Abstract
Background
Thrombocytopenia is one of the most common laboratory abnormalities encountered in patients with severe sepsis. It has been reported that thrombocytopenia is linked to mortality in patients with severe sepsis. However, the mechanism of thrombocytopenia in sepsis is unknown. We hypothesized that inflammatory cytokines and microRNAs (miRNAs) are not only involved in the pathogenesis of sepsis, but also are correlated with thrombocytopenia.
Patients and methods
Eligible patients with severe sepsis were prospectively recruited and treated at our hospital between June 2012 and May 2014. The miRNA and protein expression of interleukin (IL)-18 and IL-27 were detected by real-time polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. The expression of miR-130a and miR-150 was detected by TaqMan real-time polymerase chain reaction.
Results
Sixty eligible patients were divided into two groups: 28 severe sepsis patients with thrombocytopenia and 32 severe sepsis patients without thrombocytopenia. The results demonstrated that the miRNA expression and plasma concentration of IL-18 in severe sepsis patients with thrombocytopenia were higher than those in severe sepsis patients without thrombocytopenia (P=0.015 and P=0.034, respectively), and miR-130a expression was significantly lower in severe sepsis patients with thrombocytopenia (P<0.003).
Conclusion
Our data demonstrate that severe sepsis patients with thrombocytopenia have increased plasma and miRNA expression levels of IL-18 and decreased expression of miR-130a, suggesting that IL-18 and miR-130a might be involved in the pathophysiological process of severe sepsis with thrombocytopenia.
Keywords: IL-18, miR-130a, severe sepsis, thrombocytopenia, IL-27, miR-150, pathophysiological process
Introduction
Severe sepsis is a major health care problem affecting millions of people around the world.1 Sepsis is a complex clinical syndrome characterized by severe infection in the body and bloodstream that most commonly originates in the lung, urinary tract, or abdomen. Thrombocytopenia, which is defined as a platelet count (PLT) in the peripheral blood of less than 100×109/L, is one of the most common laboratory abnormalities encountered in patients with severe sepsis.2,3 It has been reported that the incidence of thrombocytopenia in intensive care units varies from 23% to 41%.4,5 Thrombocytopenia has been reported to be directly linked to the mortality of patients with severe sepsis.5–7 Therefore, elucidating the mechanism of thrombocytopenia is very important for the identification of reliable inflammatory mediators and the development of a promising therapeutic approach for treating these patients.
Excess production of pro- and anti-inflammatory cytokines is frequently found in the circulation of septic patients. Interleukin (IL)-18, a proinflammatory cytokine, is produced by activated macrophages during endogenous inflammatory processes and plays a vital role in the pathophysiology of sepsis.8 Among critically ill patients, urinary excretion of IL-18 is significantly higher in patients with sepsis than in patients without sepsis.9–11 Several studies have shown that elevated serum levels of IL-18 are associated with poor clinical outcome in severe inflammatory and septic conditions.12–14 IL-27, a novel heterodimeric cytokine of the IL-12 family, was shown to be rapidly induced during murine experimental peritonitis induced by cecal ligation and puncture. It has been reported that IL-27 is a key negative regulator of innate immune cell function in septic peritonitis. Furthermore, in vivo blockade of IL-27 is a novel potential therapeutic target for the treatment of sepsis.15 It has been demonstrated that IL-18 and IL-27 play a role in the pathogenesis of idiopathic thrombocytopenic purpura, which is characterized by low platelet numbers in the peripheral blood.16–20 Most importantly, several studies also demonstrated that IL-27 and IL-18 are novel prognostic cytokines in infection-induced sepsis.8,21–24 Therefore, it was speculated that IL-18 and IL-27 are involved in severe sepsis with thrombocytopenia.
MicroRNAs (miRNAs) are a type of small, noncoding, single-stranded RNAs that can posttranscriptionally down-regulate specific genes by targeting miRNAs for cleavage or translational repression.25 It was demonstrated that miR-146a expression in septic patients is significantly decreased compared to the levels in normal controls,26,27 suggesting that miR-146a may be significantly associated with sepsis. Furthermore, serum miRNAs such as miR-223, miR-146a, and miR-15a are newly emerging biomarkers for sepsis,28 and miR-223, miR-15a, miR-16, miR-122, miR-193b, and mR-483-5p can be used as predictors for mortality in patients with sepsis.29 Most importantly, the plasma levels ratio for miR-150/IL-18 can be used for assessing the severity of the sepsis.30 Collectively, these studies demonstrate that miRNAs play an important role in the pathophysiology of sepsis. In the present study, we aimed to identify the miRNAs that target IL-18 and/or IL-27 to further study new functional miRNAs for the treatment of sepsis with thrombocytopenia.
Patients and methods
Study design and eligible patients
Eligible patients with severe sepsis were prospectively recruited and treated at the Intensive Care Unit of Tianjin First Center Hospital between June 2012 and May 2014. The study protocol was in accordance with the ethical guidelines of the 1995 Declaration of Helsinki and was approved by the independent ethics committees at Tianjin First Center Hospital. In addition, informed written consent was obtained from each patient. Consistent with an earlier study,31 the assessable patients were divided into two groups: sepsis patients with thrombocytopenia and sepsis patients without thrombocytopenia. Both groups received standard care and appropriate medical support based on the guidelines issued by the surviving sepsis campaign.32
Severe sepsis was defined as an inflammatory response with evidence or suspicion of microbial processes and accompanied by evidence of hypoperfusion or dysfunction of at least one organ system, which was established according to the International Sepsis Definitions Conference.32 Patients younger than 18 years old; those suffering from diabetes, malignancies, cirrhosis, chronic heart failure, chronic respiratory failure, chronic renal failure, human immunodeficiency virus infection, autoimmune diseases, or acquired immune deficiency syndrome; patients who had undergone transplantation; and patients who were receiving immunosuppressive, steroid, or radiation therapy were excluded from this study.
Their peripheral blood samples were collected and analyzed within 24 hours from the diagnosis of severe sepsis. At the same time, we determined fibrin–fibrinogen degradation product, plasminogen activator inhibitor-1, fibrinogen, prothrombin time, C-reactive protein, creatinine, albumin, white blood cell count, PLT, the Acute Physiology and Chronic Health Evaluation II score, and sequential organ failure assessment score. In addition, the following clinical parameters were recorded for each patient: age, sex, site of infection, existence of shock, the number and kind of organ dysfunction, as well as 28-day mortality.
RNA isolation, reverse transcription, and real-time polymerase chain reaction
Peripheral blood was collected into ethylenediaminetetra-acetic acid-anticoagulant vacuum tubes. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation over Ficoll-Hypaque gradients. Total RNA was isolated from 2×106 PBMCs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and then the reverse transcription (RT) reactions were carried out using the Superscript First-Strand Synthesis System (Invitrogen) following the manufacturer’s protocol. To detect the miRNA expression of IL-18 and IL-27 in PBMCs of sepsis patients, we performed real-time polymerase chain reaction (PCR) on an ABI PRISM-7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The sequences of primers were as follows: for IL-18: 5′-CCCCAATCCCTTTATTACCC-3′ (forward), 5′-CGAAGTGGTGGTCTTGTTGC-3′ (reverse); and for IL-27: 5′-CTTGGCTGGCGTCTCAGCCT-3′ (forward), 5′-CGGAGAGCAGCTTCCTGGCG-3′ (reverse). For PCR amplification, an initial denaturation at 94°C for 10 minutes was followed by 40 cycles at 94°C for 15 seconds and at 60°C for 1 minute. After PCR, a melting curve analysis was performed by increasing the temperature from 60°C to 95°C with a temperature transition rate of 0.1°C/s. Relative gene expression levels were obtained by comparing the expression of each cytokine to that of β-actin using the 2−ΔΔCt method (Ct,target gene − Ct,β-actin).
Enzyme-linked immunosorbent assay for cytokine concentrations
Plasma samples were collected after a short centrifugation and were stored at −80°C until they were analyzed. Plasma IL-18 and IL-27 concentrations were measured by using enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions (NeoBioscience Technology Co., Ltd., Shenzhen, China).
TaqMan real-time PCR for quantification of mature miRNAs
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Ten nanograms of total RNA was then used to synthesize complementary DNAs using miRNA-specific primers and an RT kit (Applied Biosystems). Quantitative RT-PCR assays were performed using a TaqMan® MicroRNA assay kit (Applied Biosystems) for miR-130a and miR-150, according to the manufacturer’s instructions. Ribonucleic acid U6B (RNU6B) small nuclear RNA was quantified as a control to normalize differences in total RNA levels. PCR amplification reactions were performed on an ABI PRISM-7500 Sequence Detection System (Applied Biosystems). An initial denaturation at 94°C for 10 minutes was followed by 40 cycles of denaturation at 94°C for 15 seconds and extension at 60°C for 1 minute. The relative gene expression values were obtained by comparing the expression of target genes to that of RNU6B using the 2−ΔΔCt method (Ct,target miRNA − Ct,RNU6B).
Sample size and statistical analysis
The all-cause mortality of severe sepsis varies in different patient populations. The 28-day mortality ranging from 20.8% to 61% has been reported.33–35 Using the 28-day mortality, the sample size was calculated to detect a 10% difference in mortality between the two groups with a two-tailed test, a significance level (α) of 5%, and a power of 80%. Thus, 60 patients with severe sepsis were needed for this study.
The results are presented as median and range as well as mean ± standard deviation values. Quantitative data were analyzed using Student’s t-test. Differences with values of P<0.05 were considered to be statistically significant. Statistical Package for the Social Sciences version 19.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis.
Results
Demographic data and baseline characteristics
A total of 67 patients with severe sepsis were recruited from June 2012 to May 2014. Seven patients were excluded either because of acute gastrointestinal hemorrhage (two patients) or receiving platelet transfusion (three patients) or insufficient data (two patients). Ultimately, 60 eligible patients were divided into two groups: 28 severe sepsis patients with thrombocytopenia and 32 severe sepsis patients without thrombocytopenia. There were no significant differences in baseline characteristics in terms of sex, age, Acute Physiology and Chronic Health Evaluation II score, sequential organ failure assessment score, C-reactive protein, creatinine, albumin, white blood cell count, site of infection, the number and kind of organ dysfunction, fibrin–fibrinogen degradation product, plasminogen activator inhibitor-1, fibrinogen other than PLT, prothrombin time, existence of shock, and 28-day mortality (Tables 1 and 2).
Table 1.
Clinical characteristics of patients
| Variable | All (60 patients) | SST (28 patients) | SS (32 patients) |
|---|---|---|---|
| Sex (male/female) | 29/31 | 13/15 | 16/16 |
| Age, median (range), years | 61 (19–82) | 60 (19–74) | 63 (23–82) |
| APACHE II score, median (range) | 22 (2–45) | 21 (2–43) | 24 (3–45) |
| SOFA score, median (range) | 7.2 (0–17) | 7.0 (0–16) | 8.0 (0–17) |
| CRP, mg/dL, median (range) | 9.6 (2.3–34.6) | 10 (2.3–34.6) | 9.4 (3.0–29.8) |
| Creatinine, median (range), mg/dL | 1.2 (0–14) | 1.0 (0.4–14) | 1.3 (0–10.9) |
| Albumin, median (range), mg/dL | 27.3 (16.0–51.8) | 28.6 (16.0–51.8) | 26.4 (17.0–50.3) |
| WBC, median (range), ×103/μL | 11.0 (2.4–31.0) | 10.1 (3.0–31.0) | 11.8 (2.4–28.5) |
| PLT, median (range), ×103/μL | – | 20.0 (10.0–67.0) | 124 (101.0–452.0)* |
| FDP (mean ± SD), μg/L | 16.7±5.2 | 17.7±2.7 | 15.9±5.4 |
| PAI-1 (mean ± SD), ng/mL | 28.6±3.5 | 30.6±3.4 | 25.3±7.2 |
| Fibrinogen (mean ± SD), g/L | 4.9±1.6 | 5.1±2.2 | 4.8±1.2 |
| PT (mean ± SD), seconds | – | 13.97±2.86 | 16.57±2.98* |
Note:
P<0.001.
Abbreviations: APACHE II score, Acute Physiology and Chronic Health Evaluation II score; CRP, C-reactive protein; FDP, fibrin-fibrinogen degradation product; PAI-1, plasminogen activator inhibitor-1; PLT, platelet count; PT, prothrombin time; SOFA score, sequential organ failure assessment score; SS, severe sepsis without thrombocytopenia; SST, severe sepsis with thrombocytopenia; WBC, white blood cell count.
Table 2.
Baseline characteristics and 28-day mortality of patients
| Variable | All (60 patients) | SST (28 patients) | SS (32 patients) |
|---|---|---|---|
| Primary infection site | |||
| Respiratory | 30 (50%) | 14 (50%) | 16 (50%) |
| Abdominal | 10 (16.7%) | 5 (17.9%) | 5 (15.6%) |
| Urinary tract | 7 (11.7%) | 3 (10.7%) | 4 (12.4%) |
| Bloodstream | 6 (10%) | 3 (10.7%) | 3 (9.4%) |
| Others | 4 (6.6%) | 2 (7.1%) | 2 (6.3%) |
| Unknown | 3 (5%) | 1 (3.6%) | 2 (6.3%) |
| Microorganism identified | |||
| Gram-negative bacilli | 25 (41.6%) | 13 (46.4%) | 12 (37.5%) |
| Gram-positive cocci | 20 (33.3%) | 9 (32.1%) | 11 (34.5%) |
| Fungus | 2 (3.3%) | 1 (3.6%) | 1 (3.1%) |
| Intracellular germs | 2 (3.3%) | 1 (3.6%) | 1 (3.1%) |
| Others | 10 (6.5%) | 4 (14.3%) | 6 (18.8%) |
| Existence of shock | |||
| Yes | 15 (25%) | 11 (39.3%)* | 4 (12.5%)* |
| No | 45 (75%) | 17 (60.7%)* | 28 (87.5%)* |
| The number of organ dysfunction | |||
| 1 | 20 (33.3%) | 8 (28.6%) | 12 (37.4%) |
| 2 | 20 (33.3%) | 10 (35.7%) | 10 (31.3%) |
| ≥3 | 20 (33.4%) | 10 (35.7%) | 10 (31.3%) |
| The kind of organ dysfunction | |||
| Arterial hypoxemia | 14 (23.3%) | 7 (25%) | 7 (21.9%) |
| Arterial hypotension | 49 (81.7%) | 22 (78.6%) | 27 (84.4%) |
| Acute respiratory failure | 14 (23.3%) | 6 (21.4%) | 8 (25%) |
| Acute renal failure | 21 (35%) | 9 (32.1%) | 12 (37.5%) |
| Impaired neurological status | 13 (21.7%) | 7 (25%) | 6 (18.8%) |
| Acute liver failure | 10 (16.7%) | 5 (17.9%) | 5 (15.6%) |
| Others | 11 (18.3%) | 5 (17.9%) | 6 (18.8%) |
| None | 0 | 0 | 0 |
| Twenty-eight days survival | |||
| Alive | 34 (56.7%) | 12 (42.9%)* | 22 (68.8%)* |
| Dead | 26 (43.3%) | 16 (57.1%)* | 10 (31.2%)* |
Note:
P<0.05.
Abbreviations: SS, severe sepsis without thrombocytopenia; SST, severe sepsis with thrombocytopenia.
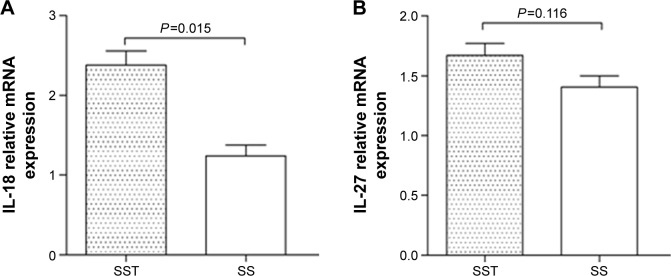
IL-18 and IL-27 miRNA expression in PBMCs of sepsis patients
The expression of IL-18 miRNA in severe sepsis patients with thrombocytopenia was higher than that in those without thrombocytopenia (P=0.015) (Figure 1). There was no significant difference in the expression of IL-27 between the two groups.
Figure 1.
miRNA expression of IL-18 and IL-27 in PBMCs of sepsis patients.
Notes: (A) miRNA expression of IL-18 in severe sepsis patients with thrombocytopenia was greater than that in those without thrombocytopenia (P=0.015). (B) There was no significant difference in the expression of IL-27 between the two groups (P=0.116).
Abbreviations: IL, interleukin; miRNA, micro RNA; PBMCs, peripheral blood mononuclear cells; SS, severe sepsis without thrombocytopenia; SST, severe sepsis with thrombocytopenia.
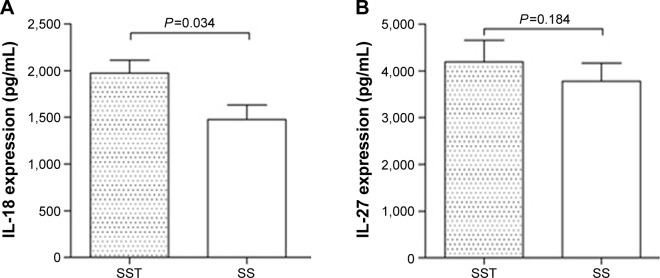
Plasma concentrations of IL-18 and IL-27 in sepsis patients
As shown in Figure 2, the plasma concentration of IL-18 in severe sepsis patients with thrombocytopenia was significantly higher than that in sepsis patients without thrombocytopenia (P=0.034). No significant difference was found in the expression of IL-27 between the two groups.
Figure 2.
Plasma concentrations of IL-18 and IL-27 in sepsis patients.
Notes: (A) The plasma concentration of IL-18 in severe sepsis patients with thrombocytopenia was found to be significantly higher than that in those without thrombocytopenia (P=0.034). (B) No significant difference in the expression of IL-27 was found between the two groups (P=0.184).
Abbreviations: IL, interleukin; SS, severe sepsis without thrombocytopenia; SST, severe sepsis with thrombocytopenia.
miR-130a and miR-150 expression in PBMCs of sepsis patients
Because miR-130a and miR-150 have been reported to target IL-18,18,25 we measured the expression of miR-130a and miR-150 in PBMCs of sepsis patients by TaqMan real-time PCR. The results demonstrated that miR-130a expression was significantly lower in severe sepsis patients with thrombocytopenia than in those without thrombocytopenia (P<0.003) (Figure 3), whereas miR-150 expression was not significantly different between the two groups (data not shown).
Figure 3.
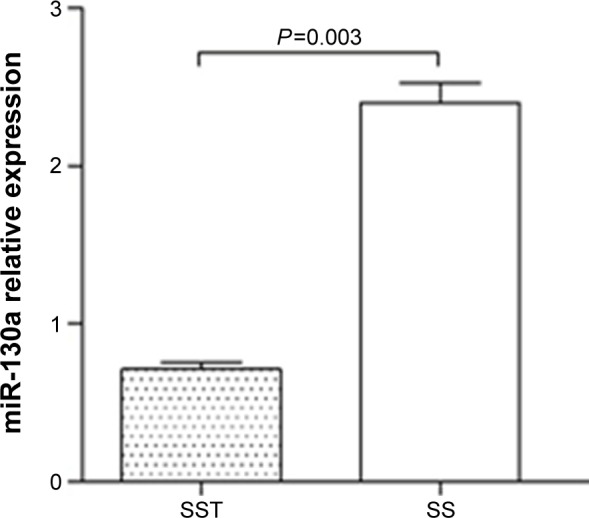
miR-130a expression in PBMCs of sepsis patients. miR-130a expression was significantly lower in severe sepsis patients with thrombocytopenia than in severe sepsis patients without thrombocytopenia (P<0.003).
Abbreviations: PBMCs, peripheral blood mononuclear cells; SS, severe sepsis without thrombocytopenia; SST, severe sepsis with thrombocytopenia.
Discussion
Sepsis is a systemic disease characterized by microbial infection and systemic inflammatory response syndrome, and is associated with high morbidity and mortality, especially in severe cases.32 Thrombocytopenia is common among severe sepsis patients,36 and a low PLT is predictive of a poor outcome.6 To date, the mechanism of severe sepsis with thrombocytopenia is still unknown. In the present study, we selected two cytokines linked to sepsis and thrombocytopenia, IL-18 and IL-27, to study the mechanism of the development of thrombocytopenia in severe sepsis patients.
It has been reported that the serum IL-18 level in sepsis patients is significantly greater than that in healthy volunteers.37 It has also been reported that a high blood IL-18 level may be an early predictor of mortality.38 The results of the present study showed that the miRNA expression and plasma concentration of IL-18 in severe sepsis patients with thrombocytopenia were higher than those in sepsis patients without thrombocytopenia (P=0.015 and P=0.034, respectively), whereas there was no significant difference in IL-27 expression between the two groups, indicating that IL-18 is involved in the development of thrombocytopenia in severe sepsis patients.
The discovery of miRNAs had led to the discovery of novel regulatory mechanisms for development of sepsis.26–30 It has been reported that IL-18 is the target gene of miR-130a and miR-150.18,30 Therefore, in this study, we also detected the expression of miR-130a and miR-150 by TaqMan real-time PCR, and the results showed that miR-130a expression was significantly lower in severe sepsis patients with thrombocytopenia, compared to that in sepsis patients without thrombocytopenia (P<0.003), indicating that miR-130a plays an important role in the pathogenesis of thrombocytopenia in severe sepsis patients.
Indeed, it is clear that knowing the primary infection site, identifying the microorganism, knowing the existence of shock, the number and kind of organ dysfunction, and clinical management of severe sepsis, including initial volemic resuscitation with goal-directed fluid challenge, diagnosis of infection with microbiological sampling coupled with imaging studies, treatment of infection with antibiotics, and so on, are very important. Even though severe sepsis is a syndrome involving the whole body, identification of these potential confounders causes important subsequent actions. For example, a severe sepsis patient with thrombocytopenia was associated with 28-day mortality, which may be due to the induction of shock (Table 2). Therefore, presence or absence of thrombocytopenia should be considered along with sepsis when approaching the evaluation or treatment of patients with severe sepsis.31
This study had potential weaknesses. First, the all-cause mortality of sepsis varies in different patient populations, which could have also affected outcome. Moreover, it involved a relatively small number of patients who were treated with heterogeneous regimens and therapies. Although the results demonstrate that severe sepsis patients with thrombocytopenia have increased plasma and miRNA expression levels of IL-18 and reduced expression of miR-130a, suggesting that IL-18 and miR-130a are involved in the pathophysiological process of thrombocytopenia in severe sepsis, further investigation is warranted to identify who would benefit most from analysis of these laboratory parameters. Taken together, these findings suggest that direct neutralization of IL-18 and upregulation of miR-130a to inhibit IL-18 indirectly may be promising therapeutic approaches of treating severe sepsis patients with thrombocytopenia.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (No. 81301624) and National Clinical Key Specialty Project Foundation of the Ministry of Health (No. 2011873). No benefits in any form have been or will be received from a commercial party directly or indirectly related to the subject of this article. We are indebted to all participating patients and colleagues at the Department of Intensive Care Unit and Key Lab for Critical Care Medicine of the Ministry of Health, Emergency Medicine Research Institute, and Tianjin First Center Hospital, who provided assistance for this study. We also thank the anonymous referee for his/her very helpful comments, which remarkably improved the quality of this paper.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors have no financial disclosures and are not using any copyrighted information in this paper. No text, text boxes, or figures in this article have been previously published or owned by another party. The authors report no conflicts of interest in this work.
References
- 1.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- 2.Vincent JL, Yagushi A, Pradier O. Platelet function in sepsis. Crit Care Med. 2002;30:S313–S317. doi: 10.1097/00003246-200205001-00022. [DOI] [PubMed] [Google Scholar]
- 3.Sakr Y. Heparin-induced thrombocytopenia in the ICU: an overview. Crit Care. 2011;15:211. doi: 10.1186/cc9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aird WC. The hematologic system as a marker of organ dysfunction in sepsis. Mayo Clin Proc. 2003;78:869–881. doi: 10.4065/78.7.869. [DOI] [PubMed] [Google Scholar]
- 5.Nijsten MW, ten Duis HJ, Zijlstra JG, et al. The TH. Blunted rise in platelet count in critically ill patients is associated with worse outcome. Crit Care Med. 2000;28:3843–3846. doi: 10.1097/00003246-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Akca S, Haji-Michael P, de Mendonca A, Suter P, Levi M, Vincent JL. Time course of platelet counts in critically ill patients. Crit Care Med. 2002;30:753–756. doi: 10.1097/00003246-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30:1765–1771. doi: 10.1097/00003246-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Tschoeke SK, Oberholzer A, Moldawer LL. Interleukin-18: a novel prognostic cytokine in bacteria-induced sepsis. Crit Care Med. 2006;34:1225–1233. doi: 10.1097/01.CCM.0000208356.05575.16. [DOI] [PubMed] [Google Scholar]
- 9.Washburn KK, Zappitelli M, Arikan AA, et al. Urinary interleukin-18 is an acute kidney injury biomarker in critically ill children. Nephrol Dial Transplant. 2008;23:566–572. doi: 10.1093/ndt/gfm638. [DOI] [PubMed] [Google Scholar]
- 10.Siew ED, Ikizler TA, Gebretsadik T, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5:1497–1505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagshaw SM, Langenberg C, Haase M, Wan L, May CN, Bellomo R. Urinary biomarkers in septic acute kidney injury. Intensive Care Med. 2007;33:1285–1296. doi: 10.1007/s00134-007-0656-5. [DOI] [PubMed] [Google Scholar]
- 12.Lamkanfi M, Sarkar A, Vande Walle L, et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochholzer P, Lipford GB, Wagner H, Pfeffer K, Heeg K. Role of interleukin-18 (IL-18) during lethal shock: decreased lipopolysaccharide sensitivity but normal superantigen reaction in IL-18-deficient mice. Infect Immun. 2000;68:3502–3508. doi: 10.1128/iai.68.6.3502-3508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamanaka K, Tanaka M, Tsutsui H, et al. Skin-specific caspase-1-transgenic mice show cutaneous apoptosis and pre-endotoxin shock condition with a high serum level of IL-18. J Immunol. 2000;165:997–1003. doi: 10.4049/jimmunol.165.2.997. [DOI] [PubMed] [Google Scholar]
- 15.Wirtz S, Tubbe I, Galle PR, et al. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203:1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan NN, Zhu XJ, Peng J, et al. Interleukin 18 and interleukin 18 binding protein in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2009;144:755–761. doi: 10.1111/j.1365-2141.2008.07520.x. [DOI] [PubMed] [Google Scholar]
- 17.Shan NN, Zhu XJ, Wang Q, et al. High-dose dexamethasone regulates interleukin-18 and interleukin-18 binding protein inidiopathic thrombocytopenic purpura. Haematologica. 2009;94:1603–1607. doi: 10.3324/haematol.2009.007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, Li H, Du W, et al. Reduced MIR130A is involved in primary immune thrombocytopenia via targeting TGFB1 and IL18. Br J Haematol. 2014;166(5):767–773. doi: 10.1111/bjh.12934. [DOI] [PubMed] [Google Scholar]
- 19.Liu XG, Ren J, Yu Y, et al. Decreased expression of interleukin-27 in immune thrombocytopenia. Br J Haematol. 2011;153:259–267. doi: 10.1111/j.1365-2141.2011.08614.x. [DOI] [PubMed] [Google Scholar]
- 20.Li HY, Zhang DL, Ge J, et al. Elevated interleukin-27 enhances the polarization of Th1/Tc1 cells and the production of proinflammatory cytokines in primary immune thrombocytopenia. Hum Immunol. 2012;73:240–247. doi: 10.1016/j.humimm.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Scicluna BP, van der Poll T. Interleukin-27: a potential new sepsis biomarker exposed through genome-wide transcriptional profiling. Crit Care. 2012;16:188. doi: 10.1186/cc11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong HR, Lindsell CJ, Lahni P, Hart KW, Gibot S. Interleukin 27 as a sepsis diagnostic biomarker in critically ill adults. Shock. 2013;40:382–386. doi: 10.1097/SHK.0b013e3182a67632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opal SM. Dual inhibition of interleukin-1β and interleukin-18: a new treatment option for sepsis? Am J Respir Crit Care Med. 2014;189:242–244. doi: 10.1164/rccm.201312-2292ED. [DOI] [PubMed] [Google Scholar]
- 24.Wong HR, Liu KD, Kangelaris KN, Lahni P, Calfee CS. Performance of interleukin-27 as a sepsis diagnostic biomarker in critically ill adults. J Crit Care. 2014;29:718–722. doi: 10.1016/j.jcrc.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 26.Wang JF, Yu ML, Yu G, et al. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun. 2010;394:184–188. doi: 10.1016/j.bbrc.2010.02.145. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Wang HC, Chen C, et al. Differential expression of plasma miR-146a in sepsis patients compared with non-sepsis-SIRS patients. Exp Ther Med. 2013;5:1101–1104. doi: 10.3892/etm.2013.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie LX. Evidence for serum miR-15a and miR-16 levels as biomarkers that distinguish sepsis from systemic inflammatory response syndrome in human subjects. Clin Chem Lab Med. 2012;50:1423–1428. doi: 10.1515/cclm-2011-0826. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie L. Serum microRNA signatures identified by Solexa sequencing predict sepsis patients’ mortality: a prospective observational study. PLoS One. 2012;7:e38885. doi: 10.1371/journal.pone.0038885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasilescu C, Rossi S, Shimizu M, et al. MicroRNA fingerprints identifies miR-150 as a plasma prognostic marker in patients with sepsis. PLoS One. 2009;4:e7405. doi: 10.1371/journal.pone.0007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiery-Antier N, Binquet C, Vinault S, et al. Is Thrombocytopenia an early prognostic marker in septic shock? Crit Care Med. 2015 Dec 14; doi: 10.1097/CCM.0000000000001520. Epub. [DOI] [PubMed] [Google Scholar]
- 32.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2012;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 33.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 34.Finfer S, Bellomi R, Blair D, et al. NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 35.Wu Q, Ren J, Wang G, et al. Evaluating the safety and efficacy of recombinant human thrombopoietin among severe sepsis patients with thrombocytopenia: study protocol for a randomized controlled trial. Trial. 2015;16:220. doi: 10.1186/s13063-015-0746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mavrommatis AC, Theodoridis T, Orfanidou A, Roussos C, Christopoulou-Kokkinou V, Zakynthinos S. Coagulation system and platelets are fully activated in uncomplicated sepsis. Crit Care Med. 2000;28:451–457. doi: 10.1097/00003246-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 37.Grobmyer SR, Lin E, Lowry SF, et al. Elevation of IL-18 in human sepsis. J Clin Immunol. 2000;20:212–215. doi: 10.1023/a:1006641630904. [DOI] [PubMed] [Google Scholar]
- 38.Emmanuilidis K, Weighardt H, Matevossian E, et al. Differential regulation of systemic IL-18 and IL-12 release during postoperative sepsis: high serum IL-18 as an early predictor of lethal outcome. Shock. 2002;18:301–305. doi: 10.1097/00024382-200210000-00002. [DOI] [PubMed] [Google Scholar]