WNV in Cx. tritaeniorhynchus mosquitoes (intrathoracic inoculation method); (a) Differential yield of JEV in mosquitoes infected simultaneously with BAGV to mosquitoes infected with JEV alone; (b) Differential yield of JEV in mosquitoes previously infected with BAGV to mosquitoes infected with JEV alone; (c) Differential yield of WNV in mosquitoes infected simultaneously with BAGV to mosquitoes infected with WNV alone; (d) Differential yield of WNV in mosquitoes previously infected with BAGV to mosquitoes infected with WNV alone. Values are mean ± SD (n=3).
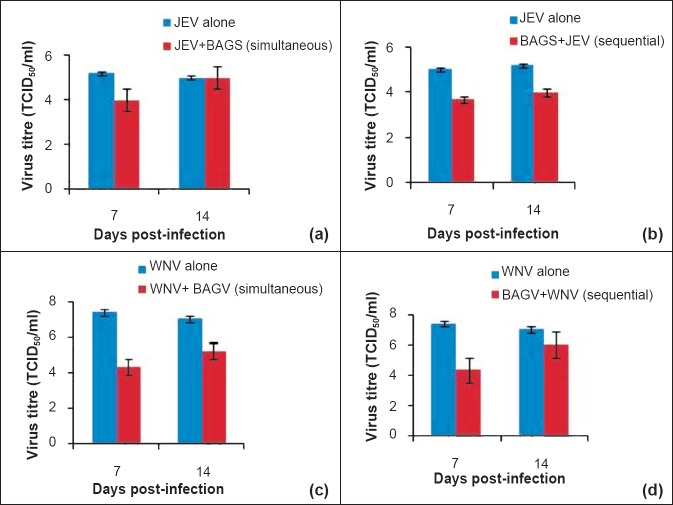
Presence of BAGV did not alter the susceptibility of Cx. quinquefasciatus mosquitoes to JEV. In both the methods of infection, reduction in JEV yield in presence of BAGV was approximately 1 log10 TCID50/ml only (Fig. 3a, b). No difference in virus yield was found on 14th day PI in simultaneously infected mosquitoes as identical titres were obtained in BAGV infected mosquitoes and normal mosquitoes (Fig. 3a) though a difference of 1 log was detected in mosquitoes previously infected with BAGV (Fig. 3b). However, susceptibility to WNV was found to be altered as remarkable reduction was observed in both simultaneously infected and mosquitoes previously exposed to BAGV (Fig. 3c, d). The reduction in virus yield was more prominent at day 7 PI than day 14 PI as the differences in titre of 3 log10 and 1log10TCID50/ml were observed, respectively.
Fig. 3.
Influence of BAGV infection in replication of JEV and WNV in Cx. quinquefasciatus mosquitoes (intrathoracic inoculation method); (a) Differential yield of JEV in mosquitoes infected simultaneously with BAGV to mosquitoes infected with JEV alone; (b) Differential yield of JEV in mosquitoes previously infected with BAGV to mosquitoes infected with JEV alone; (c) Differential yield of WNV in mosquitoes infected simultaneously with BAGV to mosquitoes infected with WNV alone; (d) Differential yield of WNV in mosquitoes previously infected with BAGV to mosquitoes infected with WNV alone. Values are mean ± SD (n=3).
Effect of BAGV in orally fed mosquitoes: Oral feeding experiments were conducted only with JEV in Cx. tritaeniorhynchus and WNV in Cx. quinquefasciatus mosquitoes in presence of BAGV. Simultaneous infection of the viruses was not conducted in the study (oral feeding). In both species of mosquitoes, BAGV was found to be playing an important role in inhibiting JEV and WNV (Fig. 4). JEV replication was found restricted to approximately 2 log10 TCID50/ml in BAGV infected mosquitoes upto 9th day PI in comparison to JEV yield in normal mosquitoes which yielded approximately 5 log10 TCID50/ml during the same period (Fig. 4a). However, from 9th day PI onwards, enhanced replication of JEV was observed yielding titres comparable to JEV yield in normal mosquitoes. In Cx. quinquefasciatus mosquitoes the influence of BAGV was found prominent in inhibiting WNV replication upto 15th day PI (Fig. 4b). The difference in virus yield was prominent during 5th to 13th day PI which showed 2-3 log10 TCID50/ml reduction in WNV titre in comparison to WNV yield in the controls.
Fig. 4.
Influence of BAGV infection in replication of JEV and WNV in Cx. tritaeniorhynchus and Cx. quinquefasciatus mosquitoes (oral route). Values are mean ± SD (n=3).
Establishment of persistent infection of BAGV in Cx. tritaeniorhynchus mosquitoes and their susceptibility to JEV: Persistent infection of BAGV was established in Cx. tritaeniorhynchus mosquitoes by serial passaging of infected mosquitoes. Presence of BAGV was detected in eggs (1.7 log10 TCID50/ml) laid by the 3rd generation of mosquitoes. The progeny of the 4th generation adults, when infected with JEV showed a gradual decline and maintained at a titre of 2 log10TCID50/ml upto 9th day PI demonstrating a reduction of approximately 3 log10 TCID50/ml in comparison to JEV yield in normal mosquitoes (Fig. 5). However, an increase in JEV titre to approximately 5 log10 was detected on 11th day PI, which was maintained upto 14th day PI.
Fig. 5.
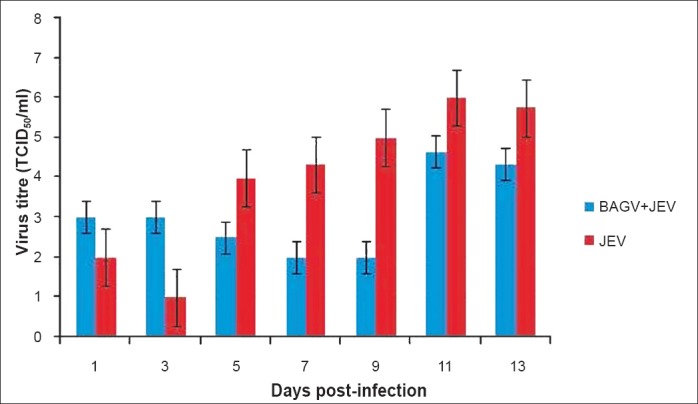
Inhibition of JEV replication in BAGV persistent Cx. tritaeniorhynchus mosquitoes (oral route). Values are mean ± SD (n=3).
Role of BAGV in the natural cycle of Cx. tritaenior-hynchus mosquitoes: During the establishment of persistent infection, it was observed that egg laying and hatching of eggs was affected by BAGV. When a virus titre of 4.7 log10 TCID50/ml was used to infect mosquitoes by oral feeding, only three egg rafts could be obtained from 43 fully engorged mosquitoes. However, none of the eggs hatched. Eggs were retained in the ovaries of the infected mosquitoes. In subsequent experiments, the virus concentration was lowered 10- and 100-folds which resulted in higher number of egg rafts (Table). However, the number of eggs laid was comparatively lower than what was obtained in normal mosquitoes (Table).
Table.
Bagaza virus effects oviposition and hatching of eggs in Cx. tritaeniorhynchus mosquitoes
Discussion
In the present study, we explored the potential of BAGV in influencing replication of JEV and WNV in Cx. tritaeniorhynchus and Cx. quinquefasciatus mosquitoes. Earlier studies have shown that mosquito cell line or a susceptible mosquito, once infected with an arbovirus, does not get infected from subsequent infection with another virus of the same group10,11,12,13,14,15,16,17. Similar phenomenon has also been observed in mosquito cell lines infected with alphaviruses18. It was observed that mosquito cell lines persistently infected with Sindbis virus altered the productivity of other togaviruses in the cell lines upon subsequent infection. Hobson-Peters et al19 demonstrated suppression of WNV and Murray Valley fever encephalitis viruses in mosquito cell cultures infected with Palm Creek virus, a new flavivirus isolated from Coquillettidia xanthogaster mosquitoes. Contradictory to the above findings, Kent et al20 could not demonstrate suppression of WNV replication in Cx. quinquefasciatus mosquitoes infected with the insect flavivirus, Cx. flavivirus Izabel. They could not find any significant impact either on virus replication or transmission of WNV in the mosquitoes previously infected with the mosquito flavivirus. Rather, they observed enhanced WNV transmission in a certain population of Cx. quinquefasciatus mosquitoes upon simultaneous infection.
In the present study using intrathoracic inoculation of mosquitoes, suppression of replication of both JEV and WNV was observed in mosquitoes on 7th and 14th day PI either infected simultaneously or sequentially. These findings demonstrated partial super-infection exclusion in Cx. tritaeniorhynchus infected sequentially with BAGV rather than simultaneous infection. Unlike JEV, significant reduction in WNV titre was observed in both Cx. tritaeniorhynchus and Cx. quinquefasciatus mosquitoes in presence of BAGV especially on 7th day PI.
In the oral feeding experiment, which is the natural route of infection, the findings were almost identical to that of inoculation method. Partial exclusion was observed with JEV and WNV in Cx. tritaeniorhynchus and Cx. quinquefasciatus mosquitoes, respectively. Similar results were obtained with BAGV persistent mosquitoes also. This has significance in nature as there is a possibility of mosquitoes being infected with different insect viruses and these viruses might be playing an important role in the control of pathogenic viruses21.
The major limitation of the present study was the inability to determine the individual titres of BAGV, JEV and WNV in the co-infected mosquitoes as we could not standardize real time RT-PCR for BAGV. That would have given the exact picture of the reduction in the titres of JEV or WNV. Our previous study1 with BAGV in Cx. tritaeniorhynchus and Cx. quinquefasciatus mosquitoes showed that the maximum titre BAGV yielded in the mosquitoes never exceeded 5 log10 and 4 log10 TCID50/ml, respectively in repeated experiments. That substantiated the fact that the observed reduction in the titres of JEV and WNV in the present study in co-infected mosquitoes could be due to these viruses only and not BAGV.
Another important finding of the present study was that mosquitoes infected with ≥4.7 log of BAGV, did not oviposit, but retained eggs in the ovaries. Oviposition was observed when lower concentrations of virus were administered; however, majority of the eggs did not hatch. This shows that infection with BAGV alters oogenesis and oviposition depending on the virus titre. This is also an important observation which has importance in the natural control of mosquitoes. However, laboratory and field conditions are quite different and more studies will be needed before using BAGV or other insect viruses in the natural control of mosquitoes.
The findings of the present study pointed towards the role of BAGV in the inhibition of JEV and WNV replication in two important vector mosquitoes though we could not determine the exact quantity of reduction in virus titres individually due to the lack of quantitative assay for BAGV. BAGV and other novel flaviviruses are associated with mosquitoes naturally without causing pathogenicity in the hosts. However, our preliminary studies with BAGV showed pathogenicity to Cx. tritaeniorhynchus mosquitoes which affected oviposition and hatching of eggs that clearly showed that BAGV was not a co-evolved symbiont of Cx. tritaeniorhynchus. Other studies have demonstrated the isolation and characterization of a number of novel flaviviruses, of which, a few are known to enhance/suppress subsequent infection with flaviviruses of public health importance19,20,21. The precise mechanism of inhibition of replication of a second member of the same family is not yet understood fully. To understand the host competition by different viruses, studies at the receptor and cellular levels are warranted. With the advancements made in molecular virology, it will be possible to identify the factors in the near future. The unfolding of the mechanism, therefore, would have great epidemiological significance as viruses of less pathogenicity could be used as a tool to control/limit the spread of highly pathogenic mosquito borne viruses such as JEV, WNV and other encephalitis causing viruses.
Acknowledgment
The authors acknowledge the Director, NIV, Pune for support, and thank Drs Atanu Basu and D. Cecilia, for valuable suggestions. Technical support rendered by Shri K.N. Lekharaj is acknowledged.
Footnotes
Conflicts of Interest: None.
References
- 1.Sudeep AB, Bondre VP, Mavale MS, Ghodke YS, George RP, Aher RV, et al. Preliminary findings of Bagaza virus (Flavivirus: Flaviviridae) growth kinetics, transmission potential & transovarial transmission in three species of mosquitoes. Indian J Med Res. 2013;138:257–61. [PMC free article] [PubMed] [Google Scholar]
- 2.Kuno G, Chang GJ. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152:687–96. doi: 10.1007/s00705-006-0903-z. [DOI] [PubMed] [Google Scholar]
- 3.Diallo M, Nabeth P, Ba K, Sall AA, Ba Y, Mondo M, et al. Mosquito vectors of the 1998-1999 outbreak of Rift Valley fever and other arboviruses (Bagaza, Sanar, Wesselsbron and West Nile) in Mauritania and Senegal. Med Vet Entomol. 2005;19:119–26. doi: 10.1111/j.0269-283X.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 4.Digoutte JP. Bagaza (BAG) strain: Dak Ar B 209. Am J Trop Med Hyg. 1978;27:376–7. [Google Scholar]
- 5.Traore-Lamizana M, Zeller HG, Mondo M, Hervy JP, Adam F, Digoutte JP. Isolations of West Nile and Bagaza viruses from mosquitoes (Diptera: Culicidae) in central Senegal (Ferlo) J Med Entomol. 1994;31:934–8. doi: 10.1093/jmedent/31.6.934. [DOI] [PubMed] [Google Scholar]
- 6.Agüero M, Fernández-Pinero J, Buitrago D, Sánchez A, Elizalde M, San Miguel E, et al. Bagaza virus in partridges and pheasants, Spain, 2010. Emerg Infect Dis. 2011;17:1498–501. doi: 10.3201/eid1708.110077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bondre VP, Sapkal GN, Yergolkar PN, Fulmali PV, Sankararaman V, Ayachit VM, et al. Genetic characterization of Bagaza virus (BAGV) isolated in India and evidence of anti-BAGV antibodies in sera collected from encephalitis patients. J Gen Virol. 2009;90:2644–9. doi: 10.1099/vir.0.012336-0. [DOI] [PubMed] [Google Scholar]
- 8.Rosen L, Gubler D. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg. 1974;23:1153–60. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- 9.Reed LJ, Muench H. A simple method for estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 10.Altman RM. The behavior of Murray Valley encephalitis virus in Culex tritaeniorhynchus Giles and Culex pipiens quinquefasciatus Say. Am J Trop Med Hyg. 1963;12:425–34. [PubMed] [Google Scholar]
- 11.Zebovitz E, Brown A. Interference among group A arboviruses. J Virol. 1968;2:1283–9. doi: 10.21236/ad0844170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton BT. Heterologous interference in Aedes albopictus cells infected with alphaviruses. J Virol. 1979;30:45–55. doi: 10.1128/jvi.30.1.45-55.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamberlain RW, Sudia WD. Dual infections of eastern and western equine encephalitis viruses in Culex tarsalis. J Infect Dis. 1957;101:233–6. doi: 10.1093/infdis/101.3.233. [DOI] [PubMed] [Google Scholar]
- 14.Rozeboom LE, Kassira EN. Dual infections of mosquitoes with strains of West Nile virus. J Med Entomol. 1969;6:407–11. doi: 10.1093/jmedent/6.4.407. [DOI] [PubMed] [Google Scholar]
- 15.Sundin DR, Beaty BJ. Interference to oral superinfection of Aedes triseriatus infected with La Crosse virus. Am J Trop Med Hyg. 1988;38:428–32. doi: 10.4269/ajtmh.1988.38.428. [DOI] [PubMed] [Google Scholar]
- 16.Zou G, Zang B, Lim PY, Yuan Z, Bernard KA, Shi PY. Exclusion of West Nile virus superinfection through RNA replication. J Virol. 2009;83:11765–76. doi: 10.1128/JVI.01205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pesko K, Mores CN. Effect of sequential exposure on infection and dissemination rates for West Nile and St. Louis encephalitis viruses in Culex quinquefasciatus. Vectorborne Zoonotic Dis. 2009;9:281–6. doi: 10.1089/vbz.2007.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karpf AR, Lenches E, Strauss EG, Strauss JH, Brown DT. Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. J Virol. 1997;71:7119–23. doi: 10.1128/jvi.71.9.7119-7123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobson-Peters J, Yam AW, Lu JW, Setoh YX, May FJ, Kurucz N, et al. A new insect-specific flavivirus from Northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co-infected mosquito cells. PLoS One. 2013;8:e56534. doi: 10.1371/journal.pone.0056534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kent RJ, Crabtree MB, Miller BR. Transmission of West Nile virus by Culex quinquefasciatus Say infected with Culex flavivirus Izabal. PLoS Negl Trop Dis. 2010;4:e671. doi: 10.1371/journal.pntd.0000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolling BG, Olea-Popelka FJ, Eisen L, Moore CG, Blair CD. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology. 2012;427:90–7. doi: 10.1016/j.virol.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]