Abstract
Background:
Prevention of ventilator-associated pneumonia is a healthcare goal. Although data is inconsistent, some studies suggest that oral chlorhexidine may decrease rates of pneumonia in mechanically-ventilated patients. We sought to assess the rate of pneumonia in the Surgical Intensive Care Unit (SICU) pre and post implementation of routine chlorhexidine mouthwash prophylaxis.
Materials and Methods:
A retrospective cohort study was conducted, including patients between 1/1/2009 and 12/31/2009 who did not receive chlorhexidine mouthwash compared to patients that received prophylactic chlorhexidine mouthwash between 3/1/2010 and 2/28/2011. The primary outcome of the study was rate of probable ventilator-associated pneumonia (VAP) for the pre-chlorhexidine implementation cohort compared to post-implementation, using the 2013 Center for Disease Control definitions. Mechanically ventilated patients with respiratory cultures were screened for inclusion in the study. Secondary endpoints included duration of mechanical ventilation, in-hospital mortality, ICU and hospital length of stay. Statistical analysis was conducted by Fisher's exact test for nominal data and Mann-Whitney U test for continuous data.
Results:
A total of 1780 mechanically ventilated patients in the pre-chlorhexidine group and 1854 in the post-chlorhexidine group were screened for inclusion. Of the 601 mechanically ventilated patients that were further evaluated for inclusion; 158 patients (26.3%) had positive cultures and were included in the study (94 pre-group and 64 post-group). The rate of probable VAP was significantly decreased in the post-group compared to the pre-group (1.85% pre vs 0.81% post, P = 0.0082).
Conclusion:
Use of chlorhexidine mouthwash prophylaxis may reduce rates of probable VAP. Further study is warranted.
Keywords: Chlorhexidine, ICU, prevention, ventilator-associated pneumonia
INTRODUCTION
An estimated 300,000 patients in United States hospitals are mechanically ventilated each year, placing them at risk for ventilator-associated pneumonia (VAP).[1] Up to 20% of mechanically ventilated patients may develop VAP, resulting in prolonged hospital length of stay, increased healthcare costs, and risk of disability in those that survive hospitalization.[2,3,4,5] Overall, attributable mortality of VAP is approximately 9%. Efforts to reduce the development of VAP have primarily focused on sedation management, spontaneous breathing trials, stress ulcer and venous thromboembolism prophylaxis, head of bed elevation and oral hygiene.[6]
Chlorhexidine (Peridex®) is a topical antibiotic oral rinse with activity against both gram-positive and gram-negative organisms, which has shown efficacy in some, but not all, studies for VAP prevention.[7,8,9,10,11,12] These studies have found significant reductions in nosocomial infection rates, incidence of pneumonia and significantly less gram-negative organisms from the use of chlorhexidine 0.12% solution.[7,9] Although many studies favor chlorhexidine use, other similar studies have shown comparable rates of VAP between the chlorhexidine and control groups.[13,14] Due to limitations with available literature and lack of consistency among efficacy outcomes, the 2005 Infectious Disease Society of America (IDSA) Guidelines for hospital-acquired, ventilator and healthcare-associated pneumonia does not recommend routine use of chlorhexidine until more data becomes available.[15] In contrast, the Society of Critical Care Medicine strongly supports the use of VAP prevention bundles, which incorporates the use of topical oral chlorhexidine as an intervention championed by the Institute for Healthcare Improvement.[16]
The objective of this study was to assess the rate of probable VAP in mechanically ventilated SICU patients before and after implementation of routine chlorhexidine mouthwash prophylaxis using the 2013 Centers for Disease Control guideline definitions.
MATERIALS AND METHODS
Data collection and assessment
This retrospective cohort study assessed ventilator-associated events prior to and following the implementation of chlorhexidine mouthwash in the 44-bed surgical intensive care unit (SICU) in a University Medical Center. Institutional review board (IRB) approval was obtained from the University Office of Responsible Research Practices Institutional Review Board, and was carried out with the ethical standards set forth in the Helsinki Declaration on 1975.
Definitions
The primary outcome was the development of probable VAP based on the 2013 Center for Disease Control definition, with VAP defined as pneumonia occurring >48 hours following endotracheal intubation.[15] According to the 2013 CDC Ventilator-Associated Event (VAE) guidelines, a Ventilator-Associated Condition (VAC) is the initial surveillance tier, which confirms the patient to be mechanically ventilated for at least 2 calendar days of stable or decreasing daily minimum positive end-expiratory pressure (PEEP) or fraction of inspired oxygen (FIO2); then followed by at least 2 days of increased daily minimum PEEP ≥3 cm H2O greater than the daily PEEP during the baseline period or where the increase in the daily minimum FIO2 is ≥0.20 (20% points in oxygen concentration) greater than the daily minimum FIO2 during the baseline period. The second tier, Infection-related Ventilator-Associated Complication (IVAC) is defined as evidence of an abnormal white blood cell count (≥12,000 cell/mm3 or ≤4,000 cell/mm3) or abnormal temperature (>38°C or <36°C) and initiation of a new antibiotic that is continued for at least 4 days. Possible and probable VAP are defined as the third tier of the VAE surveillance guidelines, which attempts to identify the subset of IVAC patients with respiratory infections that include evidence of purulent respiratory secretions and/or positive results of microbiological tests performed on respiratory tract specimens. Possible VAP is the presence of purulent secretions or a positive lower respiratory tract culture. Probable VAP is defined as increased oxygenation demand as described in IVAC above with confirmation of purulent secretions and >10,000 cfu/ml from bronchoalveolar lavage (BAL).
In the SICU, diagnosis of pneumonia in mechanically ventilated patients is standardized with protected-catheter quantitative BAL cultures based on clinical and radiographical symptoms. In 2007, our institution developed and implemented a ventilator bundle that included oral care (oral care mouth swabs every 4 hours and as needed, brushing every 12 hours and subglottic suctioning every eight hours), venous thromboembolism prophylaxis, stress ulcer prophylaxis, sedation management and spontaneous breathing trials. Routine use of chlorhexidine mouthwash (15 ml, twice daily) was initiated in all mechanically ventilated patients with tracheostomies in the SICU in February 2010 as a method to reduce ventilator-associated pneumonia (VAP). This study evaluated probable and possible VAP in mechanically ventilated SICU patients with a positiveBAL prior to and following the addition of chlorhexidine mouthwash to the ventilator bundle. All mechanically ventilated patients admitted to the SICU between January 1, 2009-December 31, 2009 and March 1, 2010 – February 28, 2011 were screened for BAL cultures. The pre-group comprised patients before implementation of chlorhexidine mouthwash (January 1, 2009 – December 31, 2009) while the post-group included patients who received prophylactic chlorhexidine mouthwash every 12 hours as part of the VAP prevention protocol (March 1, 2010 – February 28, 2011). Patients were included for analysis if they were mechanically ventilated for ≥2 days, had a positive bacterial quantitative BAL culture within 2 days of the onset of worsening oxygenation. Patients were excluded from analysis if they were less than 18 or greater than 89 years of age, pregnant, incarcerated or had care withdrawn during treatment.
Data collected included demographics (age, gender, weight, height), baseline vitals/Clinical Pulmonary Infection Score (CPIS), BAL cultures and sensitivities, chest radiograph (CXR) results, ventilator status and requirements, sputum data, duration and type of antibiotic therapy, mechanical ventilator duration, length of ICU and hospital stay, and mortality. BAL cultures collected on ICU day 5 or greater will also be evaluated. All patient data were obtained from our institutional Information Warehouse (IW), electronic patient information system [Integrated Healthcare Information System (IHIS)] and Essentris medical records.
Statistical analysis
The primary outcome of the study was rate of probable VAP prior to and following chlorhexidine mouthwash implementation. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 20 (IBM Armonk, NY). Categorical variables were analyzed using Chi-square statistics and Fisher's exact test and are presented as number and percentage. Continuous data with normal distribution was assessed using the Student's t-test and is presented as mean ± standard deviations, while non-parametric data was analyzed using Mann-Whitney U and is presented as median [intra-quartile ranges (IQR)]. Prior to initiation of the study, statistical significance was set for a P value <0.05.
RESULTS
In the year prior to implementation of chlorhexidine mouthwash there were 2550 patients admitted to the SICU including 1780 patients that received mechanical ventilation for >48 hours while 2647 admissions in the post-group included 1854 patients that received mechanical ventilation for >48 hours. A total of 601 patients that had BAL cultures screened were evaluated for inclusion into this study. A total of 158 patients met inclusion criteria for analysis, with 94 patients in the pre-group and 64 patients in the post-group. Out of the 601 patients screened, 386 patients (64.2%) had no bacterial growth, 34 patients (5.6%) had non-quantitative cultures, and 23 patients (3.8%) were no longer in the SICU to complete the study.
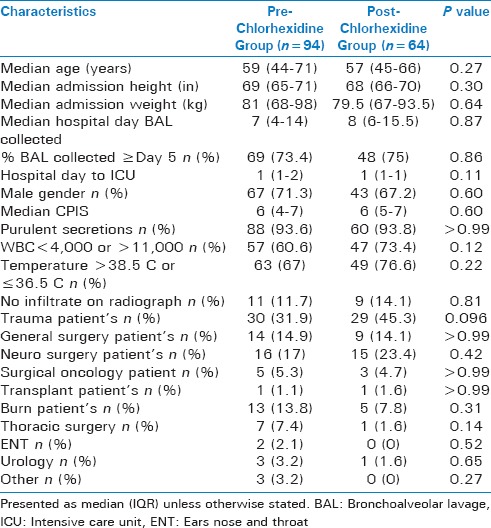
The majority of patients were male with a median age of 57 (44.5-68.5) years. Baseline characteristics did not differ significantly between groups although there was a trend towards more trauma patients in the post-group [Table 1]. The majority of patients were admitted to the SICU on hospital day 1 with a similar median time to BAL collection of 8 days in the pre-group and 7 in the post-group. Median CPIS on day of BAL collection was 6 for both groups.
Table 1.
Baseline demographics
Overall 100 patients did not experience an increase in minimum PEEP ≥3 cm H2O or FIO2 by ≥0.20 for at least 2 days (50 in pre-group and 40 in post group, P = 0.24), and 13 patients (8 pre-group and 5 post-group, P = 0.42) did not have an abnormal WBC or temperature [Figure 1]. The rate of possible VAP did not differ between groups (3 events in 1780 patients in the pre-group versus 4 in 1854 patients in the post-group, P > 0.99, Figure 2). There was a significant decrease in rate of probable VAP from 1.85% in the pre-group (33 events in 1780 patients) to 0.81% in the post-group (15 events in 1854 patients P = 0.0082). There were no significant differences in secondary outcomes between groups. The median duration of mechanical ventilation was 15 days in both the pre-group [9-33] and the post-group [10-22] (P = 0.4). Median ICU length of stay for the pre-group was 22 [13-35] days compared to 18 [13-25] days in the post-group (P = 0.18), and the median hospital length of stay was 28 [16-42] days and 23 [17-29] days (P = 0.22), respectively. There was similar mortality between both groups (25.5% pre-group versus 15.6% in the post-group, P = 0.17).
Figure 1.
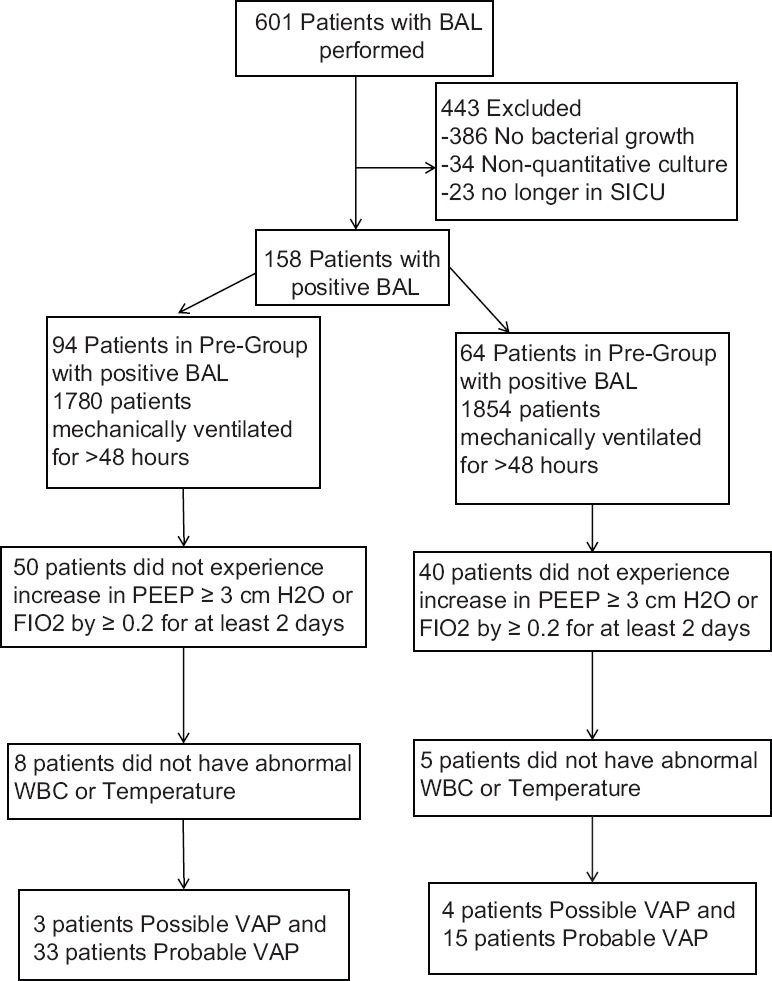
Patient Selection
Figure 2.
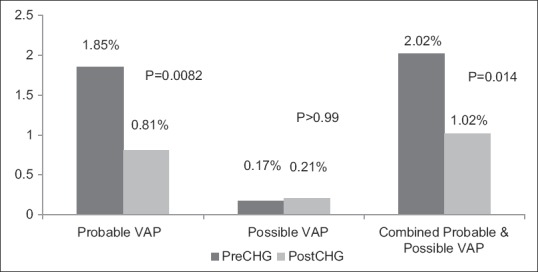
Incidence of Probable and Possible Ventilation
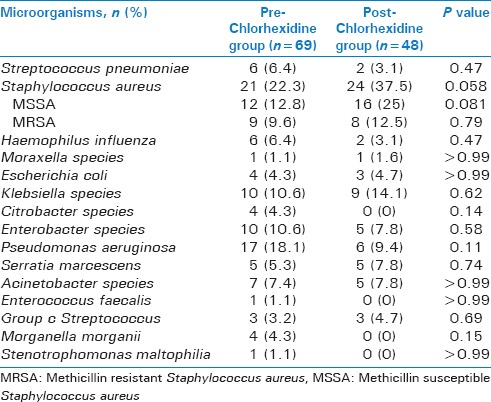
Overall microbiology was similar with the exception of significantly more Staphylococcus aureus in the post-group [Table 2]. While there was significantly more methicillin sensitive Staphylococcus aureus (MSSA) in the post-group, there was no difference between the two groups in rates of methicillin resistant Staphylococcus aureus (MRSA). There was a trend towards less Pseudomonas aeruginosa in the post-group (23.4% pre vs 10.9% post, P = 0.06). The groups also had similar rates of polymicrobial growth, at 39.4% in the pre chlorhexidine group and 31.3% in the post-group (P = 0.32). Microbial data was similar for patients that had BAL collection on SICU day 5 or later, including a trend toward increased rates of Staphylococcus aureus in the post-group, again driven by trend towards increased MSSA rather than MRSA [Table 3]. In addition, the rates or polymicrobial pneumonia and trend for less Pseudomonas aeruginosa in the post-group were also similar for patients with BAL collection on SICU day 5 or later compared to all patients regardless of BAL collection day.
Table 2.
Microorganisms for all patients
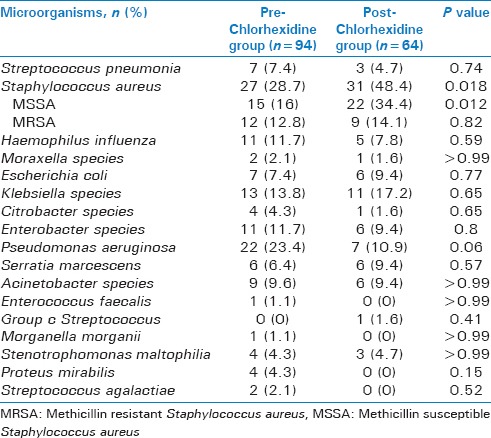
Table 3.
Microorganisms for patients with BAL collected on ICU day 5 or greater
DISCUSSION
Our results demonstrated that the routine addition of chlorhexidine mouthwash, as part of a ventilator management bundle, was associated with decreased development of probable VAP in SICU patients. There was no difference in secondary outcomes including duration of mechanical ventilation, in-hospital mortality, ICU and hospital length of stay. To our knowledge this is the first study that used the 2013 CDC VAE definitions for the diagnosis of VAP to assess the impact of oral chlorhexidine use.
Previously reported studies describing the use of chlorhexidine to prevent VAP have yielded mixed results. The findings in our study are consistent with a previously published study in mechanically ventilated cardiac surgery patients, in which patients who received chlorhexidine mouthwash demonstrated a 69.1% reduction in respiratory tract infections.[9] However, some studies infer little or no additional benefit with oral care such as prophylactic chlorhexidine in non-cardiac surgery patients, while other studies suggest that it has a positive impact on clinical outcomes.[11,17,18] A study including patients from 3 separate ICUs (medical respiratory, neurosurgical and surgical trauma) at a university medical center found no clinical benefit to chlorhexidine prophylaxis based on development of VAP through utilization of CPIS.[9] Patients that received chlorhexidine had a significantly lower CPIS at day 3, but failed to show a difference on days 5 and 7. A total of 547 patients were enrolled but 249 patients were evaluated on day 3 and 158 and 109 patients on days 5 and 7, respectively. The use of CPIS to diagnose VAP has been criticized because of inter-observer variability, and lower sensitivity and specificity in trauma, burn, surgical and medical populations.[19] Reliance on this scoring system may have contributed to conclusions opposite from our study. Although we did collect CPIS scores at the time of BAL collection, our primary endpoint utilized the CDC definition of probable VAP and a single physician interpreted chest radiographs for all analyzed patients.
In comparison to historical data, this study appeared to represent a typical SICU patient population for our institution. Overall the microbiology data was similar between groups except for the development of Staphylococcus aureus which was driven by differences in rates of MSSA. Patients are frequently admitted to our SICU after an acute event, which was reflected by many of our patient population primarily being admitted to the SICU upon hospital admission. Such acute events can put patients at risk for aspiration pneumonia which may explain the higher rate of MSSA compared to MRSA than what is normal from the institution. There was no difference in microbiological data for patients who had been in the ICU 5 or more days when BAL was obtained in total Staphylococcus aureus, MSSA or MRSA between groups. However, rates of Haemophilus influenza decreased significantly in those that had BAL obtained after five or more days in the SICU with a high trauma population, suggesting that community acquired or aspiration pneumonia may have resulted in higher rates of early pneumonias.
Similar to previously published studies, we saw a reduction in gram negative infections, specifically Pseudomonas aeruginosa.[7,9] In regards to chlorhexidine administration, there was a trend for less Pseudomonas aeruginosa in those routinely administered chlorhexidine. Traditionally, Pseudomonas aeruginosa has been the second most common cause of SICU acquired infections in our medical center. Not only was there a trend towards decreased incidence in the post-group, Pseudomonas aeruginosa dropped from second most common pathogen in the pre-group to fifth most common in the post-group. As Pseudomonas aeruginosa is one of the more common pathogens to be multidrug resistant in our institution, there may be a clinical benefit to the use of chlorhexidine in regards to resistance. Although the incidence of IVAC was decreased in the post-group, the organisms causing infection were similar in both groups.
This study is unique in the assessment tools utilized, specifically regarding the effective use of the updated CDC VAE surveillance definition for probable and possible VAP, which encompassed a standard range for temperature (>38°C, or <36°C) and WBC (≥12,000 cell/mm3 or ≤4,000 cell/mm3) with the initiation of a new antibiotic for 4 days or greater as it relates to infection risk. Much of the objective criteria used for these criteria were derived from research conducted by Klompas et al.[20] This tiered approach allows clinicians to more objectively screen patients and eliminate those patients that may have been diagnosed with VAP using CPIS or other outdated pneumonia guidelines. The number of probable VAP cases was lower in the post-group, suggesting clinical benefit of chlorhexidine mouthwash implementation based on updated objective CDC definitions.
This study was not without limitations. While the number of patients during the study period was large, strict exclusion criteria in an attempt to obtain a homogenous population resulted in a smaller sample size than expected, with less than 160 patients total included for analysis. The retrospective nature of this study has known inherent limitations, with reliance upon documentation in the electronic medical record. Information was captured within electronic progress notes, scanned documents and lab program, which required gathering data collection from a variety of systems. Literature evaluating the CDC's 2013 updated VAE surveillance guidelines utilized for the primary outcome of this study was not readily available at the time this study was being developed. Therefore, the validation of the updated CDC guidelines can further be supported by similar studies like this one. In addition, compliance was not assessed for the other individual VAP bundle components, such as spontaneous breathing trials, which could be a potential confounder if compliance changed significantly during the time period during which this study took place.
CONCLUSION
In summary, this study shows that prophylactic chlorhexidine mouthwash in addition to the use of a VAP bundle was associated with significantly lower probable VAP in mechanically ventilated SICU patients compared to patients that did not receive prophylaxis. Overall, the significant reduction in probable VAP was associated with a substantial reduction in Pseudomonas infections in the chlorhexidine group. Furthermore, the outcomes from this study are suggestive of decreased patient cost related to antibiotic management, but prospective randomized controlled trials are needed to confirm.
Financial support and sponsorship
Authors have no financial disclosures.
Conflicts of interest
Authors have no conflicts of interest to report.
REFERENCES
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–53. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 2.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 3.Krein SL, Kowalski CP, Damschroder L, Forman J, Kaufman SR, Saint S. Preventing ventilator-associated pneumonia in the United States: A multicenter mixed-methods study. Infect Control Hosp Epidemiol. 2008;29:933–40. doi: 10.1086/591455. [DOI] [PubMed] [Google Scholar]
- 4.Melsen WG, Rovers MM, Koeman M, Bonten MJ. Estimating the attributable mortality of ventilator-associated pneumonia from randomized prevention studies. Crit Care Med. 2011;39:2736–42. doi: 10.1097/CCM.0b013e3182281f33. [DOI] [PubMed] [Google Scholar]
- 5.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 6.Al-Tawfiq JA, Abed MS. Decreasing ventilator-associated pneumonia in adult intensive care units using the Institute for Healthcare Improvement bundle. Am J Infect Control. 2010;38:552–6. doi: 10.1016/j.ajic.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 7.DeRiso AJ, 2nd, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556–61. doi: 10.1378/chest.109.6.1556. [DOI] [PubMed] [Google Scholar]
- 8.Koeman M, van der Ven AJ, Hak E, Joore HC, Kaasjager K, de Smet AG, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006;173:1348–55. doi: 10.1164/rccm.200505-820OC. [DOI] [PubMed] [Google Scholar]
- 9.Munro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing, and preventing ventilator-associated pneumonia in critically ill adults. Am J Crit Care. 2009;18:428–437. doi: 10.4037/ajcc2009792. quiz 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Özçaka Ö, Başoğlu OK, Buduneli N, Taşbakan MS, Bacakoğlu F, Kinane DF. Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: A randomized clinical trial. J Periodontal Res. 2012;47:584–92. doi: 10.1111/j.1600-0765.2012.01470.x. [DOI] [PubMed] [Google Scholar]
- 11.Panchabhai TS, Dangayach NS. Role of chlorhexidine gluconate in ventilator-associated pneumonia prevention strategies in ICU patients: Where are we headed? Crit Care. 2009;13:427. doi: 10.1186/cc8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scannapieco FA, Yu J, Raghavendran K, Vacanti A, Owens SI, Wood K, et al. A randomized trial of chlorhexidine gluconate on oral bacterial pathogens in mechanically ventilated patients. Crit Care. 2009;13:R117. doi: 10.1186/cc7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellissimo-Rodrigues F, Bellissimo-Rodrigues WT, Viana JM, Teixeira GC, Nicolini E, Auxiliadora-Martins M, et al. Effectiveness of oral rinse with chlorhexidine in preventing nosocomial respiratory tract infections among intensive care unit patients. Infect Control Hosp Epidemiol. 2009;30:952–8. doi: 10.1086/605722. [DOI] [PubMed] [Google Scholar]
- 14.Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: Systematic review and meta-analysis. JAMA Intern Med. 2014;174:751–61. doi: 10.1001/jamainternmed.2014.359. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 16.Morris AC, Hay AW, Swann DG, Everingham K, McCulloch C, McNulty J, et al. Reducing ventilator-associated pneumonia in intensive care: Impact of implementing a care bundle. Crit Care Med. 2011;39:2218–24. doi: 10.1097/CCM.0b013e3182227d52. [DOI] [PubMed] [Google Scholar]
- 17.Mori H, Hirasawa H, Oda S, Shiga H, Matsuda K, Nakamura M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med. 2006;32:230–6. doi: 10.1007/s00134-005-0014-4. [DOI] [PubMed] [Google Scholar]
- 18.Nunez C. Nunez C. VAE surveillance helps clinicians improve outcomes. VAE surveillance helps clinicians improve outcomes. What the CDC's new ventilator-associated eventprotocol means. Health Manag Technol. 2013;34:16–7. [PubMed] [Google Scholar]
- 19.Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: The clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis. 2010;51(Suppl 1):S131–5. doi: 10.1086/653062. [DOI] [PubMed] [Google Scholar]
- 20.Klompas M, Magill S, Robicsek A, Strymish JM, Kleinman K, Evans RS, et al. CDC Prevention Epicenters Program. Objective surveillance definitions for ventilator-associated pneumonia. Crit Care Med. 2012;40:3154–61. doi: 10.1097/CCM.0b013e318260c6d9. [DOI] [PubMed] [Google Scholar]


