Abstract
Background & objectives:
Due to ever growing insecticide resistance in mosquitoes to commonly used insecticides in many parts of the globe, there is always a need for introduction of new insecticides for the control of resistant vector mosquitoes. In this study, larvicidal and adulticidal efficacies of three neonicotinoids (imidacloprid, thiacloprid and thiamethoxam) were tested against resistant and susceptible populations of Anopheles stephensi Liston 1901, Aedes (Stegomyia) aegypti Linnaeus, and Culex quinquefasciatus Say (Diptera: Culicidae).
Methods:
Laboratory-reared mosquito species were used. Insecticide susceptibility tests were done using standard WHO procedures and using diagnostic dosages of insecticide test papers and larvicides. Adulticidal efficacy of candidate insecticides was assessed using topical application method and larval bioassays were conducted using standard WHO procedure.
Results:
The results of topical application on 3-5 day old female mosquitoes indicated that resistant strain of An. stephensi registered lower LC50 values than the susceptible strain. Among the three insecticides tested, thiacloprid was found more effective than the other two insecticides. Culex quinquefasciatus registered lowest LC50 for imidacloprid than the other two mosquito species tested. In larval bioassays, the LC50 values registered for imidacloprid were in the order of Cx. quinquefasciatus <An. stephensi (SS) <An. stephensi (RR) <Ae. aegypti. In case of thiacloprid, the order of efficacy (LC50) was Cx. quinquefasciatus <An. stephensi (SS) <An. stephensi (RR), whereas in case of thiamethoxam, the larvicidal efficacy was in the order of An. stephensi (RR) <An. stephensi (SS) <Cx. quinquefasciatus.
Interpretation & conclusions:
The present study indicated that insecticide resistant strains of mosquito species tested showed more susceptibility to the three neonicotinoids tested, and the possibility of using neonicotinoids for the control of resistant mosquitoes should be explored.
Keywords: Aedes aegypti, Anopheles stephensi, Culex quinquefasciatus, imidacloprid, resistance, thiacloprid, thiamethoxam
Insecticides of different classes, namely organochlorines, organophosphates, carbamates and synthetic pyrethroids have been in use since last 2-5 decades in vector control programmes all over the world. Due to continued use of these insecticides, the vector species have developed multiple resistances to these insecticides. There is always a need for alternative insecticides for effective control of vector mosquitoes. The neonicotinoids are systemic toxins that target acetylcholine receptors in the insect nervous system. As per the Insecticide Resistance Action Committee Mode of Action (IRAC MoA) classification these are classified as nicotinic acetylcholine receptor (nAChR) agonists and grouped in 4A1. Imidacloprid was the first nicotinoid registered and was found effective in agriculture2. Neonicotinoids are unique from any other insecticides currently available for field use3, and have attracted attention due to their high efficacy, safety to mammals, low toxicity, no-cross resistance and unique mode of action4. These compounds have emerged as the fourth generation of pesticides replacing organophosphates, carbamates and pyrethroids, and have been used extensively for insect control4,5,6,7. Neonicotinoids cause irreversible blockage of post-synaptic nicotinergic acetylcholine receptors8. In general, these compounds possess low mammalian toxicity and are relatively non-toxic to non-target species9,10. These are highly effective in control of a wide range of insect pests11. Further, neonicotinoids are selective to insects because of the differential sensitivity of insect and vertebrate nACHR subtypes9.
Imidacloprid is a systemic, chloro-nicotinyl insecticide in use in agriculture with soil, seed and foliar applications for the control of sucking insects including rice hoppers, aphids, thrips, whiteflies, termites, turf insects, soil insects and some beetles. The chemical acts by interfering with the transmission of stimuli in the insect nervous system. It blocks nicotinergic pathway leading to the accumulation of acetylcholine, which may result in paralysis, and eventually death of the insect. It is reported effective both by contact and via stomach action12. In a study carried out by Paul et al13, imidacloprid had shown LC50 (lethal concentration) of 84 ng/ml against fourth instar larvae of Aedes aegypti Linnaeus. (Diptera: Culicidae), and in another study by Pridgeon et al14, imidacloprid registered LD50 of 7.7×10-4 μg/mg of mosquito in topical application against Ae. aegypti, 1.2×10–3 μg/mg against Culex quinquefasciatus Say (Diptera: Culicidae) and 3.8×10–4 μg/mg against Anopheles quadrimaculatus Say (Diptera: Culicidae). Rao et al15 using different analogues of imidacloprid on the larvicidal properties against Cx. quinquefasciatus showed that the analogues exerted more toxic effect than the pure imidacloprid and these showed good larvicidal efficacy on Cx. quinquefasciatus. In another study the LC50 against yellow fever mosquito was 0.03 mg/l for imidacloprid, 0.06 mg/l for acetamiprid and 0.007 mg/l for thiamethoxam and 0.11 mg/l for dinotefuran15. Bhinder et al16 compared the toxicity of imidacloprid and thiamethoxam in An. stephensi Liston (Diptera: Culicidae) and noticed measurable differences in internal transcribed spacer 2 (ITS2) sequences of control and treated mosquitoes indicating genetic damage. It was found that imidacloprid-treated mosquitoes had eight deletions, 29 insertions, 18 transitions and 33 transversions, whereas thiamethoxam-treated had 10 deletions, 8 insertions, 47 transitions and 68 transversions. All these studies demonstrated insecticidal efficacy of neonicotinoids in different combinations against different mosquito species. In the present study, an attempt was made to study the mosquito adulticidal and larvicidal efficacy of three neonicotinoids, namely imidacloprid, thiacloprid and thiamethoxam against insecticide resistant and susceptible mosquito adults and larvae.
Material & Methods
The study was conducted in the National Institute of Malaria Research (NIMR), New Delhi, India. The experiments were conducted at the Insecticide and Insecticide Resistance Laboratory recognized as WHO Collaborating Centre for Phase I Testing and Evaluation of Public Health Pesticides, at NIMR during January 2012 to February 2013. Technical grade imidacloprid (99.2%) and thiacloprid (98.4%) were provided gratis by M/s Bayer Crop Science Pvt. Ltd., Mumbai, and thiamethoxam (99.1%) by M/s Syngenta Corporation, Mumbai, India.
Mosquito species:
-
(i)
Anopheles stephensi (Sonepat): DDT-malathion-deltamethrin susceptible (SS), established in 1996 and still being colonized (306 generations as on January 2, 2012)
-
(ii)
An. stephensi (Goa): DDT-malathion-deltamethrin resistant (RR) established in 2009 and still being colonized (72 generations as on January 2, 2012)
-
(iii)
Culex quinquefasciatus (Sonepat): DDT-malathion- resistant and tolerant to deltamethrin established in 1999 and still being colonized (252 generations as on January 2, 2012)
-
(iv)
Aedes aegypti (Delhi): DDT-malathion-deltamethrin susceptible, established in 2009 (128 generations as on January 2, 2012)
All these mosquito species are being maintained as cyclic colonies in the Insectary of NIMR, New Delhi, for several years. These are characterized quarterly for insecticide susceptibility status. The mosquitoes are reared in closed rooms maintained at 27±2°C temperature and 70-80 per cent relative humidity with 14:10 h light and dark photoperiods; 10 per cent glucose soaked cotton pads are provided as food source for adults regularly and vertebrate blood for reproduction. Larvae were provided ground dog biscuits and yeast powder (3:2).
Determination of insecticide susceptibility: The laboratory-reared mosquito species were exposed to diagnostic dosages of different insecticides using WHO adult susceptibility kits and methods17 to the diagnostic dosages of DDT (4%), malathion (5%) and deltamethrin (0.05%). Insecticide test papers were procured from Vector Control Research Unit, University Sains Malaysia, Malaysia. The larval susceptibility was determined using diagnostic dosages of fenthion, malathion and temephos using WHO larval tests18. Diagnostic doses (in mg/l) used for different larvicides as recommended by WHO for different mosquito species are as follows: temephos, malathion and fenthion: 0.02, 3.125, 0.05, respectively for Ae. aegypti; 0.25, 1.0, 0.05, respectively for An. stephensi; and 0.02, 1.09, 0.05, respectively for Cx. quinquefasciatus.
Adulticidal efficacy (Topical application): These studies were carried out on the laboratory-reared DDT-malathion-deltamethrin susceptible and resistant mosquitoees. For the assays, 3-day old sugar-fed female An. stephensi, Ae. aegypti and Cx. quinquefasciatus were used. Different concentrations of insecticidal solutions (0.1 to 100 ppm) were prepared in acetone. Batches of 25 mosquitoes were anaesthetized for 25-60 sec with a steam of CO2 in an anaesthetic chamber and placed on cold plate maintained at 4°C. The weight of the average mosquito was determined in advance. Each concentration was tested against 100 mosquitoes and acetone control was run concurrently. Insecticidal solution (0.1 μl) was placed on the pronotum of thorax of the mosquito using micropipette. After applying the insecticide, the mosquitoes were transferred to holding tubes and kept for observation for 24 h in a climatic chamber maintained at 27°C temperature and 80 per cent relative humidity. Mortality was scored after 24 h and corrected by applying Abbott's formula19. The toxicity of the insecticide is expressed in ng/mg of mosquito.
Larvicidal efficacy of neonicotinoids: Larvicidal efficacy was tested by exposing late III or early IV instar larvae of different mosquito species to different concentrations of insecticidal solution in ethanol (ranging from 1 to 200 ppm). Six to eight concentrations were tested against each species and a minimum of four replicates (20 larvae in each replicate) were used for each concentration. One millilitre of the insecticidal solution was added to 99 ml of boiled and cooled tap water in a paper cup of 150 ml capacity. In each cup, 20 larvae were introduced with the help of strainer carefully. The larvae were treated in four replicates against each dose and mortality, pupal emergence, and moribund larvae were recorded at 24, 48 and 72 h intervals. The mortality data were subjected to log-probit analysis to calculate lethal doses. Dosages of neonicotinoids ranging from 0.01 to 2 mg/l were used in the studies.
Statistical analysis: Log probit analysis was used to calculate lC50 and LC90 values using SPSS v 18.0 software (SPSS Inc, Chicago, USA). Chi square test was used for testing the Pearson's goodness of fit among the dosages tested.
Results
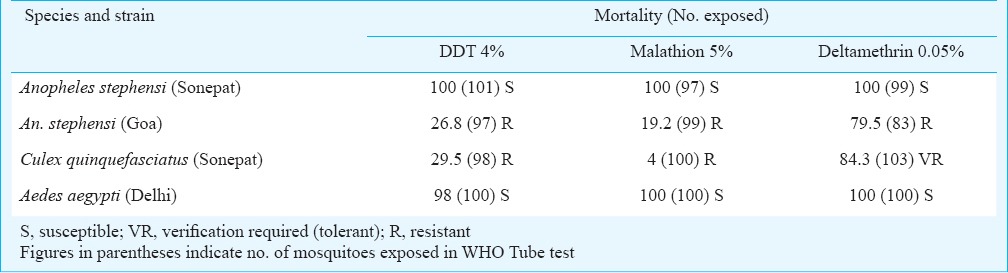
The results of insecticide susceptibility tests carried out on different strains of Ae. aegypti, Cx. quinquefasciatus and An. stephensi mosquitoes using WHO tube test are shown in Table I. The results indicated that An. stephensi (Sonepat) was susceptible to DDT, malathion and deltamethrin. An. stephensi (Goa) was resistant to DDT, malathion and deltamethrin. Culex quinquefasciatus was resistant to DDT and malathion; and verification required (tolerant) to deltamethrin (T). Aedes aegypti (Delhi) was found susceptible to DDT, malathion and deltamethrin.
Table I.
Mortality of mosquito strains exposed to WHO specified diagnostic concentration of different insecticides in WHO adult susceptibility tests
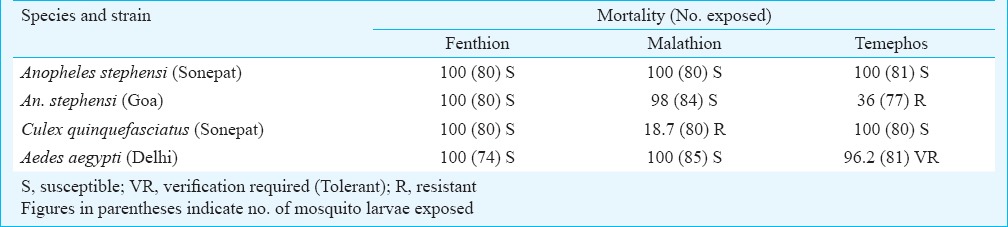
Results of larval susceptibility tests conducted on late III and early IV instar larvae of An. stephensi, Ae. aegypti and Cx. quinquefasciatus strains are shown in Table II. Susceptibility status of larvae of the three species tested to different WHO recommended diagnostic dosages of larvicides indicated that the larvae of An. stephensi (Sonepat) strain were susceptible to fenthion, malathion and temephos. The larvae recorded 100 per cent mortality in 24 h exposure to diagnostic dosages. An. stephensi (Goa) strain registered 100 per cent mortality in case of fenthion, 98 per cent mortality to malathion and only 36 per cent mortality to temephos indicating resistance to temephos. Culex quinquefasciatus larvae showed 100 per cent mortality to fenthion and temephos, whereas it was found resistant to malathion (< 20% mortality). In case of Ae. aegypti, 100 per cent mortality was reported in exposures to fenthion and malathion, whereas in case of temephos, 96.2 per cent mortality was reported indicating verification required status to this larvicide.
Table II.
Mortality of late III or early IV instar larvae of different mosquito strains exposed to WHO specified diagnostic concentrations of different larvicides in larval susceptibility tests for 24 h
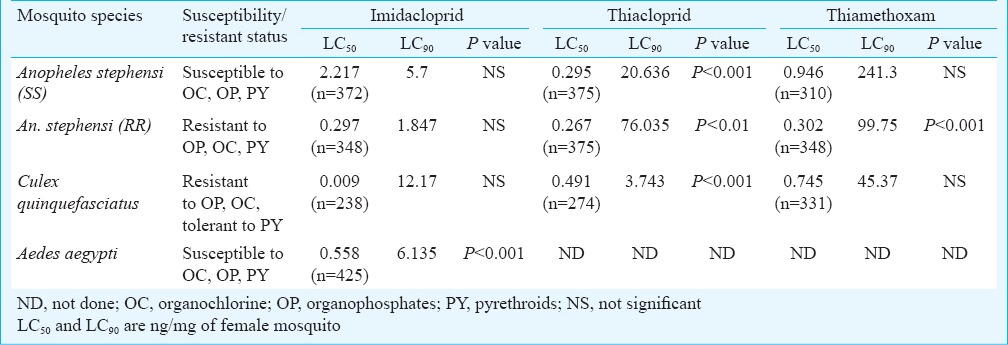
The results of topical application of three neonicotinoids against the three species of mosquitoes are shown in Table III. The LC50 values registered for An. stephensi susceptible strain against imidacloprid, thiacloprid and thiamethoxam were 2.217, 0.295 and 0.946 ng/mg of mosquito, respectively, whereas the resistant An. stephensi strain showed LC50 of 0.297, 0.267 and 0.302 ng/mg of mosquito, respectively to these insecticides. The results indicated that resistant strain of An. stephensi registered lower LC50 values than the susceptible strain. Among the three insecticides, thiacloprid was found more effective than the other two insecticides tested. Cx. quinquefasciatus registered lowest LC50 for imidacloprid than the other two mosquito species tested.
Table III.
Results of topical application of neonicotinoids against different mosquito strains
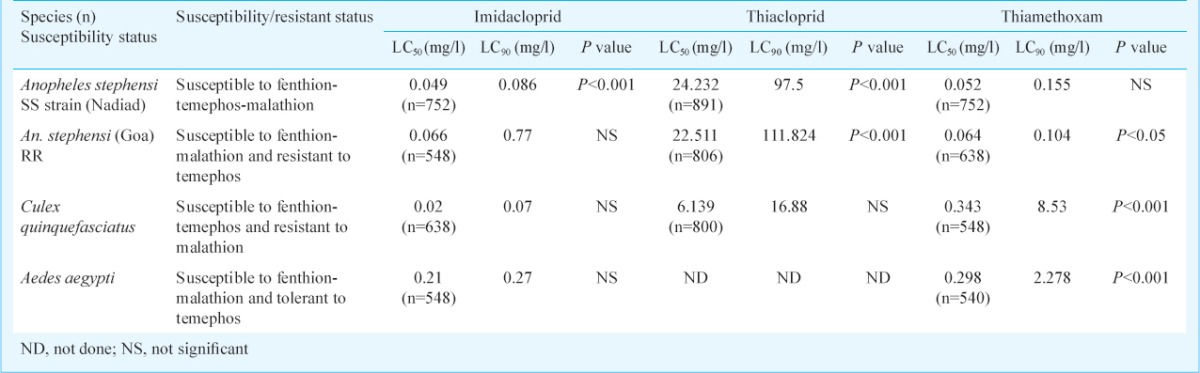
The results of larvicidal efficacy tests on three mosquito species against the three insecticides are shown in Table IV. The LC50 values registered for imidacloprid were in the order of Cx. quinquefasciatus <An. stephensi (SS) <An. stephensi (RR) <Ae. aegypti. In case of thiacloprid, the order of efficacy (LC50) was Cx. quinquefasciatus <An. stephensi (SS) <An. stephensi (RR). The larvicidal efficacy of thiamethoxam was in the order of An. stephensi (RR) <An. stephensi (SS) <Cx. quinquefasciatus.
Table IV.
Results of larval susceptibility tests with three neonicotinoids (72 h mortalities)
Discussion
Chemical insecticides are the mainstay in vector control programmes. Resistance to organochlorines such as DDT, organophosphates such as malathion and synthetic pyrethroids has been reported in many malaria vectors, and also in culicine mosquitoes due to constant selection pressure of insecticide use in agriculture as well as in public health programmes. There are limited options for management of insecticide resistance and there is a need to add new insecticides or combinations of insecticides for effective control of mosquitoes especially that have developed multiple resistances to insecticides of different classes. In the present study, an effort was made to assess the adulticidal and larvicidal efficacies of three neonicotinoids, namely imidacloprid, thiacloprid and thiamethoxam. The adulticidal toxicity (LC50) assessed by topical applications against both resistant and susceptible strains of An. stephensi was in the order of thiacloprid < thiamethoxam < imidacloprid indicating thiacloprid to be more toxic among the three neonicotinoids to adult mosquitoes. In contrast, against Cx. quinquefasciatus, imidacloprid showed increased toxicity followed by thiacloprid and thiamethoxam. In larvicidal bioassays, more toxicity was exhibited by imidacloprid followed by thiamethoxam and thiacloprid in An. stephensi, Cx. quinquefasciatus and Ae. aegypti.
The present study showed that the resistant strains registered lower LC50 values than the susceptible ones, indicating the possibility of using these to control the insecticide resistant vectors. In the study carried out by Darriet and Chandre20 using different combinations of neonicotinoids and piperonyl butoxide (PBO)+deltamethrin, the combinations produced higher mortality in resistant mosquitoes than the neonicotinoids alone.
Insecticide resistance in mosquito vectors is a growing concern in many countries, and there is an urgent need for search of new compounds with different modes of action which do not show cross resistance to insecticides being used in the vector control programmes like organochlorines, organophosphates, carbamates and pyrethroids. Absence of cross-resistance in neonicotinoids with pyrethroids, carbamates and organophosphates, and organochlorines makes them potential candidates for use in mosquito control activities21. The possibility of using neonicotinoids for mosquito control should be explored and it can be a viable option to control mosquitoes that have already become resistant to the insecticides being used. The susceptibility of insect populations to insecticides with new modes of action can be influenced by the previous exposure to insecticides22, the use of neonicotinoids for mosquito control appears to be less affected by existing resistant mechanisms because these are not being used in mosquito vector control programmes. Cross-resistance studies could not be carried out due to technical reasons and this was a limitation of the present study and certain tests could not be conducted on Ae. aegypti due to discontinuation of the colony owing to dengue threat in Delhi. Further investigations are required to study the toxicity of the compounds on non-target species that co-habit with mosquito larvae. Owing to their high mammalian safety the neonicotinoids could be an option for mosquito control.
Acknowledgment
The authors acknowledge the Indian Council of Medical Research for funding the study through intramural grants.
Footnotes
Conflicts of Interest: None.
References
- 1.Insecticide Resistance Action Committee (IRAC) Modes of action (MoA) classification scheme. (IRAC). Version 7.0, September. 2010. [accessed on July 17, 2011]. pp. 1–23. Available from: www.irac-online.org .
- 2.Palumbo JC, Horowitz AR, Prabhaker N. Insecticidal control and resistance management for Bemisia tabaci. Crop Prot. 2001;20:739–65. [Google Scholar]
- 3.Ware GW. The pesticide book. 5th ed. Fresno, CA, USA: Thompson Publications; 2000. p. 415. [Google Scholar]
- 4.Zhu Y, Lose MR, Watson GB, Sparks TC, Rogers RB, Huang JX, et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J Argic Food Chem. 2011;59:2950–7. doi: 10.1021/jf102765x. [DOI] [PubMed] [Google Scholar]
- 5.Babcock JM, Gerwick CB, Huang JX, Loso MR, Nakamura G, Nolting SP, et al. Biological characterization of sulfoxaflor, a novel insecticide. Pest Manag Sci. 2011;67:328–34. doi: 10.1002/ps.2069. [DOI] [PubMed] [Google Scholar]
- 6.Ohno I, Tomizawa M, Durkin KA, Casida JE, Kagabu S. Bis-neonicotinoid insecticides: observed and predicted binding interactions with the nicotinic receptor. Bioorg Med Chem Lett. 2009;19:3449–52. doi: 10.1016/j.bmcl.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Jeschke P, Nauen R, Schindler M, Elbert A. Overview of the status and global strategy for neonicotinoids. J Agric PC Food Chem. 2011;59:2897–908. doi: 10.1021/jf101303g. [DOI] [PubMed] [Google Scholar]
- 8.Bai D, Lummis SC, Leicht W, Breer H, Sattelle DB. Actions of imidacloprid and a related nitromethylene on cholinergic receptors of an identified insect motor neurone. Pestic Sci. 1991;33:197–204. [Google Scholar]
- 9.Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol. 2003;48:339–64. doi: 10.1146/annurev.ento.48.091801.112731. [DOI] [PubMed] [Google Scholar]
- 10.Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45:247–68. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- 11.Wollweber D, Tietjen K. Chloronicotinyl insecticides: a success of the new chemistry. In: Yamamoto I, Casida JE, editors. Nicotinoid insecticides and the nicotinic acetylcholine receptor. Tokyo, Japan: Springer; 1999. pp. 109–26. [Google Scholar]
- 12.Kidd H, James DR, editors. The agrochemicals handbook. 3rd ed. Cambridge, UK: Royal Society of Chemistry Information Services; 1994. [Google Scholar]
- 13.Paul A, Harrington LC, Scott JG. Evaluation of novel insecticides for control of dengue vector Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2006;43:55–60. doi: 10.1603/0022-2585(2006)043[0055:EONIFC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Pridgeon JW, Pereira RM, Becnel JJ, Allan SA, Clark GG, Linthicum KJ. Susceptibility of Aedes aegypti, Culex quinquefasciatus Say, and Anopheles quadrimaculatus Say to 19 pesticides with different modes of action. J Med Entomol. 2008;45:82–7. doi: 10.1603/0022-2585(2008)45[82:soaacq]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Rao MS, Murty USN, Gangadasu B, Raju BC, Ramesh CH, Kumar SB, et al. Larvicidal efficacy of neonicotinoid classes of compounds on Culex quinquefasciatus. J Entomol. 2008;5:45–50. [Google Scholar]
- 16.Bhinder P, Chaudhry A, Barna B, Kaur S. Imidacloprid and thiamethoxam induced mutations in internal transcribed spacer 2 (ITS2) of Anopheles stephensi. Toxicol Int. 2012;19:201–6. doi: 10.4103/0971-6580.97223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Test procedures for insecticide resistance monitoring in malaria vectors, bioefficacy and persistence of insecticides on treated surfaces: report of the WHO informal consultation, Geneva, 28–30 September 1998. WHO/CDS/CPC/MAL/98.12) Geneva: WHO; 1998. World Health Organization (WHO) [Google Scholar]
- 18.Geneva: WHO; 2005. World Health Organization (WHO). Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/GCDPP/2005.13; pp. 1–39. [Google Scholar]
- 19.Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–7. [Google Scholar]
- 20.Darriet F, Chandre F. Efficacy of six neonicotinoid insecticides alone and in combination with deltamethrin and piperonylbutoxide against pyrethroid-resistant Aedes aegypti and Anopheles gambiae (Diptera: Culicidae) Pest Manag Sci. 2013;69:905–10. doi: 10.1002/ps.3446. [DOI] [PubMed] [Google Scholar]
- 21.Corbel V, Duchon S, Zaim M, Hougard JM. Dinotefuran: a potential neonicotinoid insecticide against resistant mosquitoes. J Med Entomol. 2004;41:712–7. doi: 10.1603/0022-2585-41.4.712. [DOI] [PubMed] [Google Scholar]
- 22.Georghiou GP, Taylor CE. Pesticide resistance: strategies and tactics for management. Washington DC: National Academy Press; 1986. Factors influencing the evolution of resistance; pp. 157–69. [Google Scholar]


