Abstract
Chronic kidney disease (CKD) is associated with early mortality, decreased quality of life and increased health care expenditures. The aim of this study was to determine whether or not urinary NGAL (uNGAL) level is associated with renal damage and kidney disease progression in patients with CKD and to evaluate the predictive value of uNGAL in progression of CKD. Totally, 91 cases of CKD stage II, III, IV, and 50 age-matched healthy controls were enrolled. The follow-up end-point was 18 months; end-point of the study was progression to an estimated glomerular filtration rate (eGFR) of <15 ml/min and/or CKD stage V. Forty-five cases (49.4%) were progressors and 46 were nonprogressors. uNGAL levels were significantly higher in CKD subjects as compared to healthy controls (log 1.09 ± 0.22 μg/ml in controls versus log 1.22 ± 2.08 μg/ml in stage II, log 3.34 ± 2.74 μg/ml in stage III and log 3.70 ± 0.18 μg/ml in stage IV). Univariate Cox proportional hazards model showed that only eGFR (hazard ratio [HR]: 0.95; 95% confidence interval [CI]: 0.93–0.96; P < 0.001) and uNGAL (HR: 1.11; 95% CI: 1.01–1.20; P < 0.001) were significantly associated with end-point of CKD stage V, but multiple Cox proportional regression model showed significant association of uNGAL (HR: 1.11; 95% CI: 1.01–1.20; P < 0.001) and eGFR (HR: 0.962, 95% CI: 0.95–0.98; P < 0.001) with end-point of CKD stage V. This suggests that uNGAL would not be a simple surrogate index of baseline eGFR, but a marker of CKD progression beyond the information provided by eGFR estimation.
Keywords: Chronic kidney disease, progression, urinary neutrophil gelatinase-associated lipocalin
Introduction
Chronic kidney disease (CKD) is a major threat to public health problem, both in terms of prevalence of disease, cost of treatment and the co-morbidities involved. Chronic diseases are the foremost cause of death worldwide responsible for 60% of all cause of death, of which 80% deaths takes place in low and middle income group.[1] CKD is associated with early mortality, decreased quality of life and increased health care expenditures.[2,3] Various studies reported that patients with CKD suffered with cardiovascular disease and die prematurely instead of surviving long enough to face dialysis or renal transplant.[4,5]
Hypertension, proteinuria, hyperlipidemia, and inflammation are some important modifiable risk factors, but these factors are not sufficient to explain renal outcomes in patients affected with CKD.[6,7] It is now widely accepted that in some CKD-associated diseases such as diabetic nephropathy, the rate of deterioration in renal function, and the overall long-term renal outcome, are linked with the degree of renal tubulointerstitial impairment more accurately than with the severity of glomerular lesions. Various tubular proteins have been reported to be involved in the experimental pathogenesis of tubular damage and its progression to terminal fibrosis, leading to uremia.[8]
Neutrophil gelatinase-associated lipocalin (NGAL) is a small 25-kDa protein released from kidney tubular cells after harmful stimuli. It represents one of the most promising biomarkers of acute kidney injury (AKI). However, recent studies also suggest a possible role of NGAL in CKD.[9] NGAL as a biomarker in CKD would represent the ongoing process of renal damage rather than a simple marker of lost function as serum creatinine. For this reason, we evaluated urinary NGAL (uNGAL) in CKD patients in order to verify the relationship with severity of renal impairment.
The aim of this study was to determine whether or not the uNGAL level is associated with disease progression in patients with CKD stage II, III, IV and to evaluate the predictive value of uNGAL in progression of CKD.
Material and Methods
This was a prospective case-control study carried out in Department of Medicine in Nephrology Unit, K.G. Medical University, Lucknow, Uttar Pradesh, India from August 2012 to July 2014 in patients with CKD due to primary chronic glomerulonephritis. After informed written consent and ethical clearance from institutional ethics committee were obtained, total 141 subjects were enrolled for the study. Ninety-one cases of CKD stage II to IV and 50 age-matched healthy controls seeking routine health screening were enrolled. Inclusion criteria were the presence of CKD of stage II to IV according to the KDIGO guideline 2012. The diagnostic criteria of primary chronic glomerulonephritis were the presence of glomerular proteinuria and/or hematuria lasting for more than 1 year. CKD was the consequence of biopsy-confirmed glomerulonephritis. The Modification of Diet in Renal Disease formula was used to calculate the estimated glomerular filtration rate (eGFR). The staging criteria for CKD was defined for stage II as renal damage with eGFR of 60–89 ml/min/1.73 m2, stage III with eGFR of 30–59 ml/min/1.73 m2 and stage IV with eGFR of 15–29 ml/min/1.73 m2. Those with serum creatinine level of ≥6 mg/dl, eGFR of ≤15 ml/min, polycystic kidney disease, multiple myeloma, or glomerulonephritis on active immunesuppression and those who had undergone kidney transplantation were excluded from the study.
All subjects were instructed for overnight fasting for 12 h prior to blood sampling. Five ml of venous blood was obtained in sterilized vial from the cases and controls for biochemical analysis. The blood sample was centrifuged at 1500 rpm for 10 min to separate the serum, and routine hematology, biochemistry, urinalysis and urine protein measurements were performed in accordance with institutional protocols. An automated blood-cell analyzer (BC-5380, Mind Ray China) was used for routine hematology testing, and an automated clinical biochemistry analyzer (Cobas C 311 Roche-Hitachi Japan) was used for blood urea nitrogen, serum creatinine, serum uric acid, serum lipids, electrolytes and albumin. An enzyme-linked immunosorbent assay technique (ELISA) was used to measure uNGAL levels. A clean, morning midstream urine sample (5 ml) was collected into a pyrogen and endotoxin-free test tube and centrifuged at 5000 rpm for 15 min. The supernatant was transferred to an Eppendorf tube and stored at −80°C until assayed. A human NGAL ELISA kit (by Epitope Diagnostics Inc., San Diego, USA) was used for estimation of uNGAL as per producer protocol.
Follow-up and end-point
Baseline renal function tests for all patients were recorded at the time of enrollment. All of the patients had follow-up visits at the outpatient clinic or by telephonic interviews at monthly intervals, and serial renal function tests were repeated at 3 months interval. The primary follow-up end-point was 18 months and disease end-point was eGFR of <15 ml/min or CKD stage V. Patients were personally contacted in case they missed any appointment and at the study end date, to avoid eventual loss during follow-up.
Progression of chronic kidney disease
Assessed in terms of CKD stages as stage II–III, stage III–IV, stage IV–V and/or disease progression from any stage to disease end-point CKD stage V.
Statistical analysis
Continuous variable was expressed as mean ± standard deviation and compared using one-way ANOVA followed by Tukey's post-hoc test. Correlation between various kidney function parameters has been calculated using Pearson correlation coefficient. Receiver operator curve (ROC) analysis had been done to calculate the area under curve (AUC) for log NGAL and identifying the optimal NGAL cut-off values for predicting progression of CKD. Statistical significance was set at P < 0.05. All the analyses had been done using SPSS version 20.0, Support for IBM System z servers running Linux®, US and MedCalc Software bvba Acacialaan 22 8400 Ostend Belgium)
Results
This study was performed on 91 CKD subjects of different stages (stage II = 27, stage III = 33, stage IV = 31) and mean age was 43.37 ± 11.46 for stage II, 45.42 ± 12.30 for stage III and 44.35 ± 11.34 for stage IV, years respectively and 50 healthy individuals (27 men and 23 women) with mean age of 40.22 ± 12.31, years. Blood urea, serum creatinine and serum uric acid, were significantly higher and serum protein, serum albumin, eGFR were low in cases as compared to the controls [Table 1]. uNGAL levels were significantly higher in cases as compared to healthy controls (log 1.09 ± 0.22 μg/ml) in healthy controls versus log 1.22 ± 2.08 μg/ml in stage II, log 3.34 ± 2.74 μg/ml in stage III and log 3.70 ± 0.18 μg/ml in stage IV [Table 2].
Table 1.
Demographic profile of the study subjects
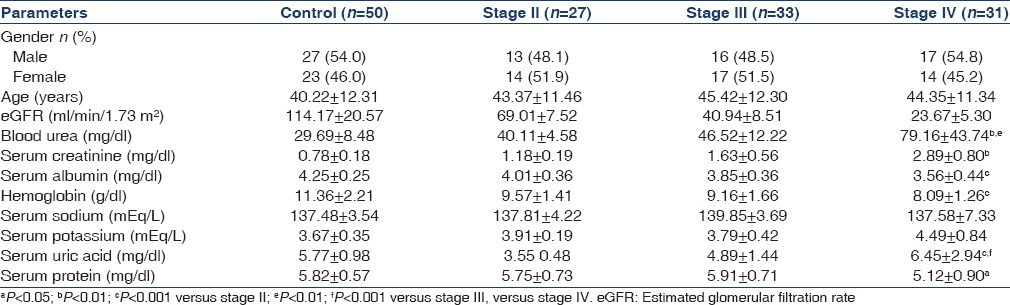
Table 2.
Disease progression in CKD stage II, III, IV
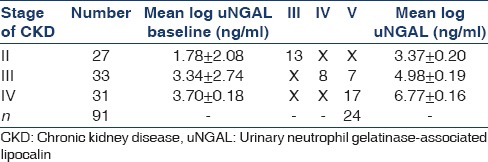
Progression to end-point (chronic kidney disease stage V)
During the observational period (follow-up of 18 months), of 91 cases, 45 cases (49.4%) were progressor and 46 cases were nonprogressor in terms of CKD stages. Total 24 cases (26.4%) reached the composite renal end-point CKD stage V. All cases who had progressed to stage V (n = 24), urgently required dialysis treatment. None of the progressors experienced a regression of serum creatinine to baseline values during the observational period. This excluded the possibility of AKI, which was mistakenly interpreted as a CKD progression. The remaining 46 patients (50.6%) who did not experience a progression in CKD completed the whole observational period (18 months) [Table 2].
Neutrophil gelatinase-associated lipocalin and progression of chronic kidney disease
Progressor subjects presented with significantly increased uNGAL values at baseline compared with nonprogressors. Mean value of uNGAL in progressors was (stage II = 3.31 ± 1.67, stage III = 4.37 ± 2.11, stage IV = 5.42 ± 2.59) whereas mean value of uNGAL in nonprogressors was (stage II = 3.09 ± 1.10, stage III = 4.04 ± 1.80, stage IV = 4.406 ± 2.13). This showed that progressors had high value of uNGAL at baseline [Table 3]. ROC for NGAL considering the progression of CKD as status variable. The AUC for NGAL was 0.778 (95% confidence interval [CI]: 0.68–0.86). A log uNGAL cut-off of log 3.5 unit had a sensitivity of 73.08% and specificity of 71.43% in predicting the progression of CKD. Above these values, patients experienced a significantly faster evolution to progression within a follow-up period of 18 months [Figure 1]. The result supports that progressor is only those patients who had elevated baseline value of uNGAL.
Table 3.
Comparison of uNGAL in progressors versus nonprogressors
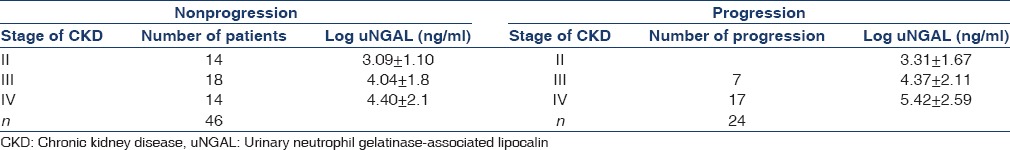
Figure 1.
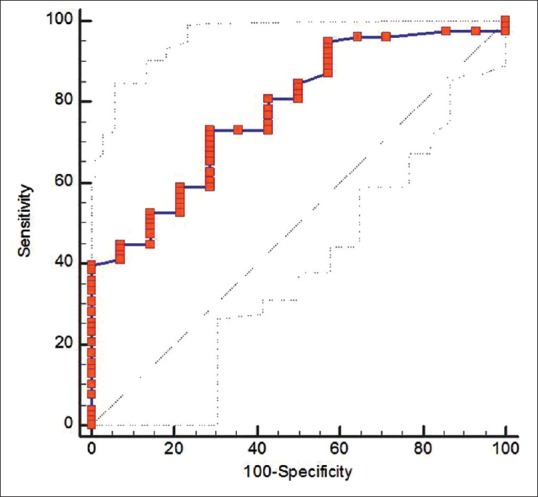
Receiver operator curve for neutrophil gelatinase-associated lipocalin (NGAL) considering the progression of chronic kidney disease (CKD) as status variable. The area under curve for NGAL was 0.778 (95% confidence interval: 0.68 – 0.86). A log NGAL cut-off of 3.51 (unit) had a sensitivity of 73.08% and specificity of 71.43% in predicting the progression of CKD
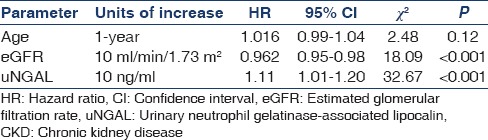
Univariate Cox proportional hazards model showed that only eGFR (hazard ratio [HR] 0.95; 95% CI: 0.93–0.96; P < 0.001) and uNGAL (HR: 1.11; 95% CI: 1.01–1.20; P < 0.001) were significantly associated with end-point of CKD whereas age, Ca×PO4 and proteinuria were not statistically significantly associated with the end-point of CKD [Table 4]. Multiple Cox proportional regression model showed significant association of uNGAL (HR: 1.11; 95% CI: 1.01–1.20; P < 0.001) and eGFR (HR: 0.962, 95% CI: 0.95–0.98; P < 0.001) with end-point of CKD [Table 5].
Table 4.
Univariate Cox proportional hazard model for progression of CKD
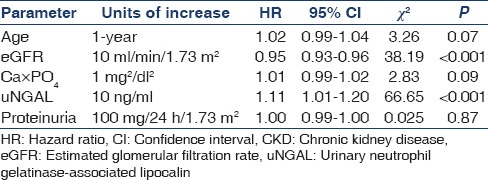
Table 5.
Multivariate Cox proportional hazard model for the progression of CKD
Discussion
Patients with CKD commonly develop different cardiovascular complication. Cardiovascular disease is the most common cause of morbidity and mortality in patients with end-stage renal disease (ESRD). Now CKD has become a major public health problem. According to annual data report of the US Renal Data System 2007, a dramatically increased overall prevalence of CKD was reported, especially for earlier stages.[10,11] With the worsening of renal function, the risk for cardiovascular disease is also increases simultaneously, and it is maximum in the terminal stage (ESRD).[12] For these reasons, search of new environmental, genetic and biological factors involved in CKD progression represent a very difficult task for researcher. It is well-accepted that traditional cardiovascular risk factors alone, such as hypertension or proteinuria, are not sufficient to fully explain pathophysiology and predict the progression of different stages of this disease.
In our study, during the follow-up of 18 months, of 91 cases, 45 cases (49.4%) were progressors and 46 cases were nonprogressors in terms of CKD stages. Total 24 cases (26.4%) reached the composite renal end-point CKD stage V. In particular, 13 cases had progressed from stage II to III, 8 cases progressed from stage III to IV. Progressor has high value of uNGAL at baseline. ROC for NGAL considering the progression of CKD shows that AUC for NGAL was 0.778 (95% CI: 0.68–0.86). A log uNGAL cut-off of log 3.5 unit had a sensitivity of 73.08% and specificity of 71.43% in predicting the progression of CKD. Above these values, patient experienced a significantly quicker evolution to end-point within a follow-up time of 18 months. Our study results are supported by Bolignano et al. who also reported that subjects with uNGAL values above 231 ng/ml showed a significantly faster progression to end-point (P < 0.0001), with a mean follow-up time of 13.2 months (95% CI: 11.9–15.9) as compared to 19.2 months (95% CI: 17.9–19.8) for uNGAL below the cut-off.[13]
The association between baseline urine NGAL levels and risk of CKD progression was strongest in the first 2 years of biomarker measurement. This study was done by Liu et al. over an average follow-up of 3.2 years, where 689 cases in which the eGFR was decreased by half or incident ESRD developed. Even after accounting for eGFR, proteinuria and other known CKD progression risk factors, urine NGAL remained a significant independent risk factor (Cox model HR 1.70 highest to lowest quartile). This study result supports our study.[14]
In a study by Wu et al. of 36 patients with drug-induced chronic tubulointerstitial nephritis (GFR 37 ± 20 ml/min/1.73 m2), urine NGAL was predictive of renal function decline and the only risk factor with a P < 0.05 in their multivariable models.[15] Viau et al. examined 87 subjects with polycystic kidney disease who had GFR 33 ± 20 ml/min/1.73 m2 and reported that urine NGAL levels were higher in patients who progressed to ESRD. No attempts were made to adjust for other known risk factors for CKD progression.[16] Nielson et al. showed in another cohort of 78 type 1 DM patients that elevated urine NGAL was not related to decline in GFR during a 4-year follow-up; it was associated with the development of ESRD, but not after adjustment (albeit CIs were wide).[17]
Univariate Cox proportional hazards model and multivariate cox proportional hazard model for progression of CKD showed that only eGFR and NGAL were significantly associated with end-point of CKD whereas the association with age, Ca×PO4 and proteinuria was not statistically significant. In multivariate analysis, Bolignano et al. found that only age and eGFR were significantly associated with end-point, whereas calcium-phosphate product, fibrinogen, CRP, proteinuria, and even age failed to reach statistical significance.[13]
In the present study, increased uNGAL was reported in CKD patients as compared to controls. The parallel elevation in uNGAL with disease severity or with increasing stages of CKD supports the hypothesis of an active tubular production, also excluding a passive consequence of reduced renal clearance capacity. The results of our study as also supported by Bolignano et al., they found that uNGAL levels were significantly higher in CKD patients than control. They also reported that in CKD patients uNGAL level may reflect the severity of renal impairment, independently predicting residual GFR even better than serum creatinine. Increase production of uNGAL in CKD could be associated with hypertension and endothelial damage.[18,19]
Recent observation as reported by Mori and Nakao “the forest fire theory” which assume that increased NGAL in CKD is the consequence of its sustained production by inflamed but viable tubular cells whereas rise in serum creatinine and decreased GFR represents general loss of viable nephrons. Thus, NGAL reflects how much kidney damage exists within CKD subjects.[20]
Limitation of our study
Present study has some limitations:
First, it was a single-center study, and relatively small sample size
The duration of follow-up time was small period
Urine NGAL concentration was ascertained only at one point in time.
Conclusion
The result of our study supports that progressor is only those patients who had elevated baseline value of uNGAL. This suggests that NGAL would not be a simple surrogate index of baseline eGFR, but also a marker on its own, predicting CKD progression beyond the information provided by GFR estimation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Geneva: WHO; 2005. World Health Organization. Preventing Chronic Disease: A Vital Investment. [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, et al. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69:350–7. doi: 10.1038/sj.ki.5000047. [DOI] [PubMed] [Google Scholar]
- 4.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 5.Goodman WG, London G, Amann K, Block GA, Giachelli C, Hruska KA, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572–9. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Kent DM, Jafar TH, Hayward RA, Tighiouart H, Landa M, de Jong P, et al. Progression risk, urinary protein excretion, and treatment effects of angiotensin-converting enzyme inhibitors in nondiabetic kidney disease. J Am Soc Nephrol. 2007;18:1959–65. doi: 10.1681/ASN.2006101081. [DOI] [PubMed] [Google Scholar]
- 7.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–19. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 8.Phillips AO. The role of renal proximal tubular cells in diabetic nephropathy. Curr Diab Rep. 2003;3:491–6. doi: 10.1007/s11892-003-0013-1. [DOI] [PubMed] [Google Scholar]
- 9.Smith ER, Lee D, Cai MM, Tomlinson LA, Ford ML, McMahon LP, et al. Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric Stages 3 and 4 chronic kidney disease (CKD) Nephrol Dial Transplant. 2013;28:1569–79. doi: 10.1093/ndt/gfs586. [DOI] [PubMed] [Google Scholar]
- 10.Bethesda, MD: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. United States Renal Data System. Annual Report of the US Renal Data System 2007. [Google Scholar]
- 11.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 12.Zoccali C. The burden of cardiovascular disease in patients with chronic kidney disease and in end-stage renal disease. Contrib Nephrol. 2008;161:63–7. doi: 10.1159/000129755. [DOI] [PubMed] [Google Scholar]
- 13.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–44. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, et al. Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int. 2013;83:909–14. doi: 10.1038/ki.2012.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Su T, Yang L, Zhu SN, Li XM. Urinary neutrophil gelatinase-associated lipocalin: A potential biomarker for predicting rapid progression of drug-induced chronic tubulointerstitial nephritis. Am J Med Sci. 2010;339:537–42. doi: 10.1097/maj.0b013e3181dd0cb1. [DOI] [PubMed] [Google Scholar]
- 16.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–76. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen SE, Hansen HP, Jensen BR, Parving HH, Rossing P. Urinary neutrophil gelatinase-associated lipocalin and progression of diabetic nephropathy in type 1 diabetic patients in a four-year follow-up study. Nephron Clin Pract. 2011;118:c130–5. doi: 10.1159/000320615. [DOI] [PubMed] [Google Scholar]
- 18.Bolignano D, Lacquaniti A, Coppolino G, Campo S, Arena A, Buemi M. Neutrophil gelatinase-associated lipocalin reflects the severity of renal impairment in subjects affected by chronic kidney disease. Kidney Blood Press Res. 2008;31:255–8. doi: 10.1159/000143726. [DOI] [PubMed] [Google Scholar]
- 19.Patel M, Sachan R, Gangwar R, Sachan P, Natu S. Correlation of serum neutrophil gelatinase-associated lipocalin with acute kidney injury in hypertensive disorders of pregnancy. Int J Nephrol Renovasc Dis. 2013;6:181–6. doi: 10.2147/IJNRD.S45523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–70. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]


