Abstract
Purpose:
To evaluate corneal changes after collagen crosslinking (CXL) therapy for keratoconus (KCN) using the Galilei dual Scheimpflug analyzer.
Methods:
This prospective, nonrandomized clinical study included 35 eyes of 32 keratoconus patients who had undergone CXL. The eyes were saturated with riboflavin solution and were subjected for 30 minutes to ultraviolet-A (UV-A) light with irradiance of 3 mW/cm2. Effectiveness of the treatment was assessed by measuring uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA), manifest cylinder/sphere, keratometry, pachymetry, posterior and anterior elevations by the Galilei dual Scheimpflug analyzer. Prior to treatment and 8 months after therapy, Scheimpflug analysis was performed using the Galilei system. The four sets of data including keratometry values, pachymetry, elevation parameters and surface indices were statistically analyzed and compared.
Results:
Mean patient age was 22.3 ± 3.8 years and mean postoperative follow-up was 8.1 ± 3.2 months. There was a significant increase in UCVA (0.54 ± 0.35 Log MAR preoperatively to 0.49 ± 0.34 LogMAR postoperatively, P = 0.01) and BCVA (0.21 ± 0.19 Log MAR preoperatively to 0.16 ± 0.17 LogMAR postoperatively, P = 0.01). Mean cycloplegic spherical equivalent refractive error was −4.13 ± 2.65 Diopter (D) preoperatively and − 4.67 ± 2.96 D postoperatively (P < 0.001). During the follow-up period, no significant difference was observed in pachymetric and elevation data postoperatively.
Conclusion:
Corneal stabilization could be achieved by collagen crosslinking therapy for keratoconus in terms of corneal thickness, keratometry values, elevation parameters and surface indices.
Keywords: Collagen Crosslinking, Galilei Dual Scheimpflug Analyzer, Keratoconus
INTRODUCTION
Corneal collagen crosslinking (CXL) with riboflavin is a new treatment modality applied to strengthen the cornea in eyes with progressive keratoconus (KCN) in which corneal thickness is >400 μm.[1] The photoactivated riboflavin enhances corneal strength and integrity by increasing collagen crosslinking and can halt the progression of keratoconus.
The Galilei dual Scheimpflug system is a noninvasive diagnostic instrument designed to analyze anterior segment characteristics including Placido-based topography, pachymetry, net corneal power, elevation maps, anterior chamber depth, and corneal wavefront.[2,3] It combines two technologies, i.e. Placido imaging, which provides curvature data, and Scheimpflug imaging, which is optimal for precise elevation measurements.
The purpose of the present study was to assess corneal changes (keratometric, pachymetric, elevation data and surface indices) after CXL therapy for keratoconus using the Galilei dual Scheimpflug analyzer. The safety and effectiveness of riboflavin ultraviolet-A (UV-A) light-induced crosslinking of corneal collagen in improving visual acuity and stabilizing the progression of keratoconus were also evaluated in this study.
METHODS
This prospective comparative study comprised of 35 (23 right and 12 left) eyes of 32 patients including 23 male and 9 female subjects with progressive keratoconus. The diagnosis of keratoconus was based on clinical findings (stromal thinning, conical protrusion, Fleischer ring, and Vogt striae) and associated characteristics on Placido disk–based topographic patterns (Tomey EM-3000, version 4.20, Premier Ophthalmic Services, Frankfurt, Germany). Inclusion criteria for CXL were corneal thickness of at least 400 µm documented by ultrasonic pachymetry and a minimum of one diopter (D) increase in keratometric values in the past 6 months. Exclusion criteria were history of any ocular surgery, previous acute corneal hydrops, dry eye syndrome and pathologic eye conditions other than keratoconus. All patients had bilateral keratoconus without subepithelial scarring and were aged more than 18 years.
All study participants were asked to discontinue wearing soft contact lenses for at least 2 weeks and rigid gas-permeable contact lenses for at least 4 weeks before the measurements. Written informed consent was obtained after explaining the purpose of the study which was approved by the Institutional Review Board of the Ophthalmic Research Center at Shahid Beheshti University of Medical Sciences, Tehran, Iran.
A complete ocular examination including slit lamp biomicroscopy, cycloplegic refraction, corrected distance visual acuity (CDVA) using a Snellen chart, keratometry, intraocular pressure measurement, and dilated fundus examination was performed.
For measurements with the Galilei dual Scheimpflug analyzer (Ziemer Ophthalmic System AG, Zurich, Switzerland), subjects were seated with their chin on a chinrest and forehead against the forehead strap while fixating on the target. Appropriate alignment of the scan center with the corneal apex was checked using an initial Scheimpflug image on the monitor. The measurement results were checked under a quality specification window; only measurements with an “OK” reading were recorded for analysis. If the comments were marked yellow or red (i.e., not OK), the measurement was repeated.
Four groups of data were then used for statistical analysis. These consisted of (1) keratometry values including the power of the flat (Kf) and steep (Ks) meridians, keratometric astigmatism and mean keratometry, (2) pachymetry data including central and minimal (at the thinnest point) corneal thickness, (3) elevation parameters including the radius of the anterior and posterior best-fit sphere (BFS) and the maximum anterior and posterior elevations at the central 3, 5, and 7 mm zones of the cornea, and (4) surface indices including inferior-superior (I-S) index, standard deviation (SD) of the corneal power, surface regularity index, surface asymmetry index, irregular astigmatism index, differential sector index, opposite sector index, center/surround index, area analyzed, keratoconus prediction index and keratoconus probability.
For maximum elevation of the cornea, a BFS was generated by the software, with the floating option over an 8-mm fit. The float map means that the reference body has no fixed center and the distance between the cornea and the sphere surface is optimized to be as small as possible. BFS setting was constant in Galilei software during both pre- and postoperative measurements. Anterior elevation was determined by taking the maximum difference in anterior elevation between the BFS and patient's cornea. Posterior elevation was determined by taking the maximum difference in posterior elevation between the BFS and patient's cornea. All measurements were obtained at the central 3, 5 and 7 mm zones of the cornea by an experienced operator using the same machine and procedures. At least 6 months after CXL procedure, the images were captured again using the Galilei dual Scheimpflug analyzer.
Surgical Technique
Under strict sterile conditions and topical anesthesia, the epithelial on the central 8-mm area of the cornea was debrided. 0.1% riboflavin (in 20% dextran solution) was applied to the cornea every 2 minutes for 30 minutes. Penetration of riboflavin into the aqueous was checked at the slit lamp. The cornea was then irradiated with UV-A light with a wavelength of 370 nm and an irradiance of 3 mW/cm2 for 30 minutes. During irradiation, riboflavin drops were applied to the cornea every 2 minutes to sustain the required concentration of riboflavin and to prevent desiccation of the cornea. At the conclusion of the procedure, topical chloramphenicol eye drops were instilled and a bandage soft contact lens was fitted. Postoperatively, chloramphenicol and betamethasone 0.1% eye drops were prescribed every 6 hours and continued for 2 weeks. Therapeutic contact lenses were removed after complete epithelial healing.
Statistical Analysis
All statistical analyses were performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). General data including age, spherical equivalent (SE) refraction, and keratometry readings as well as data obtained by the Galilei dual Scheimpflug analyzer were expressed as mean ± SD. One-way analysis of variance was used to compare these measurements between the sets of data. A paired two-tailed Student's t-test was performed to analyze postoperative changes from baseline values and to monitor postoperative changes over time. Pearson correlation coefficients (r) were used to analyze possible correlations between postoperative topography measurements and postoperative visual acuity. P values less than 0.05 were considered as statistically significance.
RESULTS
Mean age of the patients was 22.3 ± 3.8 (range, 15-31) years and mean follow-up duration was 8.1 ± 3.2 (range, 6-18) months. No significant visible haze was observed at the final postoperative examination. The surgery and the postoperative period were uneventful in all patients. The cornea re-epithelialized by one week after treatment. In the early postoperative period, all eyes had minimal anterior stromal corneal haze that resolved approximately 3 months postoperatively. Table 1 shows different topographic indices before and after CXL therapy. There was no statistically significant difference in any of these parameters.
Table 1.
Comparison of different data measured pre- and postoperatively

Visual Acuity
Mean preoperative uncorrected visual acuity (UCVA) was 0.54 ± 0.35 (range, 0-1.20) LogMAR and mean preoperative best-corrected visual acuity (BCVA) was 0.21 ± 0.19 (range, 0.0-0.68) LogMAR. Postoperatively, these values were 0.49 ± 0.34 (range, 0.0-1.20) and 0.16 ± 0.17 (range, 0.0-0.68) LogMAR, respectively, indicating a significant improvement in both UCVA (P = 0.01) and BCVA (P = 0.01) postoperatively.
After the procedure, 20 (57.1%) eyes maintained their preoperative UCVA; 6 (17.2%) eyes gained one to two lines, and 5 (14.3%) eyes gained three lines; in contrast 4 (11.4%) eyes lost one line of UCVA. Furthermore, 21 (60.0%) eyes experienced no change in BCVA, 12 (34.3%) eyes showed a gain of 1 to 3 lines and 2 (5.7%) eyes experienced a loss of one line of BCVA.
Refractive Outcomes
Mean cycloplegic SE refractive error was − 4.13 ± 2.65 (range, −12.5 to − 0.25) D, preoperatively and −4.67 ± 2.96 (range, −14.5 to 0.0) D, postoperatively (P < 0.001). Mean refractive astigmatism was −3.89 ± 1.63 (range, −8.0 to −0.50) D and −3.88 ± 1.81 (range, −9.0 to 0.0) D, respectively (P = 0.95). Refractive astigmatism decreased from 4.25 to 3.25D in 14 (40.0%) eyes and showed no change in 4 (11.4%) eyes. Deterioration of refractive astigmatism (from 0.25 to 1.0 D) was observed in 17 (48.6%) eyes. One year after CXL, manifest sphere decreased by a mean of -2.75 D in 13 (65%) eyes while no change was observed in 7 eyes (35%). Manifest cylinder decreased by a mean of -1.68 D in 15 (75%) eyes but no change occurred in 5 eyes.
Preoperative mean keratometry was 48.74 ± 3.84 (range, 43.75 to 58.87) D and remained unchanged postoperatively at 48.53 ± 3.74 (range, 43.45 to 58.54 D, P = 0.18). Keratometric astigmatism demonstrated a non-significant reduction from 4.93 ± 2.29 (range, 0.75 to 10.0) D preoperatively to 4.75 ± 2.34 (range, 0.75 to 9.0) D after the procedure.
In 19 eyes an increase in postoperative mean keratometry was observed which ranged from 0.03 to 1.16 D (group 1). Flattening of mean keratometry ranging from 0.05 to 3.98 D occurred in 16 eyes (group 2). There was no significant difference between these two subgroups in terms of preoperative parameters including BCVA, keratometry readings and pachymetry [Table 2].
Table 2.
Visual acuity, refractive outcomes, and central corneal thickness preoperatively and postoperatively
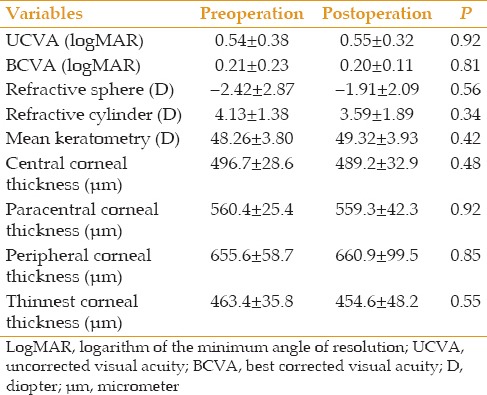
Pachymetry
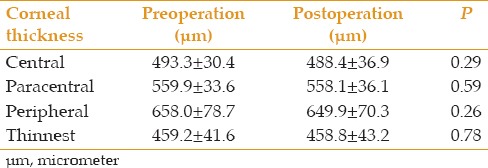
Corneal thickness at the central, paracentral and peripheral zones, as well as at the thinnest point was measured prior to the operation and at final follow-up [Table 3]. No significant difference was observed in any of these measurements.
Table 3.
Comparison of different corneal thickness measurements by the Galilei dual Scheimpflug analyzer
Correlation with Visual Acuity
The improvement in UDVA was slightly correlated with an improvement in the index of surface variance (mean change 10.50 ± 8.2; P < 0.001) from baseline to 12 month after the procedure. However, improvements in UDVA and CDVA during one year were not correlated with improvements in the minimum radius.
DISCUSSION
There is mounting evidence on the efficacy of CXL treatment[4] using the photosensitizer riboflavin and UV-A light with a wavelength of 370 nm in halting the progression of keratoconus[5,6] and corneal ectasia after refractive surgery[7,8] with minimal toxicity.[9,10,11,12] In this study, we evaluated the 1-year postoperative changes in visual acuity, keratometry, and seven anterior surface topographic indices induced by CXL. We found no significant differences in pachymetric and elevation data postoperatively. Using the Pentacam topographer, Greenstein et al reported a statistically significant decrease in the index of surface variance, index of vertical asymmetry, keratoconus index and minimum radius of curvature one year after CXL therapy. However, there was no significant change in central keratoconus index, index of height asymmetry and index of height decentration.[13] Koller et al found significant improvement in 4 out of 7 Pentacam topography indices (central keratoconus index, keratoconus index, index of height asymmetry, minimum radius of curvature) one year after CXL.[14] Using Scheimpflug elevation maps in the present study, there was no significant change in anterior or posterior corneal elevation values in the present study, which provides additional evidence for stability of the corneal surface. This observation is comparable to the results reported by Grewal et al[15] who observed no significant differences in BCVA, SE, cylinder vector, central corneal thickness, anterior corneal curvature, posterior corneal curvature, and posterior corneal elevation.
It has been well-documented that CXL almost invariably results in some central anterior corneal flattening.[16,17,18,19,20] Similarly, our results indicate that the minimum radius of curvature, defined as the inverse of corneal steepness, was increased postoperatively in agreement with the decrease in anterior surface keratometry. Improved visual acuity after CXL might be expected to result from improved topographic regularity. This study attempted to address these issues and quantitate topographic changes. Improvements in corrected or uncorrected distance visual acuity were not correlated with improvements in postoperative indices except for the index of surface variance, which demonstrated a mild significant correlation.
The results of the current study have shown that the progression of keratoconus was stopped, which is in line with other clinical reports indicating that CXL halts the progression of ectasia, improves corneal keratometry and refractive status.[7,21] However, the recent reports have not been confirmed the effectiveness of CXL in all circumstances.[22] The increasing strength in biomechanical postoperative characteristics of the cornea reduces the progression of keratoconus and ectasia which can improve the patients' keratometric and visual acuity outcomes, postoperatively.[6,15,23,24,25,26,27]
We did not encounter any complications in this case series. However, several case reports and original studies have reported complications ranging from insignificant stromal haze to sight-threatening infectious keratitis and non-infectious corneal melting. Microbial keratitis after CXL occurs rarely. Keratitis following CXL has been reported due to herpes simplex,[28] Acanthamoeba,[29] and a number of bacteria such as Escherichia coli, and staphylococcus and streptococcus species.[30,31,32] Sterile keratitis may be another complication of CXL.[33]
Limitations of our study were short-term follow-up and a relatively small number of patients.
In summary, corneal CXL is a safe and effective method for halting the progression of keratoconus which may avoid or at least delay corneal transplantation.[1,34] However, more experience is required to determine its long term efficacy and safety.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 2.Swartz T, Marten L, Wang M. Measuring the cornea: The latest developments in corneal topography. Curr Opin Ophthalmol. 2007;18:325–333. doi: 10.1097/ICU.0b013e3281ca7121. [DOI] [PubMed] [Google Scholar]
- 3.Konstantopoulos A, Hossain P, Anderson DF. Recent advances in ophthalmic anterior segment imaging: A new era for ophthalmic diagnosis? Br J Ophthalmol. 2007;91:551–557. doi: 10.1136/bjo.2006.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 5.Wollensak G. Crosslinking treatment of progressive keratoconus: New hope. Curr Opin Ophthalmol. 2006;17:356–360. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- 6.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T. Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:2035–2040. doi: 10.1016/j.jcrs.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Woodward MA, Randleman JB, Russell B, Lynn MJ, Ward MA, Stulting RD. Visual rehabilitation and outcomes for ectasia after corneal refractive surgery. J Cataract Refract Surg. 2008;34:383–388. doi: 10.1016/j.jcrs.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wollensak G, Spörl E, Reber F, Pillunat L, Funk R. Corneal endothelial cytotoxicity of riboflavin/UVA treatment in vitro . Ophthalmic Res. 2003;35:324–328. doi: 10.1159/000074071. [DOI] [PubMed] [Google Scholar]
- 10.Wollensak G, Spoerl E, Reber F, Seiler T. Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro . Eye (Lond) 2004;18:718–722. doi: 10.1038/sj.eye.6700751. [DOI] [PubMed] [Google Scholar]
- 11.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 12.Bueeler M, Spoerl E, Seiler T, Mrochen M. UV collagen crosslinking of the cornea: Safety aspects and design of a UV illumination system. In: Fabrice M, Söderberg PG, Ho A, Struck BE, Belkin M, editors. Ophthalmic Technologies XVIII. Vol. 6844. Bellingham, WA: Proceedings SPIE; 2008. pp. 112–121. [Google Scholar]
- 13.Greenstein SA, Fry KL, Hersh PS. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:1282–1290. doi: 10.1016/j.jcrs.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Koller T, Iseli HP, Hafezi F, Vinciguerra P, Seiler T. Scheimpflug imaging of corneas after collagen cross-linking. Cornea. 2009;28:510–515. doi: 10.1097/ICO.0b013e3181915943. [DOI] [PubMed] [Google Scholar]
- 15.Grewal DS, Brar GS, Jain R, Sood V, Singla M, Grewal SP. Corneal collagen crosslinking using riboflavin and ultraviolet-A light for keratoconus: One-year analysis using Scheimpflug imaging. J Cataract Refract Surg. 2009;35:425–432. doi: 10.1016/j.jcrs.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 16.Ghanem RC, Santhiago MR, Berti T, Netto MV, Ghanem VC. Topographic, corneal wavefront, and refractive outcomes 2 years after collagen crosslinking for progressive keratoconus. Cornea. 2014;33:43–48. doi: 10.1097/ICO.0b013e3182a9fbdf. [DOI] [PubMed] [Google Scholar]
- 17.Arora R, Jain P, Goyal JL, Gupta D. Comparative analysis of refractive and topographic changes in early and advanced keratoconic eyes undergoing corneal collagen crosslinking. Cornea. 2013;32:1359–1364. doi: 10.1097/ICO.0b013e3182a02ddb. [DOI] [PubMed] [Google Scholar]
- 18.Hassan Z, Szalai E, Módis L, Jr, Berta A, Németh G. Assessment of corneal topography indices after collagen crosslinking for keratoconus. Eur J Ophthalmol. 2013;23:635–640. doi: 10.5301/ejo.5000249. [DOI] [PubMed] [Google Scholar]
- 19.Touboul D, Trichet E, Binder PS, Praud D, Seguy C, Colin J. Comparison of front-surface corneal topography and Bowman membrane specular topography in keratoconus. J Cataract Refract Surg. 2012;38:1043–1049. doi: 10.1016/j.jcrs.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Piñero DP, Alio JL, Klonowski P, Toffaha B. Vectorial astigmatic changes after corneal collagen crosslinking in keratoconic corneas previously treated with intracorneal ring segments: A preliminary study. Eur J Ophthalmol. 2012;22(Suppl 7):S69–S80. doi: 10.5301/ejo.5000063. [DOI] [PubMed] [Google Scholar]
- 21.Chan E, Snibson GR. Current status of corneal collagen cross-linking for keratoconus: A review. Clin Exp Optom. 2013;96:155–164. doi: 10.1111/cxo.12020. [DOI] [PubMed] [Google Scholar]
- 22.Hafezi F, Iseli HP. Pregnancy-related exacerbation of iatrogenic keratectasia despite corneal collagen crosslinking. J Cataract Refract Surg. 2008;34:1219–1221. doi: 10.1016/j.jcrs.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 23.Vinciguerra P, Camesasca FI, Albè E, Trazza S. Corneal collagen cross-linking for ectasia after excimer laser refractive surgery: 1-year results. J Refract Surg. 2010;26:486–497. doi: 10.3928/1081597X-20090910-02. [DOI] [PubMed] [Google Scholar]
- 24.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 25.Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:691–700. doi: 10.1016/j.jcrs.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet A corneal collagen cross-linking for keratoconus in Italy: The Siena eye cross study. Am J Ophthalmol. 2010;149:585–593. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Vinciguerra P, Albè E, Trazza S, Rosetta P, Vinciguerra R, Seiler T, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009;116:369–378. doi: 10.1016/j.ophtha.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 28.Kymionis GD, Portaliou DM, Bouzoukis DI, Suh LH, Pallikaris AI, Markomanolakis M, et al. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. J Cataract Refract Surg. 2007;33:1982–1984. doi: 10.1016/j.jcrs.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 29.Rama P, Di Matteo F, Matuska S, Paganoni G, Spinelli A. Acanthamoeba keratitis with perforation after corneal crosslinking and bandage contact lens use. J Cataract Refract Surg. 2009;35:788–791. doi: 10.1016/j.jcrs.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Santonja JJ, Artola A, Javaloy J, Alió JL, Abad JL. Microbial keratitis after corneal collagen crosslinking. J Cataract Refract Surg. 2009;35:1138–1140. doi: 10.1016/j.jcrs.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 31.Zamora KV, Males JJ. Polymicrobial keratitis after a collagen cross-linking procedure with postoperative use of a contact lens: A case report. Cornea. 2009;28:474–476. doi: 10.1097/ICO.0b013e31818d381a. [DOI] [PubMed] [Google Scholar]
- 32.Pollhammer M, Cursiefen C. Bacterial keratitis early after corneal crosslinking with riboflavin and ultraviolet-A. J Cataract Refract Surg. 2009;35:588–589. doi: 10.1016/j.jcrs.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 33.Javadi MA, Feizi S. Sterile Keratitis following Collagen Crosslinking. J Ophthalmic Vis Res. 2014;9:510–513. doi: 10.4103/2008-322X.150832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type a rays induced cross-linking of corneal collagen: Preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32:837–845. doi: 10.1016/j.jcrs.2006.01.091. [DOI] [PubMed] [Google Scholar]


