Abstract
The present work was designed to assess the radioprotective effect of royal jelly (RJ) against radiation-induced apoptosis in human peripheral blood leukocytes. In this study, peripheral blood samples were obtained on days 0, 4, 7, and 14 of the study from six healthy male volunteers taking a 1000 mg RJ capsule orally per day for 14 consecutive days. On each sampling day, all collected whole blood samples were divided into control and irradiated groups which were then exposed to the selected dose of 4 Gy X-ray. Percentage of apoptotic cells (Ap %) was evaluated for all samples immediately after irradiation (Ap0) and also after a 24 h postirradiation incubation at 37°C in 5% CO2 (Ap24) by the use of neutral comet assay. Concerning Ap0, collected data demonstrated that the percentage of apoptotic cells in both control and irradiated groups did not significantly change during the study period. However, with respect to Ap24, the percentage of apoptotic cells in irradiated groups gradually reduced during the experiment, according to which a significant decrease was found after 14 days RJ consumption (P = 0.002). In conclusion, the present study revealed the protective role of 14 days RJ consumption against radiation-induced apoptosis in human peripheral blood leukocytes.
Keywords: Apoptosis, comet assay, ionizing radiation, leukocytes, royal jelly
Introduction
While passing through the living tissues, photons of ionizing radiation (IR) can produce reactive oxygen species (ROS), classically known as free radicals.[1] Cell dysfunction, cell damage, and cell death could be induced by the interaction of these free radicals with critical bio-macromolecules including proteins, lipids and DNA present in the cell.[2,3] The production of excessive amount of ROS such as hydroxyl radical leads to oxidative stress,[4] which is considered one of the factors initiating apoptosis (programmed-cell death).[5] Despite the fact that apoptosis is not the only mode of cell death,[6] it has a pivotal contributing role in radiation-induced cell killing effect[5,7] particularly in some cells such as lymphocytes.[8,9]
Regarding the central role of free radicals in IR-induced cellular insults, any molecule with antioxidant capacity and free radical scavenging ability would be considered a promising radioprotector. Although natural radioprotective agents have less efficiency compared to the synthetic ones, there is a trend in using natural origins due to their low toxicity.[2]
Royal jelly (RJ) is a natural food supplement mainly secreted from the hypopharyngeal and mandibular glands of honeybees (Apismellifera). It consists of different substances including water, proteins, sugar, vitamins, and free amino acids.[10,11] Features including the efficient antioxidant capacity, free radicals scavenging ability, and also oxidative stress-modulating effect[12,13,14] have made RJ attract special attention in many radiotherapy-related studies.
The normalization in oxidative stress and biochemical as well as hematological markers, in irradiated rats treated with RJ, was ascribed to the RJ antioxidant capacity.[14] Furthermore, the protective effect of RJ on radiation-induced oxidative stress was investigated in head and neck irradiated rats.[15] In addition, oral administration of RJ to whole-body irradiated rats, 10 days before and after irradiation, led to a marked increase in antioxidant activities and resulted in a substantial reduction in oxidative stress parameters such as malondialdehyde in their lung and liver samples.[16] Furthermore, studying the protective effect of RJ on radiation-induced oral mucositis in rats revealed its moderating role in oral mucositis-related biochemical and histopathological parameters.[17] Moreover, two clinical trials regarding chemoradiotherapy-induced oral mucositis demonstrated an improvement in the signs and symptoms of oral mucositis and its healing time in RJ-administered head and neck cancer patients.[18,19] Although there are several reports on the radioprotective effect of RJ in experimental animals,[14,15,16,17] there are few clinical trials regarding its radiation-modifying effect,[18,19] against radiation-induced apoptosis in particular.
Based on earlier studies indicating the protective effect of RJ against radiation-induced side effects and regarding the role of apoptosis as a biological dosimeter in some radiation-related studies,[20,21] this work intended to show the radioprotective effect of RJ against radiation-induced apoptosis in human peripheral blood leukocytes with a method combining both the in vivo administration of RJ and in vitro testing of the radioprotective effect. For assessing apoptosis, neutral comet assay was performed for all collected samples. This cytogenetic method has been widely used to measure IR-induced DNA damage and apoptosis in individual cells.[8,22,23,24,25]
Materials and Methods
Study population
This is a quasi-experimental study with six healthy male volunteers (mean age 26 ± 4 years and mean body mass index 20.68 ± 2.23) recruited in April and May 2015. The aim of the study with its benefit and risk were explained to the participants. Afterward, informed consent was obtained from all individual participants. The participants were nonsmokers with no history of antibiotics and RJ consumption or medical radiation exposure during last 2 months prior to the blood samplings, and they were asked to keep their usual diets during the course of study.
The study has been registered with an IRCT2014090819091N1 number in Iranian Registry of Clinical Trial and all its ethical points have been considered and approved by the Ethics Committee of Babol University of Medical Sciences, Babol, Iran.
Royal jelly administration
The 1000 mg RJ soft gelatin capsules were purchased from Marnys® Company, Spain. All participants took one 1000 mg RJ capsule orally per day for 14 consecutive days.
Blood sampling
To examine the protective effect of RJ against radiation-induced apoptosis during a 14 days RJ consumption period, peripheral blood samples were collected from all participants on days 0, 4, 7, and 14 of the study. At each sampling time, 2 ml of venous blood was obtained by venipuncture into the heparinized tube from each participant. The collected heparinized samples were equally divided into two aliquots as control and irradiated groups.
Irradiation
Five minutes prior to irradiation, the cell containing microtubes were placed in an ice water phantom and were irradiated using photon mode (6 MV) of a CLINAC (Clinical Siemens Primus Linac). Radiation dose was 4 Gy with the dose rate of 1.8 Gy/min.
Comet assay
In this study, apoptotic as well as nonapoptotic cells in both control and irradiated groups were assessed by the use of neutral comet assay[23] with some modifications, immediately after irradiation and also after a 24 h postirradiation incubation at 37°C in a humidified atmosphere of 95% air and 5% CO2.
All materials were purchased from Merck Company, Germany unless otherwise mentioned. The two-window roughened side of the comet assay microscopic slides (Sotooneh, Iran) was precoated with a layer of 1% normal melting point agarose (Fermentas, Lithuania) dissolved in distilled water. 10 μl of whole blood was mixed with 140 μl of 0.75% low melting point agarose (Fermentas, Lithuania) dissolved in phosphate buffer saline. 50 μl of the mixture was overlaid on top of each window of the frosted microscope slides, covered with a coverslip and kept at 4°C for about 3 min to let the gel solidify. Then, the coverslips were gently removed and the slides were immersed in the freshly prepared lysing buffer (2.5 M NaCl, 0.1 M Na2 EDTA, 10 mM tris-base, 1% Triton X-100, 10% dimethyl sulfoxide, pH 10) for 30 min at 4°C in the dark to digest DNA-bound proteins in order not to limit the DNA migration in the electric field.
Following lysing step, the slides were transferred into the tris-borate-EDTA (TBE) electrophoresis buffer (90 mM tris-base, 90 mM Boric acid and 2.5 mM Na2 EDTA, pH 8.3–8.4) for 15 min at 4°C to allow the slides to be rinsed. Afterward, the slides were transferred to a submarine horizontal electrophoresis chamber filled with the TBE electrophoresis buffer. Electrophoresis was carried out for 10 min at 20 V and 9 mA at 4°C. Subsequently, the slides were rinsed with distilled water for 5 min so as to anneal DNA and were finally dehydrated in 96% ethanol at room temperature. The air-dried slides were stained with 20 μg/ml ethidium bromide (Sigma) dissolved in distilled water and covered with coverslips for observation. The slides were viewed, and images were captured at ×200 magnification using a fluorescent microscope (E-800, Nikon, Japan) equipped with an excitation filter (510–550 nm) and barrier filter (590 nm) attached to a charge-coupled device camera.
In the comet assay, apoptotic cells appear with a diffuse “fan-like” tail and a small head while normal cells have minimal DNA diffusion with a more defined head [Figure 1]. Slides were analyzed visually for the percentage of apoptotic cells (Ap %); the ratio of apoptotic cells multiplied by 100 to the number of total cells examined.[26] Approximately, 500–700 cells were analyzed for each slide and at least 1000 cells were counted for each sample.
Figure 1.
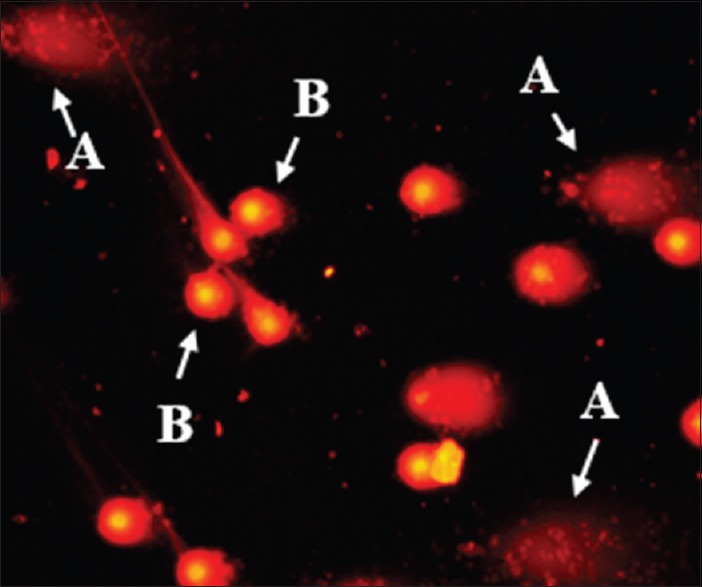
A photomicrograph of apoptotic (A) and normal (B) peripheral blood leukocytes (at ×200)
Six experimental parameters were assessed to characterize cellular radiation effects immediately after irradiation (Ap0) and also after the 24 h postirradiation incubation (Ap24), including: (1 and 2) Baseline Ap % detectable in control cells that had not been irradiated (Ap0C, Ap24C), (3 and 4) induced Ap % measured in irradiated groups (Ap0Rad, Ap24Rad) and (5 and 6) percentage of net induced apoptotic cells (NAp %) calculated by subtracting the baseline Ap % from Ap % measured in irradiated groups (NAp0 = Ap0Rad − Ap0C, NAp24 = Ap24Rad − Ap24C).
Statistical analysis
Data were analyzed using the SPSS version 16 software package for Windows (SPSS Inc., Chicago), and the figure was drawn by the use of GraphPad Prism version 6 software (La Jolla California, USA). The nonparametric Mann–Whitney U-test and Kruskal-Wallis analyses were used to determine the significance between two and more than two groups, respectively. P < 0.05 was considered as significant. All data were expressed as a mean ± standard deviation.
Results
Prior to the beginning of the study to determine the optimum dose of radiation for inducing apoptosis, an individual whole blood sample was divided into four portions. One was kept as control, and three others were irradiated with different doses of radiation ranging from 2 to 8 Gy and Ap0 and Ap24 were investigated by the neutral comet assay. Data obtained from measuring Ap0 demonstrated no significant differences among various doses of radiation, while, concerning Ap24, 4 Gy of X-ray induced a deliberate amount of Ap % [Figure 2]. Therefore, the dose of 4 Gy was selected to irradiate samples in the subsequent experiments.
Figure 2.
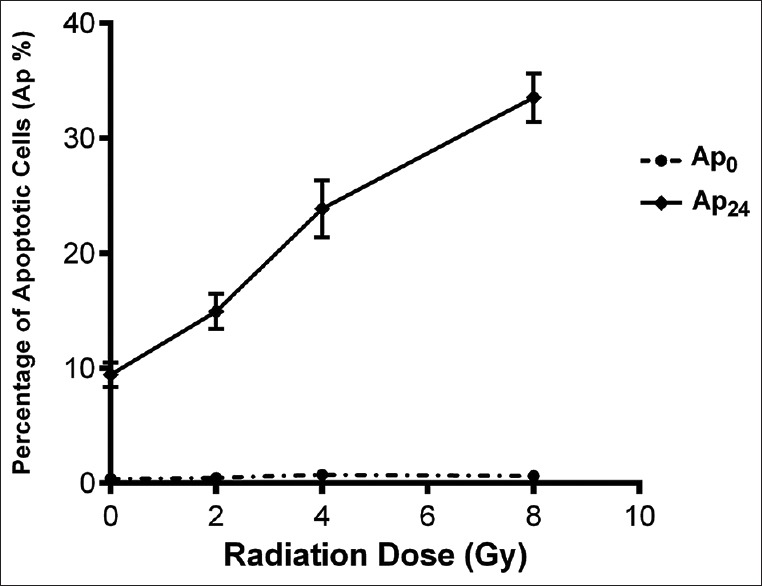
Percentage of apoptotic cells (Ap %) induced with different doses of X-ray (2–8 Gy), immediately after irradiation (Ap0) and after the 24 h postirradiation incubation (Ap24) measured by using neutral comet assay. Error bars indicate the standard deviation of mean values
Results are shown in Table 1. Considering Ap0C and Ap0Rad, no significant difference was found between the two mentioned parameters on various sampling days (P > 0.05). In addition, data did not show a substantial difference, either among Ap0C or among Ap0Rad of different days (P > 0.05).
Table 1.
Comparison of the mean percentage of the apoptotic cells assessed immediately after irradiation and also after the 24 h postirradiation incubation in both control and irradiated groups on different blood sampling days measured by the use of neutral comet assay
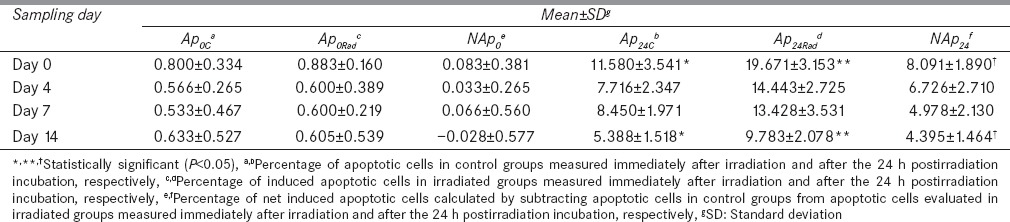
After the 24 h postirradiation incubation, both Ap24C and Ap24Rad noticeably showed higher Ap % in comparison with their counterparts, which were immediately measured after irradiation (Ap0C and Ap0Rad, respectively), at each sampling time (P < 0.05) [Table 1]. In contrast to Ap0Rad, which was not markedly different from Ap0C, Ap24Rad was significantly higher than Ap24C on all days (P < 0.05). Based on obtained results, both Ap24C and Ap24Rad gradually reduced during the experiment [Table 1], so a significant decrease was found in each stated parameter after 14 days RJ consumption (P = 0.004 and 0.002, respectively).
In order to directly investigate the role of RJ in attenuating radiation-induced apoptosis, NAp0 and NAp24 were also calculated for each sampling time. The results revealed that the mean value of NAp24 was 8.091 ± 1.890, 6.726 ± 2.710, 4.978 ± 2.130, and 4.395 ± 1.464 on days 0, 4, 7, and 14, respectively. Thus, a marked decrease was found in NAp24 after 14 consecutive days of RJ consumption (P = 0.009) [Table 1]. However, the mean value of NAp0 was 0.083 ± 0.381, 0.033 ± 0.265, 0.066 ± 0.560 and − 0.028 ± 0.577 on days 0, 4, 7, and 14, respectively and in contrast to NAp24, NAp0 did not significantly alter during the study period (P > 0.05).
Discussion
Cellular adaptation strategies, leading to cell survival or cell death, in response to the IR in mammalian cells, involve activation of DNA repair pathways, cell cycle checkpoints, and apoptosis.[6] DNA, as the main target of IR, could be influenced by undesirable effects of IR through direct as well as indirect pathways. The latter is a more prominent mechanism due to the presence of water in high concentrations throughout DNA. Indeed, by splitting water molecules, IR could be able to generate free radicals including the hydroxyl radical, which is highly reactive with neighboring macromolecules such as DNA.[1] Based on the fact that excessive amount of ROS, which leads to oxidative stress, has a crucial role in the cellular apoptotic response,[5] the antioxidants and free radical scavengers are capable of postponing or inhibiting apoptosis.[27]
RJ is a natural food supplement produced by honeybees, with many pharmacological activities.[11] Several studies have shown its antioxidative activity and free radical scavenging ability with different methods.[10,12,28,29,30,31]
In this study, no serious side effects of RJ consumption were observed during the course of study. In a randomized placebo-controlled double-blind trial carried out by Morita et al., no serious adverse effects of daily RJ consumption for 6 months were reported.[32]
Concerning Ap0C measured on each sampling day, it could be concluded that 14 days RJ consumption did not significantly change background apoptosis during the course of study.
With regard to irradiated groups, results showed that Ap0Rad were not considerably different from controls [Table 1]. In agreement with the findings, Tarang et al. found that Ap % in irradiated groups immediately after irradiation were almost similar to those obtained from control samples.[24] Very low and dose-independent Ap0Rad could be justified by the fact that apoptosis process might be initiated after irradiation, but it still has not been fulfilled to become morphologically detectable.[33] Hence, after irradiation, whole blood samples were incubated at 37°C in 5% CO2 for 24 h to measure Ap24Rad, as well. The 24 h incubation was selected to evaluate Ap24Rad since radiation-induced apoptosis would be measurable after this period of time[26] and the differences between control and irradiated samples could be detectable at this time point. In addition, by the use of neutral comet assay, other investigators performed the evaluation of IR-induced apoptosis after a 24 h postirradiation incubation.[25,34]
In the present trial, incubation of control groups for 24 h at 37°C in 5% CO2 markedly raised Ap24C, so that it reached a maximum of around 11.5% on day 0. This spontaneously occurring apoptosis in control groups could primarily be ascribed to the natural ageing and death of the lymphocytes and granulocytes.[35]
As anticipated, data revealed a dramatic increase in Ap24Rad compared to Ap0Rad on each sampling day. Although Ap % was elevated after the 24 h postirradiation incubation in both control and irradiated groups, Ap24Rad was considerably higher than Ap24C on different sampling days [Table 1]. The higher amount of Ap24Rad compared to Ap24C on each sampling day confirmed the role of radiation in inducing apoptosis. In agreement with these findings, several studies have proven the induction of apoptosis by IR in different cell types and with different approaches.[5,6,7,23]
The decrease seen in Ap24C during the study period could probably be justified by the increase in pro-inflammatory cytokines[35] and/or changes in the expression of apoptosis-related genes including p53, as the apoptosis-inducing gene, and Bcl-2, as the survival-related gene.[36] Since RJ has both anti-inflammatory and immunomodulatory activities leading to the diminution of pro-inflammatory cytokines,[11,37] it could be presumed that the effect of RJ on the expression of apoptosis-related genes might be a more justifiable explanation for the reduction in Ap24C. Supporting these resulting data, by investigating apoptosis in liver and kidney of cisplatin-treated rats, Karadeniz et al., found that positive reactions of Bcl-xL, as an important member of Bcl-2 family, were increased in the RJ-treated group compared to the control one.[38]
Since IR principally induces apoptosis through ROS production leading to oxidative stress,[5] the oxidative stress-modulating role of RJ would be considered the possible explanation for the decrease in Ap24Rad during the course of study. In line with these findings, the ameliorative effect of RJ administration against radiation-induced oxidative stress, biochemical impairments and histological changes in irradiated rats was previously reported.[14] Moreover, less oxidative stress was observed in the RJ-treated irradiated group in comparison with the RJ-nontreated irradiated group in rats undergoing head and neck irradiation.[15] In addition, in another study, a substantial reduction was stated in both oxidative stress and biochemical parameters in RJ-treated whole body irradiated rats compared to the RJ-nontreated whole body irradiated group.[16]
The exact molecular mechanism of the radioprotective effects of RJ is not clear. Nevertheless, previous studies demonstrated that antioxidant capacity and free radical scavenging ability of RJ is mainly due to its protein fractions and its polyphenolic compounds including flavonoids.[10,11,29] Moreover, it has already been well-documented in the literature that the phenolic compounds are known to counteract oxidative stress by acting as powerful natural antioxidants.[39]
The decrease observed in NAp24 after 14 days RJ consumption could confirm our initial results and could emphasize on the beneficial effect of RJ against radiation-induced ROS. However, because of not being able to detect radiation-induced apoptosis immediately after irradiation, NAp0 did not significantly change during the course of study.
Electron Microscopy, DNA laddering, flow cytometry, TUNEL assay, in situ end labelling method and comet assay are various approaches applied for detecting apoptotic cells,[40] among which, comet assay is a rapid and simple technique for measuring DNA damage level and detecting apoptosis in individual cells.[22] The consistency of this genotoxicity assay for the detection of apoptotic cells was evaluated in previous studies.[23,26,41] In addition, an in vitro study with respect to investigating radioprotective effects of Vitamin C and famotidine against radiation-induced apoptosis in human peripheral blood leukocytes was previously conducted by the use of neutral comet assay.[8] Since healthy participants cannot be exposed to IR so as to study radioprotective effects, the in vivo/in vitro method could be applied in clinical practice.
Although the same sample size was used in some similar papers,[8,19,42] it should be noted that the present research is limited by its sample size. Moreover, using a single dose of 1000 mg of RJ per day is another limitation of the study since it was ideal to administer different daily doses of RJ to different groups and compare resulting data. In addition, not measuring the expression of apoptosis-related genes by the molecular biological studies and not investigating the concentrations of polyphenolic compounds and/or the other antioxidants in collected whole blood samples during the course of study are the other limitations. Furthermore, not recruiting the positive control to establish the role of elevated levels of antioxidants as a result of RJ ingestion in obtained blood samples could be considered to be another limitation of the present trial. Therefore, this work will be conducted in future to elucidate the precise mechanism of the action of RJ on inhibition of apoptosis in human blood leukocytes.
Conclusions
From the delineated data in this study, it could be concluded that IR could induce apoptosis in human peripheral blood leukocytes through the formation of free radicals leading to cellular damage. Furthermore, it could be concluded that RJ was effective in modifying radiation-induced apoptosis probably through its antioxidant capacity and free radical scavenging property.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors wish to express their gratitude to all participants for their contributions to the study, to Ms. Rameshgar for her valuable technical assistance and cooperation, to the personnel of the radiotherapy ward of Imam Khomeini Hospital (Sari, Iran), to Ms. Abbasabadi for organizing the irradiation time, to Dr. Ghasemi and Dr. Jorsaraei for their kind advice and to Dr. Evangeline Foronda for proofreading the article.
References
- 1.Hubenak JR, Zhang Q, Branch CD, Kronowitz SJ. Mechanisms of injury to normal tissue after radiotherapy: A review. Plast Reconstr Surg. 2014;133:49e–56e. doi: 10.1097/01.prs.0000440818.23647.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuntic VS, Stankovic MB, Vujic ZB, Brboric JS, Uskokovic-Markovic SM. Radioprotectors – The evergreen topic. Chem Biodivers. 2013;10:1791–803. doi: 10.1002/cbdv.201300054. [DOI] [PubMed] [Google Scholar]
- 3.Tahamtan R, Shabestani Monfared A, Tahamtani Y, Tavassoli A, Akmali M, Mosleh-Shirazi MA, et al. Radioprotective effect of melatonin on radiation-induced lung injury and lipid peroxidation in rats. Cell J. 2015;17:111–20. doi: 10.22074/cellj.2015.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley PA. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 5.Shinomiya N. New concepts in radiation-induced apoptosis: ’ Premitotic apoptosis’ and ’ postmitotic apoptosis’ . J Cell Mol Med. 2001;5:240–53. doi: 10.1111/j.1582-4934.2001.tb00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross GM. Induction of cell death by radiotherapy. Endocr Relat Cancer. 1999;6:41–4. doi: 10.1677/erc.0.0060041. [DOI] [PubMed] [Google Scholar]
- 7.Verheij M, Bartelink H. Radiation-induced apoptosis. Cell Tissue Res. 2000;301:133–42. doi: 10.1007/s004410000188. [DOI] [PubMed] [Google Scholar]
- 8.Mozdarani H, Ghoraeian P. Modulation of gamma-ray-induced apoptosis in human peripheral blood leukocytes by famotidine and Vitamin C. Mutat Res. 2008;649:71–8. doi: 10.1016/j.mrgentox.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Borzoueisileh S, Monfared AS, Abediankenari S, Mostafazadeh A. The assessment of cytotoxic T cell and natural killer cells activity in residents of high and ordinary background radiation areas of Ramsar-Iran. J Med Phys. 2013;38:30–3. doi: 10.4103/0971-6203.106602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagai T, Inoue R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004;84:181–6. [Google Scholar]
- 11.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Alvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73:R117–24. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 12.Nagai T, Inoue R, Suzuki N, Nagashima T. Antioxidant properties of enzymatic hydrolysates from royal jelly. J Med Food. 2006;9:363–7. doi: 10.1089/jmf.2006.9.363. [DOI] [PubMed] [Google Scholar]
- 13.Silici S, Ekmekcioglu O, Kanbur M, Deniz K. The protective effect of royal jelly against cisplatin-induced renal oxidative stress in rats. World J Urol. 2011;29:127–32. doi: 10.1007/s00345-010-0543-5. [DOI] [PubMed] [Google Scholar]
- 14.Azab KS, Bashandy M, Salem M, Ahmed O, Tawfik Z, Helal H. Royal jelly modulates oxidative stress and tissue injury in gamma irradiated male Wister Albino rats. N Am J Med Sci. 2011;3:268–76. doi: 10.4297/najms.2011.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cihan YB, Cihan C, Mutlu H, Unal D. Effect of royal jelly on serum trace elements in rats undergoing head and neck irradiation. Kulak Burun Bogaz Ihtis Derg. 2013;23:37–43. doi: 10.5606/kbbihtisas.2013.77753. [DOI] [PubMed] [Google Scholar]
- 16.Cihan Y, Ozturk A, Gokalp SS. Protective role of royal jelly against radiation-induced oxidative stress in rats. Int J Hematol Oncol. 2013;23:79–87. [Google Scholar]
- 17.Cihan Y, Deniz K. The effects of royal jelly against radiation-induced acute oral mucositis. Int J Hematol Oncol. 2014;24:45–53. [Google Scholar]
- 18.Erdem O, Güngörmüs Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist Nurs Pract. 2014;28:242–6. doi: 10.1097/HNP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi K, Kogashiwa Y, Moro Y, Kohno N. The effect of topical application of royal jelly on chemoradiotherapy-induced mucositis in head and neck cancer: A preliminary study. Int J Otolaryngol 2014. 2014 doi: 10.1155/2014/974967. 974967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boreham DR, Gale KL, Maves SR, Walker JA, Morrison DP. Radiation-induced apoptosis in human lymphocytes: Potential as a biological dosimeter. Health Phys. 1996;71:685–91. doi: 10.1097/00004032-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Menz R, Andres R, Larsson B, Ozsahin M, Trott K, Crompton NE. Biological dosimetry: Phe potential use of radiation-induced apoptosis in human T-lymphocytes. Radiat Environ Biophys. 1997;36:175–81. doi: 10.1007/s004110050069. [DOI] [PubMed] [Google Scholar]
- 22.Fairbairn DW, Olive PL, O’ Neill KL. The comet assay: A comprehensive review. Mutat Res. 1995;339:37–59. doi: 10.1016/0165-1110(94)00013-3. [DOI] [PubMed] [Google Scholar]
- 23.Wada S, Khoa TV, Kobayashi Y, Funayama T, Yamamoto K, Natsuhori M, et al. Detection of radiation-induced apoptosis using the comet assay. J Vet Med Sci. 2003;65:1161–6. doi: 10.1292/jvms.65.1161. [DOI] [PubMed] [Google Scholar]
- 24.Tarang A, Mozdarani H, Akbari MT. Frequency of background and radiation-induced apoptosis in leukocytes of individuals with alpha-thalassemia variants, assessed by the neutral comet assay. Hemoglobin. 2009;33:247–57. doi: 10.1080/03630260903039586. [DOI] [PubMed] [Google Scholar]
- 25.Shahidi M, Mozdarani S, Shammas S. Interindividual differences in radiation-induced apoptosis of peripheral blood leukocytes in normal individuals and breast cancer patients. Int J Radiat Res. 2012;9:237–44. [Google Scholar]
- 26.Wilkins RC, Kutzner BC, Truong M, Sanchez-Dardon J, McLean JR. Analysis of radiation-induced apoptosis in human lymphocytes: Flow cytometry using Annexin V and propidium iodide versus the neutral comet assay. Cytometry. 2002;48:14–9. doi: 10.1002/cyto.10098. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi H, Kosaka N, Nakagawa S. alpha-Tocopherol protects PC12 cells from hyperoxia-induced apoptosis. J Neurosci Res. 1998;52:184–91. doi: 10.1002/(SICI)1097-4547(19980415)52:2<184::AID-JNR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Jamnik P, Goranovic D, Raspor P. Antioxidative action of royal jelly in the yeast cell. Exp Gerontol. 2007;42:594–600. doi: 10.1016/j.exger.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Liu JR, Yang YC, Shi LS, Peng CC. Antioxidant properties of royal jelly associated with larval age and time of harvest. J Agric Food Chem. 2008;56:11447–52. doi: 10.1021/jf802494e. [DOI] [PubMed] [Google Scholar]
- 30.Cemek M, Aymelek F, Büyükokuroglu ME, Karaca T, Büyükben A, Yilmaz F. Protective potential of Royal Jelly against carbon tetrachloride induced-toxicity and changes in the serum sialic acid levels. Food Chem Toxicol. 2010;48:2827–32. doi: 10.1016/j.fct.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Buratti S, Benedetti S, Cosio MS. Evaluation of the antioxidant power of honey, propolis and royal jelly by amperometric flow injection analysis. Talanta. 2007;71:1387–92. doi: 10.1016/j.talanta.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Morita H, Ikeda T, Kajita K, Fujioka K, Mori I, Okada H, et al. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr J. 2012;11:77. doi: 10.1186/1475-2891-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–50. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Kizilian-Martel N, Wilkins RC, Mclean JR, Malone S, Raaphorst GP. Prediction of radiosensitivity by measurement of X-ray induced apoptosis in human blood using the comet assay. Anticancer Res. 2003;23:3847–54. [PubMed] [Google Scholar]
- 35.McNamee JP, Bellier PV, Kutzner BC, Wilkins RC. Effect of pro-inflammatory cytokines on spontaneous apoptosis in leukocyte sub-sets within a whole blood culture. Cytokine. 2005;31:161–7. doi: 10.1016/j.cyto.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Majewska E, Sulowska Z, Baj Z. Spontaneous apoptosis of neutrophils in whole blood and its relation to apoptosis gene proteins. Scand J Immunol. 2000;52:496–501. doi: 10.1046/j.1365-3083.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- 37.Gasic S, Vucevic D, Vasilijic S, Antunovic M, Chinou I, Colic M. Evaluation of the immunomodulatory activities of royal jelly components in vitro. Immunopharmacol Immunotoxicol. 2007;29:521–36. doi: 10.1080/08923970701690977. [DOI] [PubMed] [Google Scholar]
- 38.Karadeniz A, Simsek N, Karakus E, Yildirim S, Kara A, Can I, et al. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid Med Cell Longev 2011. 2011 doi: 10.1155/2011/981793. 981793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddhuraju P. The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (Jacq.) Marechal seed extracts. Food Chem. 2006;99:149–57. [Google Scholar]
- 40.Archana M, Yogesh TL, Kumaraswamy KL. Various methods available for detection of apoptotic cells – A review. Indian J Cancer. 2013;50:274–83. doi: 10.4103/0019-509X.118720. [DOI] [PubMed] [Google Scholar]
- 41.Olive PL, Frazer G, Banáth JP. Radiation-induced apoptosis measured in TK6 human B lymphoblast cells using the comet assay. Radiat Res. 1993;136:130–6. [PubMed] [Google Scholar]
- 42.Nascimento PA, da Silva MA, Oliveira EM, Suzuki MF, Okazaki K. Evaluation of radioinduced damage and repair capacity in blood lymphocytes of breast cancer patients. Braz J Med Biol Res. 2001;34:165–76. doi: 10.1590/s0100-879x2001000200003. [DOI] [PubMed] [Google Scholar]


