Abstract
Purpose:
To evaluate the feasibility of screening for retinopathy of prematurity (ROP) by assessing the pupillary response to mydriatics.
Methods:
This observational case series included 134 eyes of 67 premature infants with birth weight less than 2,000 grams and gestational age less than 33 weeks. A composite eye drop composed of phenylephrine 1%, tetracaine and tropicamide 0.5% was applied 3 times within 5-minute intervals and pupil diameters were measured. The eyes were examined by experienced ROP specialists using an indirect ophthalmoscope. Zone and stage of ROP, presence of plus disease and need for treatment were recorded. The relationship between the pupillary response to mydriatics, and presence and severity of ROP was evaluated. Logistic regression was used for statistical analysis.
Results:
According to receiver operating characteristic (ROC) curve analysis, final pupil diameter after mydriatic administration was found the most accurate factor among other factors to recognize ROP zone I from zones II and III (Area under ROC: 0.92 [95%CI: 0.85-0.98]). The best cutoff value for final pupil diameter was 5.6 mm, because it could differentiate involvement of zone I from zones II and III with sensitivity of 80% and specificity of 100%.
Conclusion:
Response of the pupil to mydriatic eye drops may be useful as a less invasive method for rough estimation of ROP in high risk babies who need emergent attention; however, it cannot be considered as a screening test due to its low sensitivity.
Keywords: Mydriatic Eye Drops, Predictive Value, Pupillary Response, Retinopathy of Prematurity, Screening
INTRODUCTION
Retinopathy of prematurity (ROP) is a potentially blinding condition but may be prevented with early detection and treatment.[1] The routine procedure for ROP screening requires insertion of an eyelid speculum and scleral indentation for better visualization of the peripheral retina. Vulnerable premature infants seem to undergo significant distress and may sustain potentially serious complications such as apnea and intraventricular hemorrhages secondary to such examinations.[1,2,3,4] Therefore researchers always seek methods to substitute examinations or reduce the number of sessions. The WINROP screening algorithm uses a less invasive method for diagnosis of ROP by measuring factors such as weight and insulin-like growth factor 1.[5,6,7]
A relationship seems to exist between pupil rigidity on one hand and high stages of ROP and plus disease (fulminant ROP) on the other hand.[8] In the present study, we evaluated whether poor pupillary response to mydriatics can predict the presence of ROP.
METHODS
This observational case series included 134 eyes of 67 premature infants with birth weight less than 2,000 grams and gestational age less than 33 weeks who were admitted at our ROP screening clinic. Informed consent was obtained from the parents or guardians of the infants prior to the study. The whole process of the study and the data collection scheme were approved by the ethics committee, conformed to local regulations and were compliant with principles of the Declaration of Helsinki.
Neonates with anterior segment anomalies, microcornea (less than 8 mm), iris coloboma, aniridia, posterior synechiae, pupillary membranes, history of any eye surgery, use of mydriatic or miotic eye drops, and stage 5 ROP were excluded from the study. The first examination of each patient was considered for the purpose of the study and there was no subject with gestational age more than 38 weeks at the time of this examination.
Baseline horizontal pupillary diameter was measured with a ruler that allowed a reading accuracy of 0.5 mm under the maximum illumination of a portable indirect ophthalmoscope. The measurements were obtained by two individuals and average values were calculated and considered as baseline pupil diameter. Phenylephrine 1%, tetracaine and tropicamide 0.5% eye drops were mixed for maximal benefit. This composite eye drop was administered three times within five-minute intervals and pupil diameter was measured after instillation of each drop and continued every 10 minutes until the diameter reached at least 6 mm, or until 60 minutes had elapsed in patients whose pupil diameter did not reach 6 mm (non-responders). We used tetracaine as a topical anesthetic and also for better penetration of mydriatics through the corneal epithelium.
Meanwhile, all eyes were examined by experienced ROP specialists using an indirect ophthalmoscope and a 20-diopter lens. Zone and stage of ROP, presence of plus disease and the necessity for treatment were recorded. Finally, the relationship between pupillary response to mydriatics, and presence and severity of ROP was evaluated. Pupillary response was evaluated by two factors; the time to reach maximal pupil diameter and the extent of pupil diameter changes (i.e., final pupil diameter minus baseline pupil diameter). The correlation between pupillary response and iris pigmentation, plus disease, zone of involvement, stage of ROP, birth weight, gestational age, gender and baseline pupil diameter were studied.
Statistical Analysis
Data was analyzed using STATA version 11 (StataCorp, College Station, TX, USA). Categorical variables were presented in numbers (%) while continuous data were presented using median (interquartile range: [IQR]) or mean (±SD) values as appropriate. Since the normality of distribution was violated, non-parametric tests including Mann-Whitney U and Kruskal-Wallis H were used to compare the study groups. Categorical variables were tested employing Pearson's Chi square test. Bonferroni correction was used to adjust for multiple comparisons. Spearman's rank correlation test was performed to assess the correlation between variables. In order to assess the independent association of variables with the response to mydriatic administration (as a categorical variable: Below 6 mm or over 6 mm), we performed logistic regression analysis; variables with P values less than 0.2 in univariate regression analysis were included in the multivariate model. For categorical variables with more than 2 layers, dummy variables were created in the logistic regression model and the first layer of the variable was considered as the reference value. P values less than 0.05 were considered as statistically significant. Receiver operating characteristics (ROC) curve analysis was undertaken to evaluate the predictive accuracy of the response to mydriatic administration for diagnosis of ROP, plus disease, stage and zone of ROP and also ROP requiring treatment.
RESULTS
Out of 67 infants (134 eyes), 52 (38.81%) subjects were female and mean birth weight was 1,452 ± 345.7 g and mean final pupil diameter after mydriatic administration was 6.04 ± 0.88 mm. ROP was diagnosed in 52 (38.81%) subjects using indirect ophthalmoscopy. A total of 26 (19.4%) subjects had a poor response to mydriatic administration (less than 6 mm). Baseline characteristics of cases are detailed in Table 1.
Table 1.
Characteristics of patients
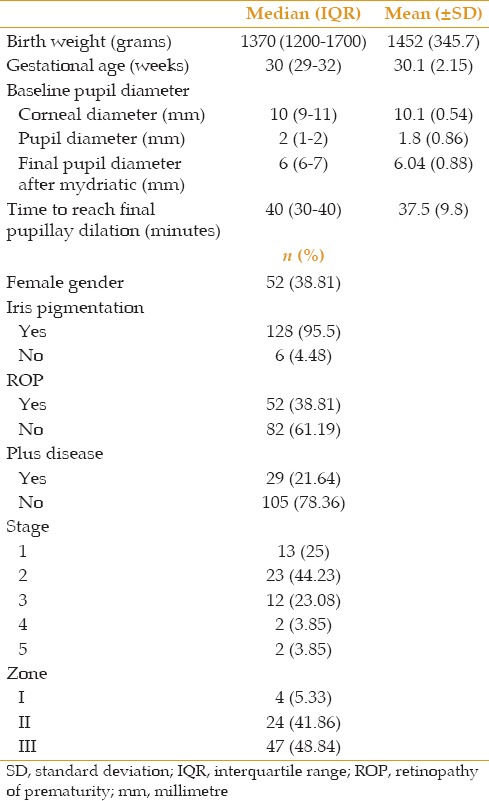
Final pupil diameter after mydriatic administration as a continuous variable was not associated with gender or iris pigmentation. However, final pupil diameter was significantly smaller in patients with ROP than those without ROP (5.8 ± 0.96 vs. 6.4 ± 0.61 mm, P = 0.001, Table 2) as well as in those with plus disease as compared to subjects without plus disease (5.38 ± 0.98 vs. 6.2 ± 0.77 mm, P < 0.001, Table 2). Mean final pupil diameter also varied significantly between different stages and zones of ROP (P = 0.001 and P < 0.001, respectively, Table 2). Considering the response to mydriatic administration as a categorical variable (defined as less than 6 mm and over 6 mm), we observed no significant difference between responders and non-responders with respect to mean (or median) birth weight, gestational age, or corneal and pupil diameter [Table 3].
Table 2.
Comparing mean final pupil diameter with respect to gender, iris pigmentation, ROP, plus disease. Stage and zone of ROP
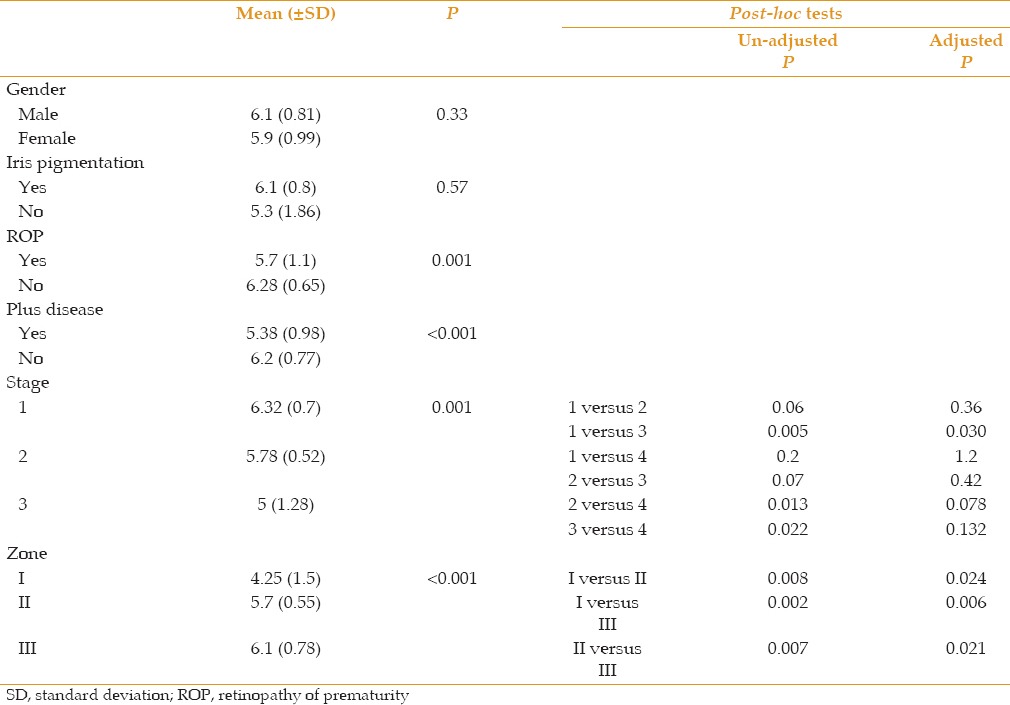
Table 3.
Response to mydriatic administration (defined as below 6 mm or over 6 mm) with respect to other characteristics of subjects
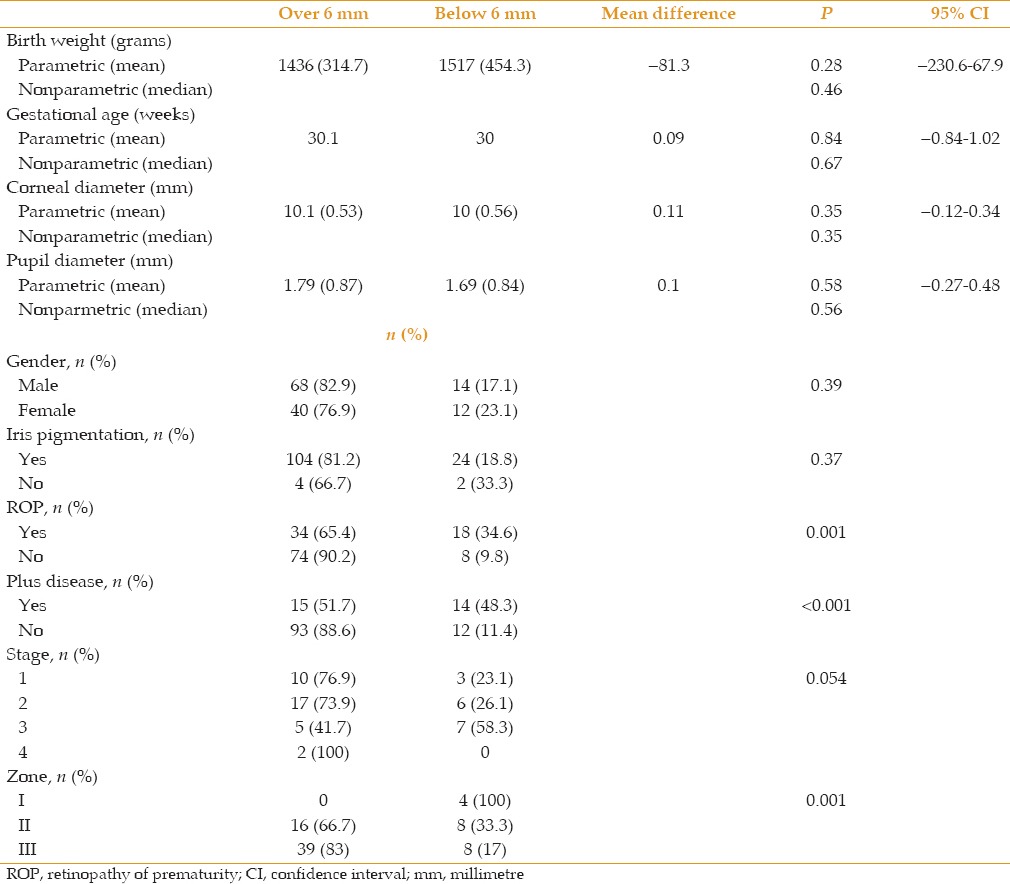
The percentage of response to mydriatic administration did not differ between male and female subjects, cases with light or dark iris pigmentation and those with different stages of ROP [Table 3]; however, this did not appear to be true regarding the presence of ROP and plus disease and also between different zones of ROP [Table 3]. The percentage of response to mydriatic administration was significantly lower in patients with ROP (as compared to non-ROP subjects) or plus disease (as compared to those without plus disease). None of the patients with involvement of zone I responded to mydriatics, while 66.7% and 83.3% of subjects with involvement in zones II and III responded to mydriatics, respectively. Final pupil diameter after mydriatic administration was significantly and inversely correlated with the presence of ROP and plus disease, stage of ROP and also directly with involved zones in ROP [Table 4].
Table 4.
Spearman's rank correlation coefficient between final pupil diameter after mydriatic administration and other variables
Mean time for reaching final pupil diameter was not correlated with the presence of ROP and plus disease, stage of ROP and involved zones. Area under ROC (AUROC) curve for diagnosis of ROP was 0.60 (Confidence interval (CI), 95%:0.51-0.69). The best cutoff value for this time was 40 minutes for the diagnosis of ROP with sensitivity and specificity of 61.8 and 57.3%, respectively [Table 5].
Table 5.
ROC curve analysis to evaluate the predictive accuracy of time to highest pupil diameter after mydriatic administration
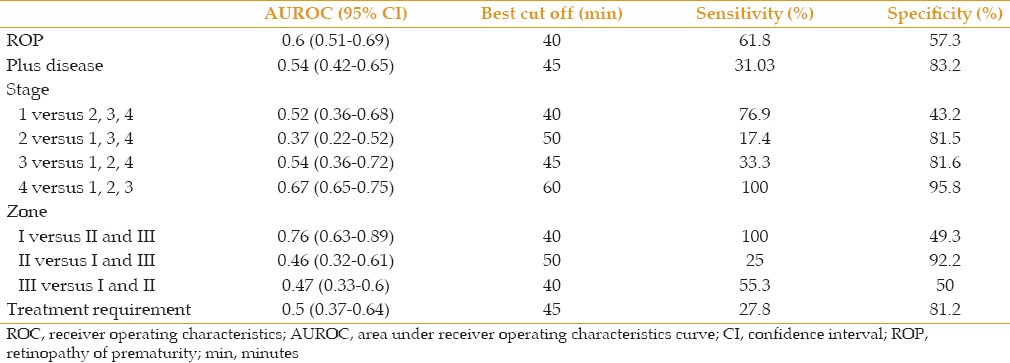
Table 6 outlines the results of logistic regression analysis to find correlations of the response to mydriatic administration (as a categorical variable: Less than 6 mm or more than 6 mm). In univariate analysis, the presence of ROP, presence of plus disease and stage of ROP were associated with the response to mydriatic administration with P value less than 0.2 and accordingly were included in multivariate analysis. However, their association lost significance in multivariate analysis which might partly be explained with small sample size. Tables 7 and 8 describe the results of ROC curve analysis to evaluate the predictive accuracy of final pupil diameter after mydriatic administration as a continuous variable [Table 7] and response to mydriatic administration as a categorical variable (below 6 mm and above 6 mm) [Table 8].
Table 6.
Logistic regression analysis of response to mydriatic administration (defined as below 6 mm and over 6 mm) and other variables
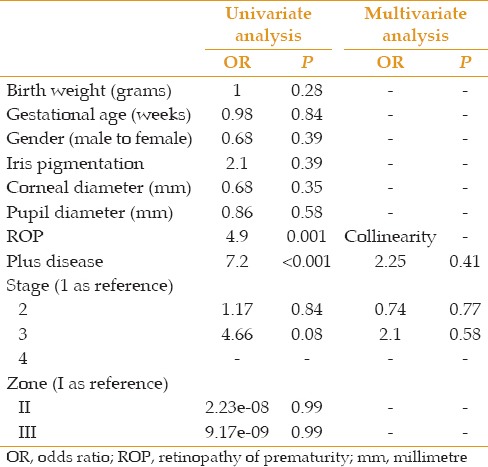
Table 7.
ROC curve analysis results to evaluate the predictive accuracy of final pupil diameter after mydriatic administration as a continuous variable
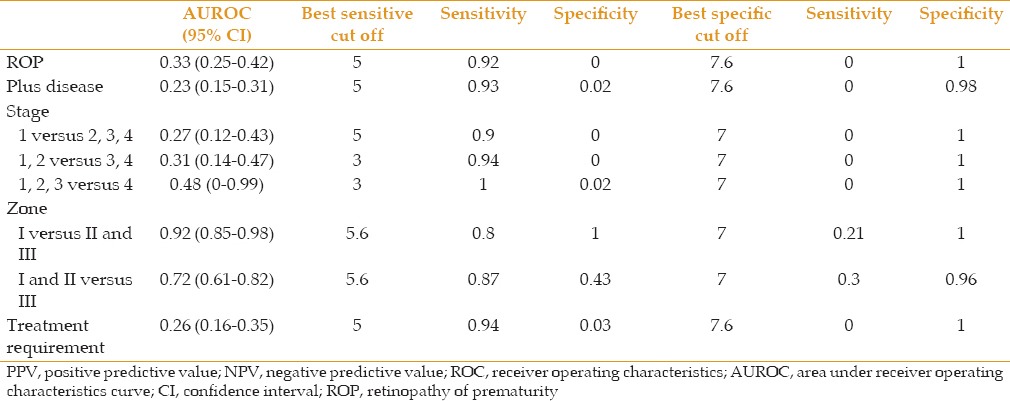
Table 8.
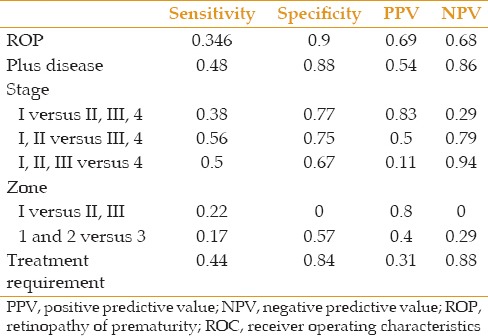
ROC curve analysis results to evaluate the predictive accuracy of response to mydriatic administration as a categorical variable (defined as below 6 mm and over 6 mm)
Among all parameters including final pupil diameter and extent of changes after mydriatic administration and time to reach to final pupil diameter, final pupil diameter was the most accurate factor distinguishing between involvement of zone I from zones II and III (AUROC: 0.92, CI 95%:0.85-0.98). The best cutoff value was 5.6 mm which was able to distinguish involvement of zone I from zone II and III with sensitivity of 80% and specificity of 100%. The sensitivity and specificity for the cutoff value of 5.6 mm to diagnose involvement of zones I and II from III were 87% and 43%, respectively. The predictive accuracy of final pupil diameter after mydriatic administration was considerably low for diagnosing ROP, plus disease and also to differentiate stages of ROP. However, cutoff value of 5 mm had sensitivity of 92% and 93% to detect ROP and plus disease, respectively (but with very low specificity). The best sensitive and specific cutoff values to distinguish different stages of ROP are presented in Table 7.
We found the cutoff pupil diameter of 5.6 mm having the best capability to detect premature infants requiring treatment with a sensitivity of 94% (with very low specificity).
The sensitivity of the response to mydriatic drops (defined as below 6 mm or above 6 mm) for ROP diagnosis was not acceptable. The sensitivity of the pupil diameter less than 6 mm was 34.6% and 48% for ROP and plus disease, respectively. Its specificity was measured 90% for ROP and 88% for plus disease [Table 8]. The predictive values of the response to mydriatic agents to differentiate between stages and zones of ROP are presented in Table 8. Pupil dilation due to mydriatic administration was able to detect patients with an indication for treatment with sensitivity of 44% and specificity of 84%.
DISCUSSION
The present study demonstrated that poor pupil dilatation after applying mydriatic eye drops is a sign of ROP and its severity. The percentage of patients with ROP or plus disease who responded to mydriatic administration (>6 mm) was significantly lower than subjects without ROP or plus disease. Although poor pupillary dilation was a strong predictor of lower zones of ROP involvement (zone I versus zones II and III), such a strong correlation for distinguishing between different stages of the disease did not exist.
It may be assumed that the degree of iris pigmentation may affect final pupillary diameter; however, in our cases heavier or lighter iris pigmentation did not show any significant association with the response to mydriatic agents. The explanation might be that all of our patients were from Persian ethnicity and consequently, there were no detectable differences in their physiologic response to mydriatic eye drops based on the level of iris pigmentation. The other reason may be the small number of patients with pupillary dilation. Congenital pupillary membranes may cause poor pupillary response independent of ROP, thus patients with this entity excluded from our study.[9,10]
Since routine procedures for ROP examination are painful and there is no standard protocol for effective pain management[11] and because local anesthetics can only partially decrease pain which may have adverse systemic effects on these neonates,[12] finding more gentle ways to predict ROP and the necessity for treatment is valuable.
There are various regimens for pupillary dilation in ROP examination. We used a mixture of phenylephrine 1%, tetracaine and tropicamide 0.5% which was administered three times every 5 minutes and waited up to a maximum of 60 minutes to determine whether the pupil reaches a 6 mm diameter. Different regimens have been studied in the literature. Chew et al mentioned three different mydriatic regimens for ROP screening in premature infants with dark irides: (1) Cyclopentolate 1% and phenylephrine 2.5%, (2) tropicamide 1% and phenylephrine 2.5%, and (3) normal saline, phenylephrine 1% and cyclopentolate 0.2%. A composition of cyclopentolate 0.2% plus phenylephrine 1% eye drops was considered as the best mydriatic regimen in premature infants with dark irides.[13] Applying eye drops three times is stated appropriate for achieving a pupil diameter of 6 mm and more which is considered adequate for examining the peripheral retina.[14]
Pupillary response to mydriatic agents has been studied for evaluating its predictive value in some diseases. For instance, according to Koc et al, poor pupillary response in diabetes mellitus was found to be correlated with the duration of diabetes mellitus and the presence of diabetic retinopathy.[15] Similar studies also found that the extent of pupillary dilation may be a screening test for duration of diabetes and diabetic neuropathy and retinopathy.[16,17] Sharma et al studied screening of ROP in developing countries and reported resistance to pupil dilation in Stage 5 ROP.[17] The other survey conducted by Astasheva et al evaluated the prevalence and risk factors of fulminant ROP (plus disease) and showed that pupil rigidity is an absolute sign of plus disease.[18]
Our findings showed that a cutoff value of 5.6 mm for final pupil diameter may best distinguish between involvement of zone I form zones II and III due to its high sensitivity (80%) and specificity (100%). Choosing higher cutoff points may lead to missing neonates who require early treatment due to its low sensitivity although the specificity is still remaining high.
Small sample size is one of the limitations of our study. Furthermore, measuring pupil diameter with accuracy of 0.1 mm is not possible with a ruler, thus using pupillometer in future studies may be suggested for more accurate measurements. By calculating the area under curve, we attained a cutoff pupil diameter of 5.6 mm; otherwise, measuring with a ruler could not have allowed the accuracy of 0.6 decimal fractions. Other parameters such as time to reach maximum pupil diameter and extent of changes in pupil diameter were not strong enough to diagnose ROP and plus disease. They do not have acceptable sensitivity and specificity for a high predictive value.
In summary, among parameters representing the pupillary response to mydriatic eye drops, final pupil diameter had the best predictive accuracy for distinguishing zone I ROP from zone II and III disease. However, since the sensitivity of this test was not 100%, it cannot be considered a suitable screening test or a substitute for fundus examination using indirect ophthalmoscopy which is the gold standard for ROP screening. Due to the fact that diagnosis of all cases of ROP is crucial, it is not desirable to miss even one case. Therefore, according to our findings, pupillary response to mydriatic eye drops can be considered as a less invasive method for rough estimation of emergent cases of ROP but not as a screening test.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Mitchell AJ, Green A, Jeffs DA, Roberson PK. Physiologic effects of retinopathy of prematurity screening examinations. Adv Neonatal Care. 2011;11:291–297. doi: 10.1097/ANC.0b013e318225a332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belda S, Pallás CR, De la Cruz J, Tejada P. Screening for retinopathy of prematurity: Is it painful? Biol Neonate. 2004;86:195–200. doi: 10.1159/000079542. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73:1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- 4.Ballabh P. Intraventricular hemorrhage in premature infants: Mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González Viejo I, Ferrer Novella C, Pueyo Royo V. The WINROP algorithm and other innovations in the screening for retinopathy of prematurity. Arch Soc Esp Oftalmol. 2013;88:43–44. doi: 10.1016/j.oftal.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Zepeda-Romero LC, Hård AL, Gomez-Ruiz LM, Gutierrez-Padilla JA, Angulo-Castellanos E, Barrera-de-Leon JC, et al. Prediction of retinopathy of prematurity using the screening algorithm WINROP in a Mexican population of preterm infants. Arch Ophthalmol. 2012;130:720–723. doi: 10.1001/archophthalmol.2012.215. [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, Löfqvist C, Hellström A, Heo H. Efficacy of the screening algorithm WINROP in a Korean population of preterm infants. JAMA Ophthalmol. 2013;131:62–66. doi: 10.1001/jamaophthalmol.2013.566. [DOI] [PubMed] [Google Scholar]
- 8.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 9.Lambert SR, Buckley EG, Lenhart PD, Zhang Q, Grossniklaus HE. Congenital fibrovascular pupillary membranes: Clinical and histopathologic findings. Ophthalmology. 2012;119:634–641. doi: 10.1016/j.ophtha.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesarwani S, Murthy R, Vemuganti GK. Surgical technique for removing congenital fibrovascular pupillary membrane, with clinicopathological correlation. J AAPOS. 2009;13:618–620. doi: 10.1016/j.jaapos.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Samra HA, McGrath JM. Pain management during retinopathy of prematurity eye examinations: A systematic review. Adv Neonatal Care. 2009;9:99–110. doi: 10.1097/ANC.0b013e3181a68b48. [DOI] [PubMed] [Google Scholar]
- 12.Kwon HJ, Kim HY. A pharmacologic pupillary test in the diagnosis of diabetic autonomic neuropathy. Korean J Ophthalmol. 2009;23:291–295. doi: 10.3341/kjo.2009.23.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chew C, Rahman RA, Shafie SM, Mohamad Z. Comparison of mydriatic regimens used in screening for retinopathy of prematurity in preterm infants with dark irides. J Pediatr Ophthalmol Strabismus. 2005;42:166–173. doi: 10.3928/01913913-20050501-05. [DOI] [PubMed] [Google Scholar]
- 14.Punyawattanaporn A, Tengtrisorn S, Sangsupawanich P. Pupil dilatation after single and triple doses of mydriatic agent in preterm infants. J Med Assoc Thai. 2009;92:1458–1462. [PubMed] [Google Scholar]
- 15.Koc F, Kansu T, Kavuncu S, Firat E. Topical apraclonidine testing discloses pupillary sympathetic denervation in diabetic patients. J Neuroophthalmol. 2006;26:25–29. doi: 10.1097/01.wno.0000204648.79744.71. [DOI] [PubMed] [Google Scholar]
- 16.Zaczek A, Zetterström C. The effect of phenylephrine and pilocarpine on pupil size and aqueous flare intensity in patients with diabetes mellitus. Acta Ophthalmol Scand. 1998;76:413–416. doi: 10.1034/j.1600-0420.1998.760405.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma R, Gupta VP, Dhaliwal U, Gupta P. Screening for retinopathy of prematurity in developing countries. J Trop Pediatr. 2007;53:52–54. doi: 10.1093/tropej/fml071. [DOI] [PubMed] [Google Scholar]
- 18.Astasheva IB, Sidorenko EI. Fulminant retinopathy of prematurity (“plus-disease”): Incidence, risk factors, diagnostic criteria, and variations in course. Vestn Oftalmol. 2002;118:5–9. [PubMed] [Google Scholar]


