Abstract
Purpose:
To explore the relationship between lens power and refractive error in older adults following age-related hyperopic shifts.
Methods:
From the Shahroud Eye Cohort Study, subjects aged 55-64 years without clinically significant cataracts (with nuclear opacity of grade 0 to 1) were included to maximize the proportion of subjects with age-related hyperopic shifts that normally occur between 40 to 60 years of age, before interference from the myopic shift due to nuclear cataracts. Mean axial length (AL) values, corneal power, anterior chamber depth, lens thickness, and lens power were analyzed and compared among three refractive groups (myopes, emmetropes, and hyperopes).
Results:
A total of 1,006 subjects including 496 (49.63%) male subjects were studied. Corneal power was similar in all refractive groups. Hyperopes had + 1.69 diopters higher mean spherical equivalent refractive error and − 0.50 mm shorter AL than emmetropes. Myopes had 0.67 mm longer AL than emmetropes. Hyperopes had significantly increased lens thickness as compared to emmetropes (4.42 vs. 4.39 mm respectively). In this adult sample, the hyperopic group had lower lens power (+22.29 diopters vs. +22.54 diopters in emmetropes, P = 0.132). Myopes had similar lens power as emmetropes.
Conclusion:
Axial length is the principal determinant of refractive errors. Lens power may have importance in determining hyperopia in adults free of cataract.
Keywords: Cross-sectional Study, Hyperopia, Lens Power
INTRODUCTION
Based on classical studies on adult eyes performed by Tron[1] and Stenstrom,[2] it has been generally accepted that among the ocular components of the eye, axial length (AL) shows the strongest correlation with refractive error, whereas corneal power and lens power show much weaker correlations. In the mentioned studies, the distribution of the optical components was normal, contrasting with the tight, leptokurtic distribution of refraction (i.e., a more acute peak around the mean and flatter tails). Since random association of normally distributed optical components would not produce the observed distributions of refraction, these early studies led to the concept of an active process of emmetropization, where the optical components of refraction appear to develop in concert.
In particular, active matching of AL to corneal power has been implicated since the ratio of AL to corneal radius (CR) of curvature (AL/CR) shows a more leptokurtic distribution and a stronger correlation with refraction than does AL by itself.[3] In contrast, the role of changes in lens power in determining the refractive state of the eye at rest has received little emphasis, although François and Goes[4] in 1977 reported that lens power may be involved after they studied the ocular components of 100 emmetropic eyes with the newly developed ultrasonic biometry.
Studies on refractive development in children have confirmed the importance of AL and the AL/CR ratio, and in addition have considered changes in lens power. Sorsby et al.,[5,6] had showed that from the age of three, the lens lost power with age as the eye increased in AL. They also found that changes in corneal power had little impact on refractive development after the age of three. They also noted in cross-sectional data on subjects aged 20-60 that eyes in the emmetropic range had a normal distribution of ALs and that there lens power was lower in longer eyes.[7,8] Their findings therefore supported an important role for changes in lens power in the final determination of refractive status. The importance of axial elongation and the contribution of reductions in lens thickness and lens power during the development of refraction during childhood has subsequently been confirmed by Larsen[9,10] and more recently by Mutti et al.[11,12,13]
Gordon and Donzis[14] analyzed ocular components in 148 eyes, from newborn to 36-year-old adults, and found an age-related increase in AL with reductions in corneal and lens powers, with the most dramatic changes occurring in the 1st year or two of life. Subsequent studies have confirmed the rapid development of kurtosis in the distribution of refraction postnatally.[11,15] Thus, the overall picture of refractive development during childhood is of rapid matching of AL to the power of the refractive surfaces, followed by continuing axial elongation which can be balanced, at least in some cases, by reductions in lens power. As a result, in young adults, hyperopic eyes have, on average, slightly higher lens power than emmetropic or myopic eyes,[13] and in the population as a whole (if mainly composed of emmetropic eyes), shorter eyes have, on average, higher lens power than longer eyes.[7]
The lens thins and flattens until around the age of 10.[10,12,16] From this age onward, lens curvatures steepen (and the lens becomes axially thicker),[17] which might be expected to produce a more powerful lens and a myopic shift in refraction with ageing.[18,19] However, quite substantial hyperopic shifts in refraction are commonly observed in adults, even with cycloplegic refraction.[20] The conflict between these two observations has become known as the lens paradox, which could be explained by loss of effective refractive index with age.[19,21] Recent studies on ocular components in children and adults[13,22] have shown that the calculated effective index of the lens decreases with increasing age, consistent with this hypothesis.
The final refractive state of the older adult eye thus depends on a complex set of changes in the ocular components which continue throughout childhood and adult life. Although there is now a number of population-based studies on refraction and ocular biometry in older adults, the Reykjavik Eye Study was the first to report lens power.[23] It found a negative correlation between lens power and refractive error; that is, in contrast to the situation at the end of childhood, the lens has less power in hyperopic eyes and vice versa, in myopic eyes. But they also reported a negative correlation between lens power and AL, such that shorter eyes (which would normally be expected to be more hyperopic) would have higher lens power. The authors recognized this paradox but did not explore it further.
The present analysis from another population-based study, the Shahroud Eye Cohort Study (ShECS), was limited to subjects aged 55-64 years to assess continuing hyperopic shifts in refraction, and excluded subjects with nuclear cataracts, which are well-known to be associated with myopic shifts.
METHODS
The ShECS population consists of residents in Shahroud aged 40-64. A total of, 5,190 subjects of both genders participated in this study. The detailed methodology has been previously published.[23] Multistage sampling was applied to the 40 to 64-year-old population of Shahroud, a city in the North of Iran. The study was approved by the ethics committee of Shahroud University of Medical Sciences and adhered to the tenets of the Declaration of Helsinki for research in human subjects.
For analysis of the ocular components of refraction, only subjects aged 55-64 years with nuclear opacity no more than grade 0 and 1 (i.e. without clinically significant cataract) were included to maximize the inclusion of subjects with the age-related hyperopic shifts occurring between ages 40-60, before myopic shifts due to nuclear cataracts develop.
Corneal power was calculated using the CR and assuming a refractive index of 1.3315 as proposed by Olsen.[24,25] Crystalline lens power calculation was based on measurements of distance cycloplegic refraction, corneal power, anterior chamber depth, lens thickness, and AL using the formula proposed by Bennett.[26] The A and B constants in this formula were calculated using Gullstrand's reduced eye model.[26,27]
As described by Grosvenor and Scott,[3] the emmetropic eye has an AL/CR near 3:1. In children, hyperopic eyes tend to have lower AL/CR ratio due to a short AL or a flatter cornea. The AL/CR ratio is apparently set by the end of childhood since CR does not change much after the first few years of life, while AL stabilizes in early adulthood. In an attempt to distinguish subjects who would have been hyperopic from childhood from those who were initially emmetropic or possibly mildly myopic and became hyperopic later in life due to hyperopic shifts, a cut-off point for AL/CR ratio was established at 2.92. Which represents subtraction of one standard deviation (SD) from a mean (±SD) value of 3.04 ± 0.11.
As the ocular components had normal distributions, Pearson correlations were calculated (r values are presented). One-way analysis of variances (ANOVA) with post-hoc Scheffe tests was employed for mean values of ocular parameters grouped by refractive error categories. The definitions of refractive error categories [Table 1]. P values < 0.05 were considered as significant.
Table 1.
Characteristics of subjects in each refractive group
RESULTS
A total of 1,006 subjects aged 55 to 64 years with nuclear opacity grade 0 and 1, including 496 (49.63%) men were studied. The distribution of data was quite symmetrical for spherical equivalent and principal ocular components of refraction [Figures 1-4] As presented in Table 2, only refractive error and AL/CR had high kurtosis.
Figure 1.
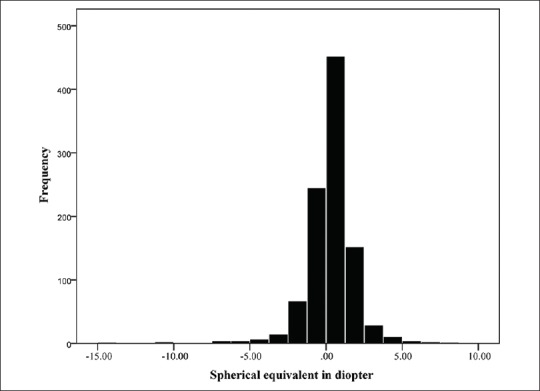
Distribution of refractions in this study.
Figure 4.
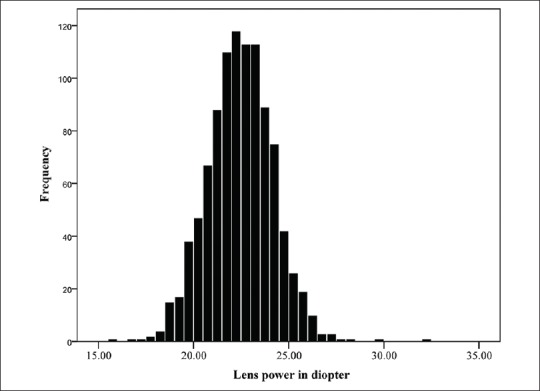
Normal distribution of lens power.
Table 2.
Means and kurtosis of studied variables in the analyzed participants
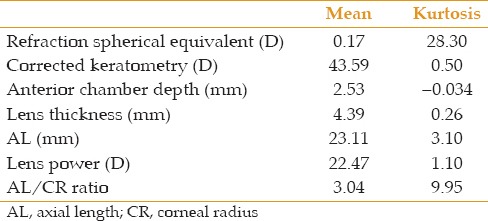
Figure 2.
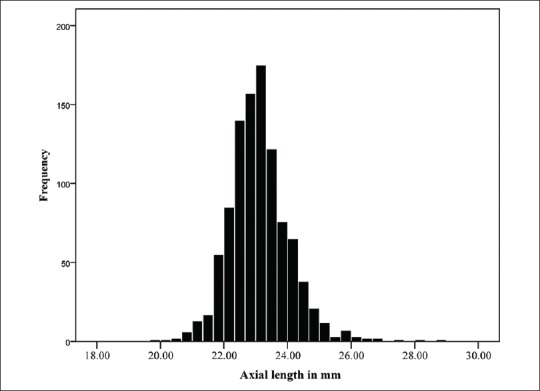
Normal distribution of axial length.
Table 1 shows the number of subjects in each refractive group and their definitions, with the mean spherical equivalent, corneal power, AL, lens thickness, and calculated lens power. As expected, myopic eyes were longer, hyperopic eyes were shorter, and interestingly there was no difference in corneal power between emmetropes and hyperopes.
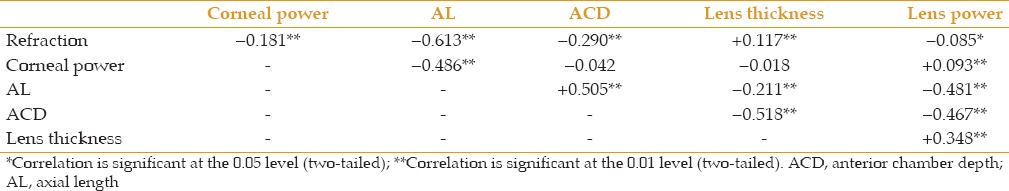
Table 3 shows correlation coefficients between the optical components that contribute to refraction. The correlation between AL and refraction was strongly negative, as expected. The correlation between AL and lens power was also strongly negative. In other words, longer eyes were more myopic and had lower lens powers. Correlations of refraction and AL with lens thickness were much weaker, but lens power and lens thickness were moderately correlated; in other words, thicker lenses had more power.
Table 3.
Correlation coefficients (r) between ocular components
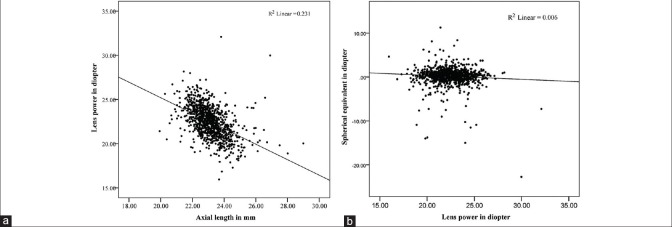
Some important relationships between variables are illustrated graphically in Figures 5 and 6. Figure 5a shows that longer eyes had lower calculated lens powers and shorter eyes had higher calculated lens powers. In an apparent contradiction, Figure 5b shows that more myopic eyes tended to have higher lens powers, and more hyperopic eyes tended to have lower lens powers, but the correlation and regression coefficients were low and not statistically significant. Figure 6 shows that lens power and lens thickness were positively correlated, with thicker lenses having higher lens power, despite the documented loss of lens power as it thickens during adult life.
Figure 5.
(a) Negative correlation between lens power and axial length (P < 0.001) (b) negative correlation between lens power and refraction (P = 0.08).
Figure 6.
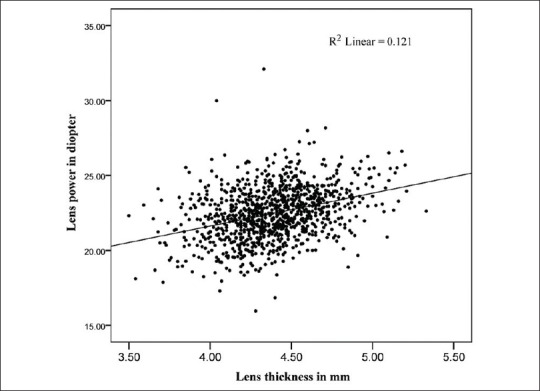
Positive significant correlation between lens power and lens thickness (P < 0.001).
When examined as refractive groups, there were significant differences in AL between the three refractive groups [Table 1]. Compared to emmetropes and hyperopes, myopes showed lower lens thickness [Table 1] while hyperopes had the lowest lens power although this difference was not significant [Table 1 and Figure 7].
Figure 7.
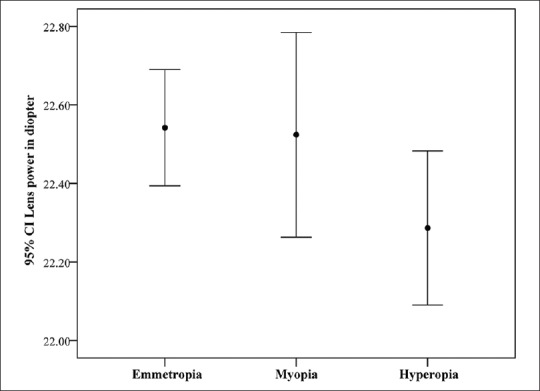
Lower mean lens power in hyperopes as compared to emmetropes (analysis of variances P = 0.132) (95% confidence interval shown by error bars).
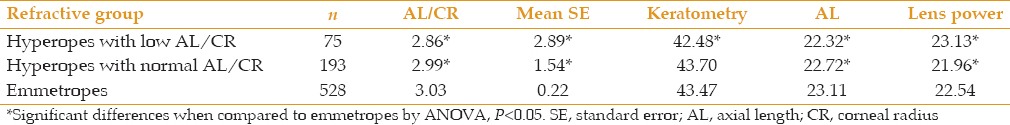
It has been shown that hyperopic subjects in early adulthood have low AL/CR.[3] AL/CR ratios are likely to become stable in adult life since neither AL nor CR changes significantly. Given the hyperopic shift that take place in adult life, hyperopic subjects at the age of 55-64 consist of two groups: The first are subjects who were hyperopic at the end of childhood with low AL/CR ratios, while the second group consists of individuals who were emmetropic at the end of childhood, with medium AL/CR ratios, but developed hyperopic shifts during adult life. Hyperopic subjects were thus analyzed using an AL/CR cut-off of 2.92 as explained earlier. As evident form Table 4, 75 out of 269 hyperopes (28%) had AL/CR ratio ≤ 2.92. These subjects had, on average, less powerful corneas, shorter AL, and interestingly, higher lens power than emmetropes. In contrast, the group of hyperopes with more emmetropic AL/CR ratios had AL and corneal powers similar to emmetropic subjects, but lower lens powers, suggesting that they may have been emmetropes who had lost lens power during adult life.
Table 4.
Comparison of ocular components for three refractive groups based on AL/CR ratio
DISCUSSION
The principal findings of this study confirm previously reported correlations between refractive components of the eye in an adult population. Only subjects aged 55-64 with low amounts of nuclear opacity in the ShECS population were considered for this analysis. This group was chosen to search for the impact of hyperopic shifts and avoid cataract-related myopic shifts. The main ocular component correlated with refractive error was AL. The correlation between lens power and refractive error was very low and negative; hyperopic eyes had lower lens power.
Studying ocular refractive components in the Reykjavik Eye Study, Olsen et al.,[24] reported relationship between the different ocular parameters in an adult population, showing a significant negative correlation (r=−0.23) between spherical equivalent refraction and calculated lens power treated as continuous variables (myopic eyes had higher lens power and vice versa, hyperopic eyes had lower lens power). In the same study, the negative correlation between lens power and AL in the whole sample demonstrated that eyes with longer ALs also had lower lens powers (and vice versa), with a significant greater correlation of −0.44.
The present data from ShECS confirms the negative relationship between lens power and AL but differs from the results of the Reykjavik Eye Study in finding little correlation between lens power and refraction as originally described by Tron in younger subjects.[1] Nevertheless, the same paradox exists, as longer eyes would be expected to be more myopic with lower lens power in childhood. The difference may be due to the fact that the Reykjavik Eye Study analysis did not exclude cataract subject, and the myopic shifts related to nuclear cataract may have produced a group of older myopic subjects with high lens power that would make the correlation between refraction and lens power even more negative as was found in the Central India Eye and Medical Study where cataract subjects were included.[28]
The situation in adults, therefore, appears to be different from that at the end of childhood,[13,29,30,31,32] where hyperopic eyes have greater lens power, emmetropic eyes have less lens power, and myopic eyes have the least. The relationships at the end of childhood result from children starting at birth with hyperopic eyes of varying sizes. The matching of AL to the refractive surfaces over the first 1-2 years of life leads to a tight and kurtotic distribution of refractive error, which is centered around mild hyperopia, such that shorter eyes tend to have higher lens powers for a given level of ametropia, thus establishing the basic association between hyperopia, higher lens power, and shorter AL. Subsequent axial elongation during childhood tends to result in myopic shifts, but these are partly compensated for by reductions in lens power, which only strengthen the association between hyperopia, high lens power, and shorter AL (or, in the same manner, of myopia, low lens power, and longer AL).
In adult life, when corneal power and AL have stabilized, there may be a continuous slow reduction in lens power, which in to be responsible for well-documented hyperopic shifts found with ageing. These appear to be larger in hyperopic and emmetropic eyes than in myopic eyes,[33] reducing the positive correlation between lens power and refractive status, while maintaining the correlation between higher lens power and shorter ALs driven by this high correlation in emmetropic eyes.
It is noteworthy that population-based studies in older adults, including the Reykjavik Eye Study,[24] the Central India Eye and Medical Study,[28] the Latino Eye Study[34] and the present one show that hyperopic eyes have lower lens power. In a population-based study of cycloplegic refractive error in adults in Tehran,[35] the prevalence of cycloplegic hyperopia >+1 diopters increased from 10.5% to 53.3% between ages 20 and 70. This 40% increase in cycloplegic hyperopic refractive error prevalence, at an age where corneal power and AL seem to be stable, can only be attributed to a decrease in lens power.[35]
The mechanisms involved in decreasing lens power with age are under study. It is widely believed that as new fibers grow in the surface of the lens and older ones become compacted in the deeper layers, they gain refractive index, and thus a gradient of refractive index is established from the surface to the center of the lens.[36] This gradient gives the lens an internal power greater than that due to its surface curvature alone.[37,38] This gradient index structure becomes less effective with ageing, as has been recently shown using magnetic resonance imaging, reducing the lens effective refractive index by a steepening of the gradient.[39] In theory, a lens that loses effective refractive index could lose power, so emmetropic eyes could become hyperopic.[35]
The positive correlation we observed between lens thickness and lens power [Figure 3] is interesting as it is known from geometrical optics that if curvatures remained unchanged, a thinner homogeneous lens would have more power than a thicker lens, setting an inverse correlation between lens thickness and lens power in a homogeneous lens. The positive correlation between lens power and lens thickness described in this study has also been reported in children,[40] and may be explained by lens growth and compaction. A thicker lens is undoubtedly a lens that has had a higher rate of growth than a thinner one. If compaction of lens fibers is a self-regulated process due to aging, this increased rate of growth could make the gradient index shallower, thereby increasing lens power. It is difficult to understand how lens power and AL reciprocally adapt to each other showing a high negative correlation. It is possible that changes in lens power are broad developmental phenomena, rather than finely-tuned emmetropization mechanisms. In this sense, there was no correlation between refraction and lens power in this sample [Table 3]. Nevertheless, differences in lens power and lens thickness appear to play a role in final determination of hyperopic spherical equivalent refraction. Myopic eyes in this study had thinner lenses than other refractive groups but did not show lower lens power [Figure 7].
Figure 3.
Normal distribution of corneal power.
Our findings suggest that hyperopic subjects in an older population are a mixture of two groups of individuals: one group are people who were hyperopes since childhood, with relatively short ALs or flat corneas (low AL/CR ratio) and high powered lenses; the other group are subjects who may have been emmetropic at the end of adolescence (with medium AL/CR) and then developed decreased lens power due to lower effective refractive index of the lens. The correlation between lens power and refraction in adults could be positive or negative according to how many hyperopic subjects were hyperopes since childhood or had become hyperopic through loss of lens power. The analysis in Table 3 shows that >70% of adult hyperopes in this study had normal AL/CR, possibly representing emmetropes that became hyperopes with aging.
One limitation of this study is that the lens power calculations were based on ocular biometric parameters and manifest distance refraction, without direct measurement of lens curvature. This method of calculating lens power, which was developed by Bennett, was tested and the verification suggested an inherent error of ±1 diopter when calculating individual lenses,[27,41] but is very accurate for the calculation of mean values of lens power.[42] Very few studies have given data for calculated lens power and calculated effective refractive index based on biometry, refraction and lens radii measurement.[11,13,22] Those studies have shown that there are inter- and intra-individual variations in calculated lens power and calculated lens effective refractive index. Without lens radii measurements, the present study has not been able to estimate lens refractive index, and hence, but the method was very accurate for calculation of mean values of lens power.[42] Very few studies have provided data for calculated lens power and effective refractive index of the lens based on biometry, refraction and lens radii measurements.[11,13,22] Those studies have shown that there are inter- and intra-individual variations in calculated lens power and effective refractive index of the lens. Without lens radii measurements, the present study has not been able to estimate refractive index of the lens, and hence, for lens power formula, Gulstrand's schematic eye values were used.
In summary, comparing studies in children with our findings in older adults, the general impression is that AL represents the principal variable determining refractive error, since correction of hyperopic errors in infants is largely achieved through axial elongation, and myopic refractive errors are produced by axial elongation. Corneal power stabilizes quite early in development, but major changes in lens power during childhood and during the course of adult life. In childhood, axial elongation is accompanied by reductions in lens power, which can partially compensate for the tendency toward myopic shifts, while in adult life, reductions in lens power may continue, resulting in hyperopic shifts. These reductions in lens power obviously have a major impact on final refractive status, but there is not strong correlation between lens power and refraction (when cataract subjects are excluded) and the distribution of lens power is normal rather than kurtotic, all of which suggest that these mechanisms do not refine refractions with the precision given by the regulation of AL growth.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Tron EJ. The optical elements of the refractive power of the eye. In: Ridley F, Sorsby A, editors. Modern Trends in Ophthalmology. London: Butterworth and Co; 1940. pp. 245–255. [Google Scholar]
- 2.Stenstrom S. Variations and correlations of the optical components of the eye. In: Sorsby A, editor. Modern Trends in Ophthalmology. II. London: Butterworth and Co; 1948. pp. 87–102. [Google Scholar]
- 3.Grosvenor T, Scott R. Role of the axial length/corneal radius ratio in determining the refractive state of the eye. Optom Vis Sci. 1994;71:573–579. doi: 10.1097/00006324-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 4.François J, Goes F. Ultrasonographic study of 100 emmetropic eyes. Ophthalmologica. 1977;175:321–327. doi: 10.1159/000308676. [DOI] [PubMed] [Google Scholar]
- 5.Sorsby A, Benjamin B, Sheridan M, Stone J, Leary GA. Refraction and its components during the growth of the eye from the age of three. Memo Med Res Counc. 1961;301(Special):1–67. [PubMed] [Google Scholar]
- 6.Sorsby A, Leary GA. A longitudinal study of refraction and its components during growth. Spec Rep Ser Med Res Counc (G B) 1969;309:1–41. [PubMed] [Google Scholar]
- 7.Sorsby A. Emmetropia and its aberrations. Trans Opthal Soc U K. 1956;76:167–169. [PubMed] [Google Scholar]
- 8.Carroll JP. Component and correlation ametropia. Am J Optom Physiol Opt. 1982;59:28–33. doi: 10.1097/00006324-198201000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Larsen JS. The sagittal growth of the eye. IV. Ultrasonic measurement of the axial length of the eye from birth to puberty. Acta Ophthalmol (Copenh) 1971;49:873–886. doi: 10.1111/j.1755-3768.1971.tb05939.x. [DOI] [PubMed] [Google Scholar]
- 10.Larsen JS. The sagittal growth of the eye. II. Ultrasonic measurement of the axial diameter of the lens and the anterior segment from birth to puberty. Acta Ophthalmol (Copenh) 1971;49:427–440. doi: 10.1111/j.1755-3768.1971.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 11.Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–3080. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- 12.Mutti DO, Zadnik K, Fusaro RE, Friedman NE, Sholtz RI, Adams AJ. Optical and structural development of the crystalline lens in childhood. Invest Ophthalmol Vis Sci. 1998;39:120–133. [PubMed] [Google Scholar]
- 13.Jones LA, Mitchell GL, Mutti DO, Hayes JR, Moeschberger ML, Zadnik K. Comparison of ocular component growth curves among refractive error groups in children. Invest Ophthalmol Vis Sci. 2005;46:2317–2327. doi: 10.1167/iovs.04-0945. [DOI] [PubMed] [Google Scholar]
- 14.Gordon RA, Donzis PB. Refractive development of the human eye. Arch Ophthalmol. 1985;103:785–789. doi: 10.1001/archopht.1985.01050060045020. [DOI] [PubMed] [Google Scholar]
- 15.Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–1628. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- 16.Shih YF, Chiang TH, Lin LL. Lens thickness changes among schoolchildren in Taiwan. Invest Ophthalmol Vis Sci. 2009;50:2637–2644. doi: 10.1167/iovs.08-3090. [DOI] [PubMed] [Google Scholar]
- 17.Brown N. The change in lens curvature with age. Exp Eye Res. 1974;19:175–183. doi: 10.1016/0014-4835(74)90034-7. [DOI] [PubMed] [Google Scholar]
- 18.Koretz JF, Handelman GH. How the human eye focuses. Sci Am. 1988;259:92–99. doi: 10.1038/scientificamerican0788-92. [DOI] [PubMed] [Google Scholar]
- 19.Brown NP, Koretz JF, Bron AJ. The development and maintenance of emmetropia. Eye (Lond) 1999;13(Pt 1):83–92. doi: 10.1038/eye.1999.16. [DOI] [PubMed] [Google Scholar]
- 20.Fotouhi A, Morgan IG, Iribarren R, Khabazkhoob M, Hashemi H. Validity of noncycloplegic refraction in the assessment of refractive errors: The Tehran Eye Study. Acta Ophthalmol. 2012;90:380–386. doi: 10.1111/j.1755-3768.2010.01983.x. [DOI] [PubMed] [Google Scholar]
- 21.Pierscionek BK. Presbyopia – Effect of refractive index. Clin Exp Optom. 1990;73:23–30. [Google Scholar]
- 22.Dubbelman M, Van der Heijde GL. The shape of the aging human lens: Curvature, equivalent refractive index and the lens paradox. Vision Res. 2001;41:1867–1877. doi: 10.1016/s0042-6989(01)00057-8. [DOI] [PubMed] [Google Scholar]
- 23.Fotouhi A, Hashemi H, Shariati M, Emamian MH, Yazdani K, Jafarzadehpur E, et al. Cohort profile: Shahroud Eye Cohort Study. Int J Epidemiol. 2013;42:1300–1308. doi: 10.1093/ije/dys161. [DOI] [PubMed] [Google Scholar]
- 24.Olsen T, Arnarsson A, Sasaki H, Sasaki K, Jonasson F. On the ocular refractive components: The Reykjavik Eye Study. Acta Ophthalmol Scand. 2007;85:361–366. doi: 10.1111/j.1600-0420.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- 25.Olsen T. On the calculation of power from curvature of the cornea. Br J Ophthalmol. 1986;70:152–154. doi: 10.1136/bjo.70.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett AG. A method of determining the equivalent powers of the eye and its crystalline lens without resort to phakometry. Ophthalmic Physiol Opt. 1988;8:53–59. doi: 10.1016/0275-5408(88)90089-0. [DOI] [PubMed] [Google Scholar]
- 27.Gullstrand A. Helmholtz's Handbuch der Physiologischen Optik (English translation edited by Southall JP Optical Society of America, 1924) 3rd ed. Vol. 1. Hamburg and Leipzig; 1909. Appendix II: Procedure of the rays in the eye. Imagery-Laws of the first order; pp. 350–358. [Google Scholar]
- 28.Iribarren R, Morgan IG, Nangia V, Jonas JB. Crystalline lens power and refractive error. Invest Ophthalmol Vis Sci. 2012;53:543–550. doi: 10.1167/iovs.11-8523. [DOI] [PubMed] [Google Scholar]
- 29.Garner LF, Yap M, Scott R. Crystalline lens power in myopia. Optom Vis Sci. 1992;69:863–865. doi: 10.1097/00006324-199211000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Ooi CS, Grosvenor T. Mechanisms of emmetropization in the aging eye. Optom Vis Sci. 1995;72:60–66. doi: 10.1097/00006324-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Ip JM, Huynh SC, Kifley A, Rose KA, Morgan IG, Varma R, et al. Variation of the contribution from axial length and other oculometric parameters to refraction by age and ethnicity. Invest Ophthalmol Vis Sci. 2007;48:4846–4853. doi: 10.1167/iovs.07-0101. [DOI] [PubMed] [Google Scholar]
- 32.Goss DA, Van Veen HG, Rainey BB, Feng B. Ocular components measured by keratometry, phakometry, and ultrasonography in emmetropic and myopic optometry students. Optom Vis Sci. 1997;74:489–495. doi: 10.1097/00006324-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Grosvenor T, Skeates PD. Is there a hyperopic shift in myopic eyes during the presbyopic years? Clin Exp Optom. 1999;82:236–243. doi: 10.1111/j.1444-0938.1999.tb06654.x. [DOI] [PubMed] [Google Scholar]
- 34.Iribarren G, Iribarren R, Torres M. Lens power in an adult population: The Los Angeles Latino Eye Study. Invest Ophthalmol. 2010;51 ARVO E-abstract 1717. [Google Scholar]
- 35.Hashemi H, Iribarren R, Morgan IG, Khabazkhoob M, Mohammad K, Fotouhi A. Increased hyperopia with ageing based on cycloplegic refractions in adults: The Tehran Eye Study. Br J Ophthalmol. 2010;94:20–23. doi: 10.1136/bjo.2009.160465. [DOI] [PubMed] [Google Scholar]
- 36.Augusteyn RC. Growth of the lens: In vitro observations. Clin Exp Optom. 2008;91:226–239. doi: 10.1111/j.1444-0938.2008.00255.x. [DOI] [PubMed] [Google Scholar]
- 37.Atchison DA, Charman WN. Thomas Young's contribution to visual optics: The Bakerian Lecture “on the mechanism of the eye”. J Vis. 2010;10:16. doi: 10.1167/10.12.16. [DOI] [PubMed] [Google Scholar]
- 38.Donders FC. London: New Sydenham Society; 1864. On the Anomalies of Accommodation and Refraction of the Eye. [Google Scholar]
- 39.Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Invest Ophthalmol Vis Sci. 2008;49:2531–2540. doi: 10.1167/iovs.07-1443. [DOI] [PubMed] [Google Scholar]
- 40.Iribarren R, Morgan IG, Chan YH, Lin X, Saw SM. Changes in lens power in Singapore Chinese children during refractive development. Invest Ophthalmol Vis Sci. 2012;53:5124–5130. doi: 10.1167/iovs.12-9637. [DOI] [PubMed] [Google Scholar]
- 41.Dunne MC, Barnes DA, Royston JM. An evaluation of Bennett's method for determining the equivalent powers of the eye and its crystalline lens without resort to phakometry. Ophthalmic Physiol Opt. 1989;9:69–71. doi: 10.1111/j.1475-1313.1989.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 42.Rozema JJ, Atchison DA, Tassignon MJ. Comparing methods to estimate the human lens power. Invest Ophthalmol Vis Sci. 2011;52:7937–7942. doi: 10.1167/iovs.11-7899. [DOI] [PubMed] [Google Scholar]