Abstract
Background & objectives:
Dengue infection is endemic in several areas and the dengue virus is transmitted by Aedes mosquitoes. Thus, it becomes important to understand the breeding ecology of dengue vector and characterize the physicochemical parameters of its breeding habitat. The objective of this study was to analyze the physicochemical parameters of the breeding habitat of the dengue vector and to find out the nutrient composition of the habitat in and around Kolkata, West Bengal. In addition, a geographic information system (GIS) was used to map the disease prone areas for its effective management and prevention.
Methods:
Water samples were collected from various breeding habitats of Aedes mosquitoes of Kolkata and adjoining areas and were analysed for various physicochemical properties like acidity, alkalinity, hardness, electrical conductivity, total dissolved solids, concentration of chloride (Cl-), sodium (Na+), potassium (K+), fluoride (F-) in relation to larval prevalence.
Results:
Parameters like water pH, total dissolved solids, total hardness, electrical conductivity, concentration of chloride, sodium and potassium were seen to vary throughout the year. Certain parameters were found to be dependent on container type, like concentration of fluoride. Significant positive correlations were seen between per dip larval density and total dissolved solids (TDS) and electrical conductivity.
Interpretation & conclusions:
Water pH, electrical conductivity, total dissolved solids were seen to play a major role in the ovipositional preferences. Container type did not seem to affect TDS. Tyres had the highest TDS in most of the cases. Nutrient composition like sodium concentration was mostly found in the coconut shells, potassium concentration also showed the same. Thus, container type and various parameters and nutrients play a major role in determining where a gravid female mosquito will lay its eggs. It was observed that by altering various chemical and physical properties of breeding habitats it was possible to control the larvae survivability.
Keywords: Aedes aegypti, breeding habitat, GIS application, immature, Kolkata, physicochemical parameters
The role of Aedes aegypti and Ae. albopictus in the transmission of dengue and Chikungunya viruses is well documented. Human ecology, habits and behaviour greatly influence mosquito distribution, species relative abundance and survival1,2. Locations of probable breeding sites and water body conditions often lead mosquito groups and subgroups and species to choose their preferred habitats3. Widespread deforestation, climate change and increase in global trade have forced the mosquitoes worldwide to adapt to breeding in domestic and semi-domestic artificial container habitats4,5. The container inhabiting Aedes mosquito species is known to follow visual or olfactory cues to appropriate water containers and then use both chemical and physical factors in the water for selecting it for oviposition6. Some of the commonly used cues are colour and optical density of water, oviposition substrate, temperature, olfactory cues, and chemical cues provided by mosquito larvae7. Successful larval control requires a knowledge of the breeding ecology of mosquitoes including types of and preferences for larval habitats, spatial and temporal distribution of breeding sites, as well as the physical, biological and chemical characteristics of the habitats.
Aedes population as well as their density are the two main indicators that determine the risk of dengue in an area. The technique of measuring larvae and adult mosquito population requires enormous effort and time and this has to be done continuously. At the same time, the decision to implement vector control activities must be carried out immediately so that the incidence of dengue can be prevented. With the advancement of information technology, especially geographic information system (GIS), management and prevention activities for dengue can be done immediately. The use of GIS allows to integrate the environmental and time elements related to mosquito breeding and disease spreading without depending on indicators like mosquito population and mosquito density. GIS is a computer-based system that can integrate various spatial and non-spatial data to study the mosquito habitats. Several studies have been conducted utilizing GIS to analyze Aedes breeding habitats and dengue fever risk in India and various other countries8,9,10,11,12. We, therefore, undertook this study to determine the physical and chemical characteristics of mosquito breeding sites in dengue affected areas of Kolkata and its adjoining areas and spatial and temporal distribution of such breeding sites.
Material & Methods
Study area: The study was carried out from November 2012 to November 2014 in selected random sites of Kolkata and adjoining areas known for Ae. aegypti infection. Cross-sectional random sampling is required in case of mapping of the vector borne diseases to improve the efficacy of the vector survellience. Moreover, spatial mapping requires random site sampling so that it does not lead to biased samples13. Sites were selected on the basis of previous records of dengue infection in those areas. The selected sites were Jadavpur and Salt Lake City and adjoining areas from greater Kolkata, Singur and Santragachhi of Hooghly and Howrah districts, respectively for the summer season collection. During the monsoon season, Kamarhati (KMH), Dakshineswar (DAK), Belgharia (BEL) from North Kolkata and Jadavpur (JDP), Tollygunj (TOL) and Baghajatin (BGJ) from South Kolkata were selected for the study. Winter collection was done from Santragachhi, Udaynarayanpur, Jadavpur and Tollygunj. Kolkata has a tropical wet and dry climate with a maximum temperature during the summer months of May - June rising up to 24 - 42°C and the minimum temperature falling during winter months of December - January up to 8-26°C on an average. Relative humidity varied from 65-85 per cent. From June to September average rainfall in Kolkata is 158 mm. Howrah has a mean annual temperature, rainfall, and relative humidity of 32-39°C, 1461 mm, and 75 per cent, respectively. Hooghly has a tropical climate. The annual mean temperature is 26.8°C, although monthly mean temperatures range from 16 to 33°C and the maximum temperature in Hooghly often exceeds 38°C. Maximum rainfall occurs during the monsoon in August and the average annual total is above 1,500 mm.
Mosquito larval collection, identification and processing: In each of the selected sites mosquito larval sampling was done. During each survey, the habitat was first examined visually for the presence of any larvae, and then the larvae were captured by using a standard dipper which also varied according to the habitat size. As there is no fixed recommended dipper for the collection of Aedes larvae, dippers of varied sizes were used for different habitats (11.5 cm diameter and 350 ml capacity; 10 ml dipper of 2.5 cm diameter), pipettes and white plastic pans14,15. The conventional mosquito breeding habitats studied were earthen containers, coconut shells, tyres, tree holes, and plastic containers. The number of each type of habitat was counted and number of larvae present in each container was also noted down. Larvae were collected by dipping method and an average of 10-30 dips were taken depending upon the habitat size. The total number of dips made and the total number of larvae present in that habitat were noted down so as to calculate the per dip larval density. All larval samplings were done during morning (0900-1200 h) or in the afternoon (1400-1700 h)15. The mosquito larvae recovered were immediately preserved using 70 per cent alcohol and were brought to the Parasitology and Microbiology Research laboratory, department of Zoology, University of Burdwan, Burdwan, West Bengal, and kept for identification. Adult mosquitoes were collected using hand collection method from the various resting places, human habitations like brick houses and temporary hutments, cattle sheds and indoors during early morning hours (0500-0700 h) and afternoon (1700-1900 h) throughout the year, twice every month. They were immediately preserved for further taxonomic identification. Co-ordinates of the adult mosquito collection were also noted down by the same method for mapping.
Collection and fixing of water samples for physicochemical analysis: Water samples were collected in 500 ml sample containers from the breeding ground of all the selected sites where larvae were found. The water collected was fixed immediately using standard procedures for studying of various parameters of water16. Parameters like water temperature, environment temperature, relative humidity, pH, total dissolved solids (TDS) were measured on spot only using normal mercury thermometer (Hick's, India) hygroscope (HTC-1, China), pH meter (ELICO, India) and digital TDS meter (HI-Media, Mumbai, India), respectively. Concentrations of sodium and potassium were determined using flame photometer (Systronics Flame Photometer-130, India). All other parameters were done following the titrametric methods16.
Physicochemical analysis of water from larval habitats: Physicochemical analysis of those water samples was done which showed comparatively higher per dip larval density among all the container types surveyed. The fixed water samples were analyzed for the following parameters: acidity, alkalinity, hardness, electrical conductivity, total dissolved solids, concentration of chloride (Cl-), sodium (Na+), potassium (K+), fluoride (F-). All these analyses were done in the Parasitology and Microbiology laboratory, department of Zoology, University of Burdwan. The water parameters were analyzed following standard methods15.
Statistical analysis: Pearson's correlation was applied to find out significant association between per dip larvae density and total dissolved solids, electrical conductivity and fluoride concentration.
Results
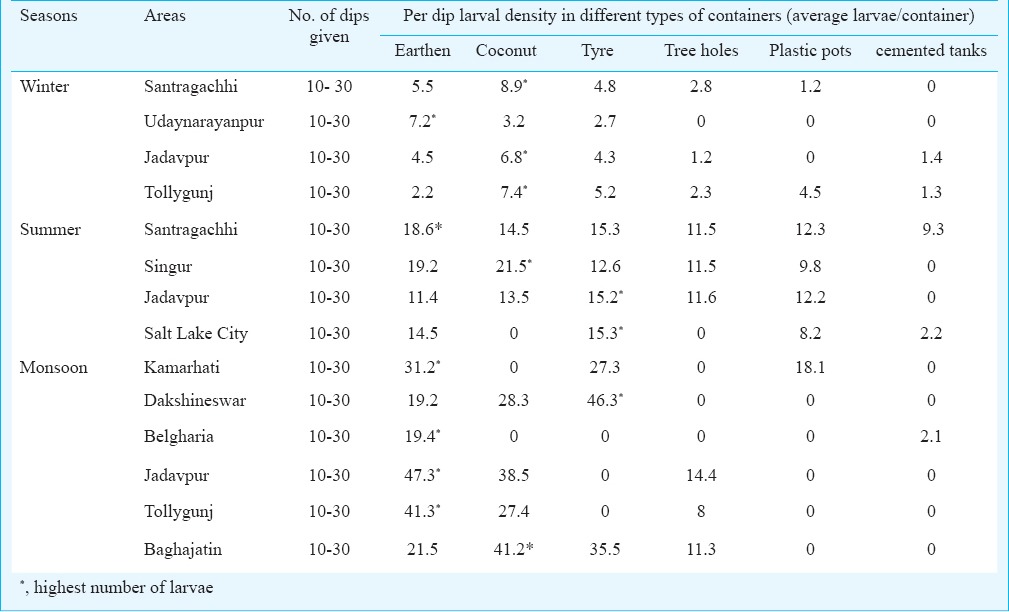
Physicochemical parameters of breeding habitats of Aedes sp. in and around Kolkata during summer, monsoon and winter seasons are shown in Tables I, II and III, respectively. Table IV shows average per dip larval density in different areas of Kolkata and adjoining areas in different containers. The environment temperature was found to be the highest in Salt Lake City and lowest in Jadavpur throughout the year, whereas the water temperature was highest in Salt Lake city (summer) and lowest in Santragachhi (winter). Water pH was found to be highest (alkaline) at Singur (8.19, summer) and lowest (acidic) in Santragachhi (5.67, summer). Highest water hardness was found in Jadavpur (3.91, summer) and lowest in Belgharia (0.23, monsoon). Baghajatin recorded the highest chloride concentration (549.5 ppm, monsoon) and whereas Udaynarayanpur had the lowest (30.74 ppm, winter). Fluoride concentration was recorded highest in Santragachhi (0.97 ppm, summer) and lowest in Baghajatin (0.12 ppm, monsoon). Potassium concentration was found to be highest in Singur (117.2 ppm, summer) and lowest in Tollygunj (7.98 ppm, winter). Sodium concentration was found to be highest in Singur (62.07 ppm, summer) and lowest in Belgharia (31.78 ppm, monsoon). Maximum TDS was found in the monsoon season in water logged tyres of Dakshineswar and minimum in the winter season in water logged coconut shells of Tollygunj whereas maximum electrical conductivity found in the monsoon season in water logged coconut shells of Baghajatin and minimum electrical conductivity found in the summer season in water logged coconut shells of Singur. During winter the highest average per dip larval density was found in the water logged coconut shells; during summer water logged tyres scored the highest and earthen containers of monsoon season showed the highest average per dip larval density.
Table I.
Physicochemical parameters of breeding habitats of Aedes sp. in and around Kolkata during summer season (November 2012 - December 2013)
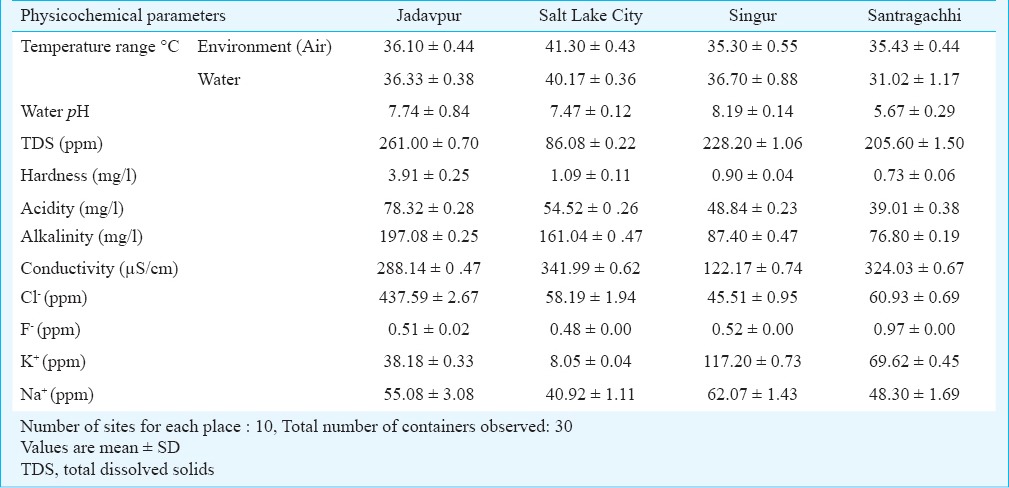
Table II.
Physicochemical parameters of breeding habitat of Aedes sp. in and around Kolkata during monsoon season (November 2012 - December 2013)
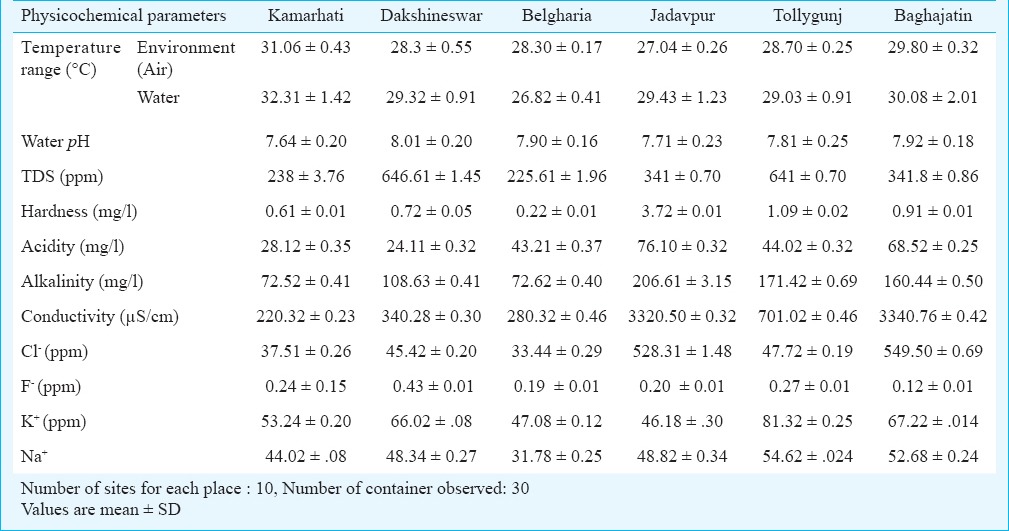
Table III.
Physicochemical parameters of breeding habitat of Aedes sp. in and around Kolkata during winter season (November 2012 - December 2013)
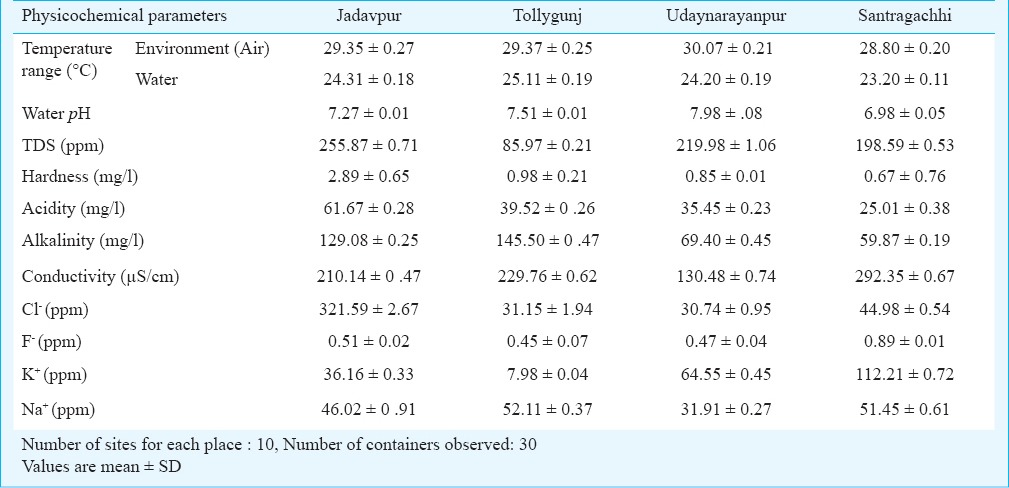
Table IV.
Per dip larval density in different areas of Kolkata and adjoining areas in different containers during the year (November 2012 - December 2013)
A significant and positive correlation was seen between per dip larval density and TDS (P=0.02, r=0.755, Fig. 1) and between per dip larval density and electrical conductivity (P=0.014, r=0.635, Fig. 2) throughout the year. A non significant negative correlation was recorded between fluoride concentration and per dip larval density (r= - 0.565, Fig. 3) throughout the year. No significant difference was found between per dip larval density and the remaining parameters.
Fig. 1.
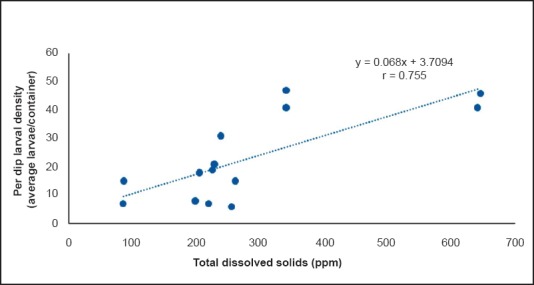
Correlation between per dip larvae density and total dissolved solids.
Fig. 2.
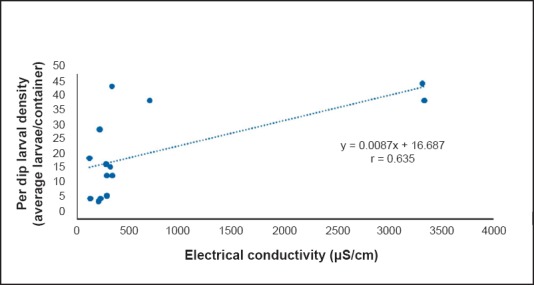
Correlation between per dip larval density and electrical conductivity.
Fig. 3.
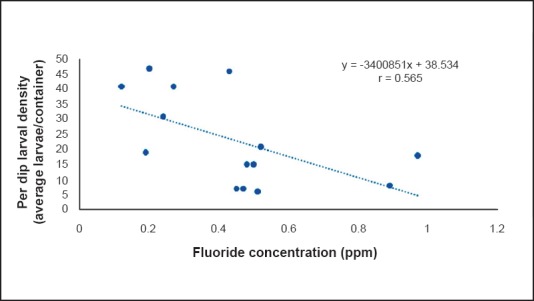
Correlation between per dip larval density and fluoride concentration.
Maximum per dip larval density was seen in the water logged coconut shells of Singur during summer, whereas larval density was maximum for earthen pots and tyres in Santragachhi and Jadavpur, Salt Lake City, respectively. Cemented tanks and tree holes had the lowest larval density. During monsoon; Kamarhati, Belgharia, Jadavpur and Tollygunj showed earthen pots to have the highest per dip larval density; whereas tyres of Dakshineswar and coconut shells of Baghajatin had the highest per dip density of larvae. Cemented tanks had the lowest larval density in almost every site, except for Belgharia. Very few larvae were found in water logged tree holes of Kamarhati and Dakshineswar. Unlike that of North Kolkata, tree holes were found positive for a significant amount of larvae in Jadavpur, Tollygunj and Baghajatin of South Kolkata. The water logged earthen pots of Udaynarayanpur showed the maximum larval density during winter, whereas in Santragachhi, Tollygunj and Jadavpur water logged coconut shells showed the highest larval density. Lowest or no larvae were found in the cemented tanks of all the four areas. No larvae were captured from the plastic pots and tree holes in case of Udaynarayanpur.
Discussion
For effective vector control measures the water ecology is essential, including the physical, biological and chemical properties of water, as well as, the spatial and temporal distribution of the mosquito breeding sites and also larval habitat preferences17. Previous study has shown that water chemistry and presence of certain nutrient concentrations are important determinant as to whether or not the female mosquitoes will oviposit and for the further development of the immatures18. The larvae are seen to adapt to a wide range of temperature. Due to the poikilothermic nature of the mosquito immatures, temperature plays an important role in their successful development. Apart from the other factors like concentration of various nutrients and minerals, temperature is the main factor responsible during larval growth and development19. Higher temperature of the water may result in rapid development of the mosquito immatures but causes decrease in their size20, while the same rise in temperature produces less adults21. The pH of water was seen to range between acidic to alkaline. MacGregor22 recorded acidophile and alkalinophile mosquito larval species, like in the present study. pH of the water was found to vary with the varying container types. This may be probably due to the nature of the container. Mosquito larvae grow optimum in water of near neutral pH 6.8 to a pH of 7.2, since this pH weakens the egg shells for emerging of the first larval instar23. As suggested by reports from Nigeria, a pH of 7.4 was found to be ideal for Aedes mosquitoes24,25. In the present study we found larvae to survive at a water pH of 8.22. This may be because with the changing environment mosquitoes are becoming resistant to their lethal environment and are starting to cope up with changes in their own genetic level. Along with these physical factors various chemical factors and nutrient composition also contribute to mosquito breeding site selection, such as total dissolved solids, total hardness, conductivity, concentration of fluoride, chloride, phosphate, sodium, potassium, etc17,26. Our study showed that water logged coconut shells and tyres were the most preferred breeding sites throughout the year, with a few exceptions of cemented tanks and earthen pots. Parameters like total dissolved solids, hardness, electrical conductivity, alkalinity, concentration of fluoride, chloride, potassium, and sodium were found to be highest in tyres and coconut shells. Coconut shells are rich in organic content and are ideal breeding place. Probably the high nutrient composition of the coconut shells attracted more of the female mosquitoes. Moreover, the small orifice of the coconuts and low illumination makes them ideal breeding spots for Aedes sp27. Discarded and unused tyres accumulate water naturally either through rain or through other means and provide a good breeding opportunity for the female mosquitoes. Tyres resist desiccation and eggs hatch after being transported from one place to another28,29. The larval density is not only habitat dependent but also depends upon the water chemistry and nutrition content. Electrical conductivity was found to be very high in the coconut shells of Baghajatin during the monsoons. Such high conductivity suggests that it must have something to do with oviposition preferences30. Thus, the present study shows that environmental manipulation can be an effective vector control measure. Altering the physicochemical parameters and removing the potential breeding sites can also be a part of the control programme.
Acknowledgment
The authors acknowledge the Department of Environment, West Bengal, for financial assistance and thank the University of Burdwan for providing facilities to carry out their work.
Footnotes
Conflicts of Interest: None.
References
- 1.Gilliet JD. In: Mosquitoes: the world naturalist. Clay R, editor. London: Weidenfeld and Nicholson; 1971. pp. 131–44. [Google Scholar]
- 2.Evans AM. London: British Museum (Natural History); 1938. Mosquitoes of the Ethiopian region. II. Anophelini - adults and early stages; pp. 368–79. [Google Scholar]
- 3.Shannon RC. The environment and behavior of some Brazilian mosquitoes. Proc Entomol Soc Wash. 1931;33:1–27. [Google Scholar]
- 4.Gubler DJ, Reiter P, Ebi KL, Yap W, Nasci R, Patz JA. Climate variability and change in the United States potential impacts on vector- and rodent-borne diseases. Environ Health Perspect. 2001;109(Suppl 2):223–33. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, Fontenille D. Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control. Parasite. 2008;15:3–13. doi: 10.1051/parasite/2008151003. [DOI] [PubMed] [Google Scholar]
- 6.Muir DA. Anopheline mosquitoes: vector reproduction, lifecycle and biotype. In: Wernsdorfer WH, McGregor I, editors. Malarial principles and practice of malariology. London: Churchill Livingstone; 1988. pp. 431–51. [Google Scholar]
- 7.Trexler JD, Apperson CS, Schal C. Laboratory and field evaluations of oviposition responses of Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) to oak leaf infusions. J Med Entomol. 1998;35:967–76. doi: 10.1093/jmedent/35.6.967. [DOI] [PubMed] [Google Scholar]
- 8.Aziz S. Geographic Information System (GIS) Application to identify high risk area of dengue and dengue hemorrhagic Fever in Georgetown, Penang. PhD Thesis. Universiti Sains Malaysia, Geography Section. 2008 [Google Scholar]
- 9.Bohra A, Andrianasolo H. Application of GIS in modeling of dengue risk based on sociocultural data: Case of Jalore, Rajasthan, India. Dengue Bull. 2002;25:92–102. [Google Scholar]
- 10.Ali M, Wagatsuma Y, Emch M, Breiman R. Use of a geographic information system for defining spatial risk for dengue transmission in Bangladesh: role for Aedes albopictus in an urban outbreak. Am J Trop Med Hyg. 2003;69:634–40. [PubMed] [Google Scholar]
- 11.Wu PC, Lay JG, Guo HR, Lind CY, Lung SC, Su HJ. Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci Total Environ. 2009;407:2224–33. doi: 10.1016/j.scitotenv.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Wen TH, Lin NH, Lin CH, King CC, Su MD. Spatial mapping of temporal risk characteristics to improve environmental health risk identification: a case study of a dengue epidemic in Taiwan. Sci Total Environ. 2006;367:631–40. doi: 10.1016/j.scitotenv.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 13.European Centre for Disease Prevention and Control (ECDC). Guidelines for the surveillance of native mosquito species in Europe. ECDC Tech. Rep. Stockholm: ECDC. 2014 [Google Scholar]
- 14.Sharma RS. Breeding habitats and natural infestations of anopheline larvae in Gurgaon urban, India. Mosq Borne Dis Bull. 1990;7:99–104. [Google Scholar]
- 15.Service WM. London: Chapman and Hall; 1993. Mosquito ecology: field sampling methods; pp. 1–988. [Google Scholar]
- 16.21st ed. Washington DC: APHA; 2005. American Public Health Association (APHA). Standard methods for the examination of water and wastewater. [Google Scholar]
- 17.Olayemi IK, Omalu IC, Famotele OI, Shegna SP, Idris B. Distribution of mosquito larvae in relation to physico-chemical characteristics of breeding habitats in Minna, North Central Nigeria. Rev Infect. 2010;1:49–53. [Google Scholar]
- 18.Piyaratne MK, Amerasinghea FP, Amerasinghe PH, Konradsen F. Physico-chemical characteristics of Anopheles culicifacies and Anopheles varuna breeding water in a dry zone stream in Sri Lanka. J Vector Borne Dis. 2005;42:61–7. [PubMed] [Google Scholar]
- 19.White GB. Anopheles gambiae complex and disease transmission in Africa. Trans R Soc Trop Med Hyg. 1974;68:278–301. doi: 10.1016/0035-9203(74)90035-2. [DOI] [PubMed] [Google Scholar]
- 20.Bayoh MN, Lindsay SW. Effect of temperature on the development of the aquatic stages of Anopheles gambiae sensu stricto (Diptera: Culicidae) Bull Entomol Res. 2003;93:375–81. doi: 10.1079/ber2003259. [DOI] [PubMed] [Google Scholar]
- 21.Bayoh MN, Lindsay SW. Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Med Vet Entomol. 2004;18:174–9. doi: 10.1111/j.0269-283X.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 22.MacGregor ME. Mosquito surveys. London: Welcome Bureau of Scientific Research; 1927. p. 282. [Google Scholar]
- 23.Okogun GR, Anosike JC, Okere AN, Nwoke BE. Ecology of mosquitoes of midwestern Nigeria. J Vector Borne Dis. 2005;42:1–8. [PubMed] [Google Scholar]
- 24.Adebote DA, Oniye SJ, Ndams IS, Nache KM. The breeding of mosquitoes (Diptera: Culicidae) in peridomestic containers and implication in yellow fever transmission in villages around Zaria, Northern Nigeria. J Entomol. 2006;3:180–8. [Google Scholar]
- 25.Afolabi OJ, Ndams IS, Mbah CE, Kogi E. The effects of alteration of pH on the breeding characteristics of mosquitoes in phytotelmata in Ahmadu Bello University Zaria, Nigeria. Int J Biosci. 2010;5:32–6. [Google Scholar]
- 26.Oyewole IO, Momoh OO, Anyasor GN, Ogunnowo AA, Ibidapo CA, Oduola OA, et al. Physico-chemical characteristics of Anopheles breeding sites: Impact on fecundity and progeny development. Afr J Environ Sci Technol. 2009;3:447–52. [Google Scholar]
- 27.Gubler DJ. Studies on the comparative oviposition behavior of Aedes (Stegomyia) albopictus and Aedes (Stegomyia) polynesiensis Marks. J Med Entomol. 1971;8:675–82. doi: 10.1093/jmedent/8.6.675. [DOI] [PubMed] [Google Scholar]
- 28.Medlock JM, Avenell D, Barrass I, Leach S. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. J Vector Ecol. 2006;31:292–304. doi: 10.3376/1081-1710(2006)31[292:aotpfs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Straetemans M. ECDC Consultation group on vector-related risk for Chikunggunya virus transmission in Europe. Vector related risk mapping of the introduction and establishment of Aedes albopictus in Europe. Euro Surveill. 2008;13:pii 8040. [PubMed] [Google Scholar]
- 30.Chen CD, Nazni WA, Seleena B, Moo JY, Azizah M, Lee HL. Comparative oviposition preferences of Aedes (Stegomyia) aegypti (L) to water from storm water drains and seasoned tap water. Dengue Bull. 2007;31:124–30. [Google Scholar]


