PRESENTATION OF CASE
Dr. Bradford C. Dickerson: A 31-year-old right-handed man was seen in an outpatient neurology clinic of this hospital because of personality changes and progressive neurologic decline.
The patient had been well until 3 years before this presentation, when his wife noticed that he was unwilling to share a portable media player that had been given to them, whereas he had previously been very generous and caring. He began to listen obsessively to audio books, insisting that he and his wife listen while cooking or driving. He impulsively purchased items they could not afford. Formerly very social, he began spending time alone; he made mistakes at work and failed advanced-degree examinations. When his wife became pregnant, he repeatedly voiced fears about losing his job if he took paternity leave. He began drinking alcohol and smoking obsessively until he vomited. After the baby was born, he seemed to be disinterested in spending time with his wife and newborn.
The patient left his job as a high-school teacher because he planned to run a small home business and care for the infant so his wife could return to work. His clients soon complained about the poor quality of his work. He mixed the infant’s formula incorrectly and forgot to finish dressing the infant before leaving the house. His wife became concerned and began taking the baby to work, which did not bother the patient.
The patient was seen by his physician at another facility for a routine evaluation. His wife reported his withdrawn, compulsive behaviors. He had had a serious head injury with loss of consciousness in a car accident at 12 years of age and was otherwise healthy. A presumptive diagnosis of depression was made.
Eleven months after symptom onset, the patient was stopped by police for reckless driving while intoxicated, and he was taken to the emergency department of another hospital. Computed tomography of the head reportedly revealed encephalomalacia in the temporal lobes, a finding that was attributed to the head trauma that had occurred during childhood. He was admitted to a psychiatric hospital for 1 week. A diagnosis of severe depression was made, and he was released to the care of relatives; this caused him to miss Christmas with his wife and child, which did not seem to concern him.
During the next 2 months, multiple psychiatric evaluations and two neurologic evaluations were performed. A behavioral neurologic evaluation reportedly revealed mild parkinsonism, attentional and executive dysfunction, memory impairment, anomia, and a flat affect.
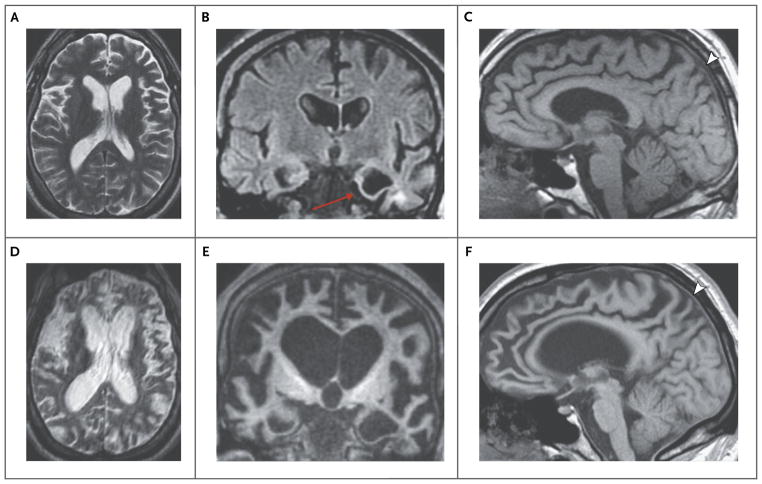
Dr. Mykol Larvie: Magnetic resonance imaging (MRI) of the head that was performed during that time revealed severe focal atrophy in the anterior left temporal lobe and relatively mild diffuse atrophy in the frontal lobes (Fig. 1A, 1B, and 1C). T2-weighted images showed a small area of hyperintensity in the white matter of the anterior left temporal lobe; the parenchymal signal was otherwise normal. The hyperintensity and focal atrophy in the left temporal lobe might be suggestive of encephalomalacia related to remote trauma, but the atrophy in the frontal lobes is not typical of traumatic brain injury and suggests a neurodegenerative process.
Figure 1. Neuroimaging Studies.
Neuroimaging studies were obtained 3 years before this evaluation. An axial T2-weighted image of the brain shows diffuse loss of parenchymal volume (Panel A). A coronal T2-weighted fluid-attenuated inversion recovery image shows severe focal atrophy in the left temporal lobe, with a small area of hyperintensity in the underlying white matter (Panel B, arrow). A sagittal T1-weighted image shows mild atrophy in the frontal lobes bilaterally (Panel C). Further neuroimaging studies were obtained during this evaluation. An axial T2-weighted image (Panel D), a coronal T1-weighted image (Panel E), and a sagittal T1-weighted image (Panel F) show marked progression of atrophy, which is now severe and has a knifelike appearance in the temporal and frontal lobes and insular cortexes. There is relative but not absolute sparing of the parietal lobes (Panels C and F, arrowheads).
Dr. Dickerson: Findings on electroencephalography were reportedly normal. Progressive behavioral deterioration continued; the patient became unable to care for himself. Seventeen months after symptom onset, he moved to another state to live with relatives and attend a full-time day program. One year later (at 31 years of age), he returned to New England to be admitted to a neurorehabilitation facility. On admission, he recognized family but was unable to communicate. He was occasionally incontinent. Three years after symptom onset, he was no longer able to walk or feed himself and ate pureed food. He was referred to the outpatient neurology clinic of this hospital. Medications included trazodone, benztropine, hydroxyzine, omeprazole, and haloperidol.
On examination, the patient was unable to follow commands and had a masked face. He was able to track a dollar bill with his eyes and had intact extraocular movements; smooth-pursuit eye movements revealed saccadic intrusions. He did not reach for the dollar bill when it was offered to him. Muscle bulk was diminished; there were no fasciculations. Tone was increased in all limbs, with mild cogwheel rigidity, occasional myoclonus, and prominent bilateral grasp reflexes. The physical examination was otherwise normal.
Additional diagnostic testing was performed.
DIFFERENTIAL DIAGNOSIS
Dr. Bruce L. Miller: In this previously healthy 28-year-old man, severe and debilitating dementia developed, with profound loss of cognitive abilities and diminished ability to move over a period of 3 years.
DEMENTIA
When I encounter a patient with dementia, I am interested in the first symptom that occurred, because this information enables me to localize the first affected area in the brain and tells me where the illness started. By tracking the patient’s symptoms and the involvement of neural circuits,1 it is often possible to deduce the pathogenesis of the illness. For example, Alzheimer’s disease usually begins in posterior brain regions that affect memory, language, and spatial function, and thus most patients with Alzheimer’s disease first have memory problems and word-finding difficulties. In this case, the first symptoms were psychiatric; loss of sympathy and empathy is strongly correlated with degeneration in the temporal lobe of the nondominant hemisphere, insular cortex, orbitofrontal cortex, and ventral striatum.2
The patient’s constellation of compulsive behaviors, apathy, and disinhibition is associated with diminished function in the circuits of the anterior cingulate, insular, and orbitofrontal cortexes that drive and regulate behavior.3 Hyperorality and loss of response to internal signals such as satiety or nausea are highly typical of involvement of the right frontoinsular cortex or hypothalamus.4 As the disease progressed, the patient became disorganized and unconcerned, exhibiting a loss of executive function that is attributable to dysfunction of frontosubcortical structures. Thus, this patient’s dementia began in the paralimbic, frontoinsular, and anterior cingulate cortexes and was possibly more severe on the right side than on the left side. The patient’s illness progressed to severe dementia over a period of approximately 3 years; after 3 years, he had features associated with parkinsonism, such as action tremor and myoclonus.
PSYCHIATRIC DISORDERS
Initially, it would have been easy to mistake this patient’s illness for a psychiatric disorder. Patients with bipolar disorder present with impulsivity, self-centeredness, and acceleration of addictive behaviors, but progressive loss of day-to-day function is unusual. Depression can be manifested by impulsivity, social withdrawal, and lack of concern for others; however, this patient never reported changes in his mood. Schizophrenia can cause profound and debilitating impairment, including apathy and executive dysfunction, but the onset of this patient’s illness occurred at an older age than would be expected in a patient with schizophrenia, and the movement abnormalities seen in this case are not compatible with a diagnosis of schizophrenia alone.
NEURODEGENERATIVE DISORDERS
Although the 3-year course of disease progression seen in this case is strikingly rapid, it is not rapid enough to be classified as a rapidly progressive dementia. Causes of rapidly progressive dementia include Creutzfeldt–Jakob disease (which usually progresses to death over a period of 9 months), viral encephalitis, primary central nervous system vasculitis, paraneoplastic syndromes, and other autoimmune encephalopathies. However, because of this patient’s relatively young age, I would perform diagnostic testing for paraneoplastic syndromes and vasculitis.5
There was no evidence of a toxic–metabolic disorder, although any patient with a progressive dementia should be screened for anemia, vitamin B12 deficiency, and thyroid, liver, or renal disease. Because of this patient’s relatively young age, I would screen for heavy metals in the urine and for syphilis. A lumbar puncture would help to rule out any infectious process (e.g., Lyme disease) and would enable measurement of β-amyloid and tau levels in the cerebrospinal fluid, which would help to rule out Alzheimer’s disease.
Disorders related to degeneration of the basal ganglia may initially affect patients by causing prominent behavioral symptoms. Huntington’s disease, a relatively common cause of dementia in this patient’s age group, often starts as a neuropsychiatric syndrome characterized by obsessive–compulsive and manic behaviors and loss of executive function. In this case, the lack of a family history of Huntington’s disease makes this inherited disease an unlikely diagnosis; furthermore, the course of disease progression was more rapid than would be expected in a patient with Huntington’s disease. Wilson’s disease should be considered in young patients with neuropsychiatric signs. Testing should include evaluation for the presence of Kayser–Fleischer rings on slit-lamp examination, a low serum copper level, and a high 24-hour urinary copper level.
Young adults with lipid-storage disorders, other late-onset inborn errors of metabolism, or white-matter degenerative diseases6 (Table 1) often present with retinopathy, hepatosplenomegaly, gaze disturbance, or white-matter disease; none of these features were present in this patient. The differential diagnosis in this case may include mitochondrial disorders, which are often inherited maternally. The patient’s mother had a mood disorder and his great-grandmother had late-onset dementia, but the patient did not have the clinical features of a mitochondrial disorder, such as short stature, loss of cardiac conduction, diabetes, retinal disease, or hearing loss.
Table 1.
Late-Onset Inborn Errors of Metabolism or White-Matter Degenerative Diseases.
| Disease | Enzyme | Clinical Syndrome | Findings on MRI |
|---|---|---|---|
| Metachromatic leukodystrophy | Arylsulfatase | Causes psychiatric symptoms progressing to dementia | Frontal changes |
| Adrenoleukodystrophy | ABCD1 peroxisomal membrane transporter protein | X-linked; occurs in young boys and heterozygotic women; symptoms are variable | Posterior changes (variable in women) |
| Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy | Notch 3 | Causes stroke, headache, neuropsychiatric symptoms, and dementia | Frontotemporal and white-matter changes |
| Nasu–Hakola disease | TREM2 (recessive); DAP12 | Causes psychiatric symptoms progressing to dementia; also causes bone cysts | Frontal atrophy and white-matter changes |
| Adult polyglucosan body disorder | Glycogen branching enzyme 1 | Causes neuropathy, incontinence, and dementia | Frontotemporal and white-matter changes |
| Neuronal ceroid lipofuscinosis | CLN1–8 and PPT1; progranulin (recessive) | Causes manic symptoms progressing to dementia, retinopathy, seizures, and granular osmiophilic deposits in white cells, skin, and neurons | Atrophy and white-matter changes |
| Gaucher’s disease | Glucocerebrosidase | Causes dementia, vertical gaze, and parkinsonism; affects liver, spleen, and bone marrow | Atrophy |
| Niemann–Pick disease type C | NPC1 and NPC2 (transport protein) | Causes schizophrenia-like symptoms, vertical gaze, parkinsonism, and cerebellar ataxia; affects liver and spleen | Atrophy |
| Adult Tay–Sachs disease | Hexosaminidase A | Causes manic symptoms, schizophrenia, dementia, amyotrophic lateral sclerosis, and retinal abnormalities | Atrophy and white-matter changes |
FRONTOTEMPORAL DEMENTIA
The features of this patient’s disorder are most consistent with a diagnosis of frontotemporal dementia, which was first described by Arnold Pick in 1892. Frontotemporal dementia targets circuits of the bilateral frontoinsular cortexes7; these were the first circuits involved in this case. Patients with frontotemporal dementia that starts on the left side of the brain present with primary progressive aphasia. Patients with frontotemporal dementia that starts on the right side of the brain present with psychiatric symptoms associated with the behavioral variant of frontotemporal dementia; the six major symptoms are disinhibition, apathy, loss of sympathy and empathy, repetitive behaviors, hyperorality, and loss of executive function. A possible diagnosis of the behavioral variant of frontotemporal dementia can be made if three of these six symptoms are present8; this patient had at least five. A probable diagnosis of the behavioral variant of frontotemporal dementia can be made if selective frontotemporal atrophy or hypometabolism is present or if the patient has a genetic mutation that is known to cause frontotemporal dementia.
Frontotemporal dementia is the most common cause of dementia in adults younger than 60 years of age9; onset of symptoms typically occurs in the sixth decade. The majority of affected patients are initially thought to have a primary psychiatric condition and are often being seen by marriage or addiction counselors, psychologists, human-relations officers, or legal counselors (as in this case). Most cases of frontotemporal dementia are sporadic, but 10 to 15% of cases are associated with an autosomal dominant genetic mutation.10 The three subtypes of frontotemporal dementia are based on the types of inclusions present in the brain: tau, TDP43 (transactive response DNA-binding protein 43), and FUS (RNA-binding protein fused in sarcoma). Tau is often associated with parkinsonism but almost never with amyotrophic lateral sclerosis (ALS), whereas TDP43 and FUS are more likely to be associated with frontotemporal dementia and ALS.
Could this patient have a tau subtype of frontotemporal dementia? Pick’s disease, the prototypical form of frontotemporal dementia, is associated with cytoplasmic and glial aggregates of tau-3R. Affected patients present with a slowly progressive dementia that typically begins in the fifth to seventh decade and is rarely familial.7 The course of disease progression in this patient is faster and the age at onset is younger than would be expected in a patient with Pick’s disease.10
Could this patient have a TDP43 subtype of frontotemporal dementia? Several forms of frontotemporal dementia are associated with TDP43, including diseases associated with mutations in the progranulin gene11 and motor neuron diseases.12 The TDP43 subtype of the behavioral variant of frontotemporal dementia is common; onset usually occurs early in the seventh decade, and the clinical features are similar to those seen in this case. This patient is younger than any patient with the TDP43 subtype of the behavioral variant of frontotemporal dementia who has been described in the literature.
ALS, which is associated with TDP43 type B, is a serious consideration in this patient. He had increased tone, suggesting possible upper-motor-neuron involvement, and it is possible that his diffuse wasting represented lower-motor-neuron disease. An electromyographic study would be useful to assess for evidence of ALS in the patient.
Familial forms of frontotemporal dementia and ALS are often caused by a hexanucleotide repeat expansion in C9ORF7213; such forms are associated with TDP43 type B. Of the patients with frontotemporal dementia associated with an autosomal dominant mutation who are being treated at the University of California, San Francisco, 53% have this mutation. Among patients with this mutation, ALS is more common than frontotemporal dementia, and the age at onset is usually older than the age at onset among patients with the two other most common mutations, of the microtubule-associated protein tau (MAPT) gene and the progranulin gene.
The FUS subtype of frontotemporal dementia is the least common of the three subtypes; it occurs in approximately 5% of patients with frontotemporal dementia. If this patient has an FUS subtype, it is more likely to be idiopathic than related to a mutation in the gene,14 since FUS mutations are more likely to cause ALS than frontotemporal dementia.15 The youngest patients with frontotemporal dementia often have an FUS subtype; onset of symptoms tends to occur in the third or fourth decade, and symptoms can include profound disinhibition, irritability, and loss of social interest. Psychosis occurs in 30% of affected patients, and movement problems emerge late in the disease course. Among affected patients, MRI findings include atrophy in the orbitofrontal cortex, ventral striatum, and anterior temporal lobe and profound atrophy of caudate nuclei.16 Because of this patient’s young age, the lack of a family history associated with his illness, and the profoundly disordered behavioral presentation, I believe an FUS subtype of the behavioral variant of frontotemporal dementia is the most likely diagnosis.
OTHER CONSIDERATIONS
Do any other disorders have features that are similar to those associated with the behavioral variant of frontotemporal dementia? The patient’s history of head injury raises the possibility of alternative diagnoses. The normal-pressure syndrome and the low-pressure syndrome can have features similar to those of the behavioral variant of frontotemporal dementia. I would also look for evidence on MRI of hydrocephalus or the sagging brain syndrome, because both of these diseases are associated with previous brain trauma. In patients with normal-pressure hydrocephalus, alterations in the flow of cerebrospinal fluid lead to disproportionate dysfunction of the frontal lobes.17 In patients with the sagging brain syndrome, injury can lead to leaks of cerebrospinal fluid, and the differential pressure can push the frontal lobes downward.18 Finally, could this patient have chronic traumatic encephalopathy? This syndrome is often seen in boxers and football players, causes progressive behavioral changes followed by motor symptoms,19 and is associated with massive aggregation of tau and TDP43. Disease progression in patients with chronic traumatic encephalopathy is slower than the progression seen in this case, although it would not be surprising to find some evidence on neuropathological examination of sequelae associated with the serious head injury that happened 17 years earlier.
SUMMARY
Additional tests should include neuroimaging and a lumbar puncture to assess for high pressure, evidence of Alzheimer’s disease, inflammation, and a massively elevated level of tau (which is a feature of Creutzfeldt–Jakob disease). The most cost-effective way to perform genetic testing for the large number of conditions that are under consideration in this case would be to perform whole-exome sequencing; the method used should accurately capture the genes associated with causes listed in the differential diagnosis, including MAPT, progranulin, and C9ORF72.
Dr. Nancy Lee Harris (Pathology): Dr. Dickerson, would you tell us your thinking when you saw this patient?
Dr. Dickerson: We also favored a diagnosis of the behavioral variant of frontotemporal dementia. Additional diagnostic evaluation included repeat brain imaging and genetic testing.
CLINICAL DIAGNOSIS
Probable behavioral variant of frontotemporal dementia.
DR. BRUCE L. MILLER’S DIAGNOSIS
Behavioral variant of frontotemporal dementia, most likely due to FUS-related neurodegeneration of an unknown cause or possibly due to a genetic mutation.
DIAGNOSTIC TESTING
Dr. Larvie: Repeat MRI revealed marked progression of atrophy in the frontal and temporal lobes and insular cortexes, with relative but not absolute sparing of the parietal lobes (Fig. 1D, 1E, and 1F). These findings are consistent with a progressive neurodegenerative disorder and typical of frontotemporal lobar degeneration.
Ms. Diane E. Lucente: The patient was seen for a genetic consultation. The patient’s mother, who was in her late 60s, had a history of psychiatric disorders, including anxiety, panic attacks, and depression; at 41 years of age, she spent 4 weeks in a psychiatric hospital for panic disorder. She was otherwise healthy. The patient’s father died in his 60s from colon cancer; he had reportedly been neurologically intact. The patient’s maternal great-grandmother had late-onset dementia. The remainder of the family history was unremarkable.
Although the family history suggested a low likelihood of an inherited disease, the patient’s relatively young age prompted us to suggest genetic testing; the patient’s wife concurred.20,21 Sequencing of the MAPT gene on chromosome 17 identified a Gly→Arg mutation at codon 389 (base 2170), a mutation known to cause frontotemporal dementia.22 The altered MAPT gene23 has several possible origins. One of the patient’s parents might have had a nonpenetrant MAPT mutation, although MAPT mutations are usually highly penetrant. The disease might have developed in the patient’s father if he had lived longer, but to our knowledge, the difference of age at disease onset among family members with MAPT mutations has never been greater than 25 years. Nonpaternity is a possible explanation, but the most likely explanation is a de novo mutation.
The patient’s child has a 50% chance of inheriting this mutation. We do not recommend genetic testing of children in families with a history of frontotemporal dementia, because no preventive treatments are available and because it is important to respect the genetic privacy of minors.
DISCUSSION OF MANAGEMENT
Dr. Dickerson: In the management of frontotemporal dementia, the priority is to make a confident clinical diagnosis, which can be challenging. Tremendous progress has been made in the 23 years since frontotemporal dementia was last discussed in this forum.24 The lack of insurance reimbursement for diagnostic testing, particularly for positron-emission tomographic (PET) imaging, can be a barrier.25 PET imaging for amyloid can play an important role in ruling out atypical forms of Alzheimer’s disease in patients with suspected frontotemporal dementia, and PET imaging for tau is now being studied in humans.26
In this case, although the clinical diagnosis of frontotemporal dementia had been made by a neurologist at another hospital 2 years before this evaluation, confirmation of the diagnosis along with the presence of a disease-causing mutation was shocking and distressing to the patient’s wife. Once the diagnosis was confirmed, we had to deliver the news that there are no disease-modifying therapies for frontotemporal dementia. Nevertheless, the symptoms of frontotemporal dementia can be treated, ideally by a multidisciplinary team of specialists.27 Treatment includes pharmacologic and nonpharmacologic management of symptoms, management of coexisting conditions, psychosocial support, and education of the family and, if possible, the patient.28
No medications have been approved for the symptomatic treatment of frontotemporal dementia, but many have shown beneficial effects in small studies.29–31 For example, selective serotonin-reuptake inhibitors and other antidepressants can modulate disinhibition and compulsive behavior, stimulants and prodopaminergic agents can sometimes reduce apathy and attentional impairment, and mood stabilizers and antipsychotic agents can ameliorate agitation and aggression. The side effects of these medications may outweigh the benefits and should always be closely monitored. This patient’s medications included benztropine for parkinsonism, trazodone and hydroxyzine for anxiety, and the antipsychotic agent haloperidol for behavioral symptoms.
Strategies for nonpharmacologic management of symptoms can include behavioral, speech and language, occupational, and physical therapy, as well as psychotherapy (for the patient or the family). The patient should also undergo an assessment of driving, financial, and health care competency and should obtain social-work assistance with arranging disability compensation and psychosocial support.32,33 Paid or volunteer companions, home health aides, and day programs or respite programs can play important roles. Finally, it is critical to assist patients and families with end-of-life care, facilitating access to palliative care resources. The Association for Frontotemporal Degeneration (www.theaftd.org) and the Alzheimer’s Association (www.alz.org) can be invaluable resources. Although research on frontotemporal dementia focuses largely on understanding the disease and offers few experimental treatment options, participation in studies can provide meaning in an otherwise tragic situation. The quality of the partnership between care providers and patients and families critically influences the experience of living with frontotemporal dementia.
This patient’s wife is with us today and will describe the experience of caring for him.
The Patient’s Wife: My husband and I met when we were college freshmen. Weeks before our fifth wedding anniversary, we found out that a baby was on the way. During my pregnancy, my gregarious husband became socially withdrawn. His behavior became erratic: he bought things we could not afford, began having trouble meeting deadlines and following the work dress code, and withdrew from his friends. He became obsessed with listening repeatedly to Harry Potter audio books, to the extent that when I was in labor, I had to ask him to put his iPod away.
By the time our child was 6 months old, my husband was functioning so poorly that I did not feel comfortable leaving them alone together. Accompanying him to a routine appointment, I said to his primary care physician, “My husband is acting so weird — does he have a brain tumor?” She was the first of eight medical and mental health professionals who failed to make the diagnosis.
Receiving an accurate diagnosis was critical not only for understanding what was happening but also for practical purposes. When the diagnosis of frontotemporal dementia was made, it allowed me to apply for Social Security disability benefits.
My husband spent the last 2 years of his life at a neurorehabilitation center. It was hard to find a facility that would take a young, strong male patient. When he arrived, he was incontinent and afraid of bathing and routinely ripped off his clothing. Eventually, he lost his ability to walk, speak, and swallow, and I became his voice. I worked with the wonderful facility and hospice staff to develop a plan that ensured his comfort and dignity while not prolonging his struggle. In his final moments, the two of us held hands and listened to our wedding song, reliving our beginning while we faced our end.
My husband died at age 33, shortly after our child’s fourth birthday and months before we might have celebrated a decade of marriage. I requested that an autopsy be performed. I am determined that frontotemporal dementia will not have the last word in our family’s story. I want our child to have a future in which frontotemporal dementia is not all-powerful and to be able to plan on a full life. The attention given to my husband’s case and to frontotemporal dementia this morning is a step toward that future.
PATHOLOGICAL DISCUSSION
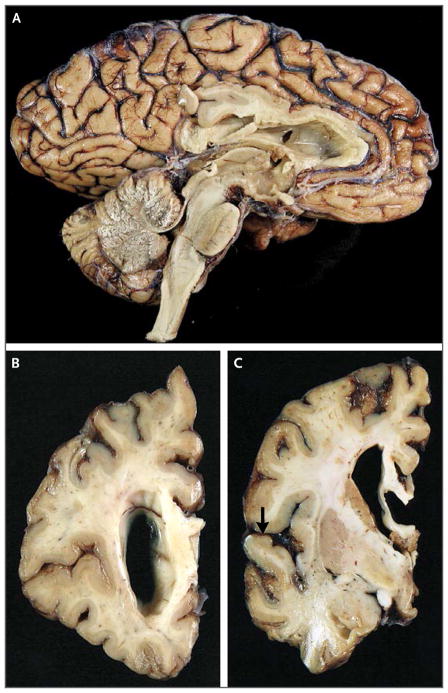
Dr. Matthew P. Frosch: On autopsy, the brain weighed 1010 g and had atrophy with a knifelike appearance in the frontal and temporal lobes. The posterior superior temporal gyrus and parietal and occipital cortexes and white matter were relatively preserved (Fig. 2).
Figure 2. Gross Neuropathological Photographs at Autopsy.
A medial aspect of the brain after fixation (Panel A) reveals moderate atrophy of the frontal and temporal lobes. A coronal section anterior to the head of the caudate (Panel B) reveals thinning of the cerebral cortex and ventricular enlargement. A coronal section obtained at the midbody of the amygdala (Panel C) reveals thinning of the cerebral cortex at the frontal and temporal lobes, with sparing of the superior temporal gyrus (arrow).
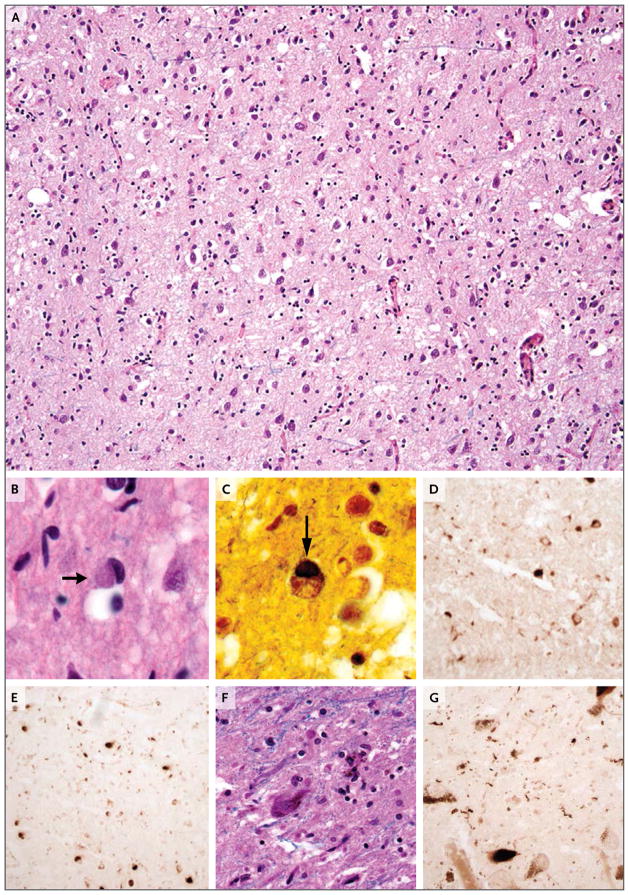
Microscopic examination (Fig. 3) revealed marked cortical thinning, spongiosis, prominent neuronal loss, and abundant reactive gliosis. Dense, smooth-contoured, rounded inclusions known as Pick bodies were seen in neuronal cytoplasm on routine staining (with a combination of Luxol fast blue and hematoxylin and eosin) and silver impregnation.34,35 This Gly389Arg MAPT mutation was present on the C-terminal side of the microtubule-binding domains; the mutation decreases interactions between tau and protein phosphatase 2A, a regulator of tau phos-phorylation.36 Staining with phosphospecific antibodies against tau was performed37; staining with AT8 (specific for phosphorylations at S199, S202, and T205) and PHF1 (specific for phosphorylations at S396 and S404) revealed tau-containing inclusions, but staining with 12E8 (specific for phosphorylations at S262 and S356) did not. These findings are consistent with those previously reported with the Gly389Arg MAPT mutation.38 Tau-containing inclusions, such as coiled bodies in oligodendrocytes, were abundant in the white matter; such white-matter lesions are present in other tauopathies with and without MAPT mutations.39 No histopathological or immunohistochemical evidence of other neurodegenerative diseases was present.40,41
Figure 3. Microscopic Neuropathological Images at Autopsy.
A combination of Luxol fast blue and hematoxylin and eosin staining of the frontal lobe shows attenuation of the neuropil, neuronal loss, and reactive gliosis (Panel A). A Pick body is evident in a cortical neuron (Panel B, arrow). Impregnation with the use of a modified Bielschowsky silver method shows a Pick body (Panel C, arrow). Immunohistochemical staining with phosphospecific antibodies against tau was performed; staining for AT8 (specific for phosphorylation at S199, S202, and T205) (Panel D) and staining for PHF1 (specific for phosphorylation at S396 and S404) (Panel E) show tau-containing inclusions. A combination of Luxol fast blue and hematoxylin and eosin staining shows neuronal loss and extracellular neuromelanin in the substantia nigra pars compacta (Panel F). Immunohistochemical staining for PHF1 shows cytoplasmic inclusions and neuropil threads in the substantia nigra that contain phosphorylated tau (Panel G).
Examination of the hippocampal formation revealed relative preservation of neurons, occasional tau-positive neuropil threads and Pick bodies in the dentate gyrus, and abundant tau-containing inclusions in the alveus and fimbria. The brain stem had neuronal loss, gliosis, tau-containing neuritic processes, and rare tau-positive cytoplasmic neuronal inclusions in the substantia nigra; Pick bodies and tau-containing dystrophic neurites were present in the basis pontis and medulla. In the spinal cord, lateral and anterior corticospinal tracts showed moderate degeneration; the number of anterior horn cells was normal, but numerous tau-containing neuropil threads were present.
The clinical and pathological findings support the diagnosis of frontotemporal lobar degeneration associated with a Gly389Arg MAPT mutation, with tau-positive inclusions (Pick bodies) and involvement of the substantia nigra.42 A condition associated with the pathological characteristics of Pick’s disease and a Gly389Arg MAPT mutation has been described previously22,43,44; cases in patients younger than this patient at symptom onset have been reported.45 Other MAPT mutations can also cause tauopathies with characteristics of Pick’s disease.35
Dr. Harris: Dr. Miller, do you have any comments?
Dr. Miller: I want to thank the patient’s wife, whose courage inspires us. You went through the tragedy that families of patients with frontotemporal dementia often endure: severe distress and often years without a diagnosis. The management of frontotemporal dementia will change over time, and as it does, we will need to make diagnoses early so we can intervene.
Dr. Harris: This conference was supported by a gift from Sarah and John Weinberg, in honor of Betty U. and William F. McNeely, both of whom were associated with the Clinicopathological Conferences (CPC) for many years. I would like to invite Dr. Weinberg, who is a retired pediatrician, to comment.
Dr. Sarah K. Weinberg: We are pleased to join you for this CPC to honor the memory of my mother and stepfather, Betty and Bill McNeely. In 1957, Betty was hired as Assistant Editor for the CPCs. In those days, two cases were discussed each week, here in the Ether Dome. The discussion was recorded on a reel-to-reel tape recorder, which was considered state-of-the-art at the time, and then transcribed by Betty. Her job was to make a readable discussion from the sometimes meandering and often inaudible recording. At the age of 16, I attended some of these CPCs. I particularly remember a case of hemochromatosis in a woman who had been taking ferrous sulfate daily for over 40 years.46 That was the start of my medical education. I subscribed to the New England Journal of Medicine through college, graduate school, and medical school, and I still subscribe today.
In the late 1960s, it was decided that a practicing internist should summarize the patients’ charts and prepare the case presentations — a job previously done by chief residents in pathology. Bill was hired for the job and continued to do it after Betty’s death in 1995, until 2003. Bill received a diagnosis of dementia, probably Alzheimer’s disease, in 2004, and he died in 2011. He donated his brain to the Massachusetts Alzheimer’s Disease Research Center with the hope of adding to our understanding of dementia.
We are proud of the dedication Bill and Betty gave to the CPCs and happy that we were able to play a role in making this CPC possible.
ANATOMICAL DIAGNOSIS
Frontotemporal lobar degeneration with tau-positive inclusions (Pick’s disease subtype) due to a Gly389Arg MAPT mutation, resulting in the behavioral variant of frontotemporal dementia with parkinsonism.
Acknowledgments
The pathological examination was supported by the Massachusetts Alzheimer’s Disease Research Center (National Institutes of Health grant P50 AG05134). Dr. Dickerson was supported by the Krupp Foundation and the National Institutes of Health (grant NS084156). This case was the first in the Betty U. and William F. McNeely Memorial Clinicopathological Conference series, supported by a gift from Dr. Sarah Weinberg and the Hon. John Weinberg.
We thank the patient’s wife for her support of our efforts to learn from her husband’s case.
Footnotes
Presented at Neurology Grand Rounds.
Dr. Miller reports receiving fees for board membership (paid to his institution) from the Larry L. Hillblom Foundation, the John Douglas French Alzheimer’s Foundation, and the Tau Consortium and consulting fees from TauRx, Allon Therapeutics, Bristol-Myers Squibb, and Eli Lilly. Dr. Dickerson reports receiving consulting fees from Merck, Forum, and the Med Learning Group and payment for development of educational presentations from the Med Learning Group. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rankin KP, Gorno-Tempini ML, Allison SC, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–56. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–25. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piguet O, Petersén A, Yin Ka Lam B, et al. Eating and hypothalamus changes in behavioral-variant frontotemporal dementia. Ann Neurol. 2011;69:312–9. doi: 10.1002/ana.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol. 2012;69:1578–82. doi: 10.1001/2013.jamaneurol.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tillema JM, Renaud DL. Leukoen-cephalopathies in adulthood. Semin Neurol. 2012;32:85–94. doi: 10.1055/s-0032-1306391. [DOI] [PubMed] [Google Scholar]
- 7.Miller BL. Frontotemporal dementia. Oxford, England: Oxford University Press; 2013. [Google Scholar]
- 8.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knopman DS, Petersen RC, Edland SD, Cha RH, Rocca WA. The incidence of frontotemporal lobar degeneration in Rochester, Minnesota, 1990 through 1994. Neurology. 2004;62:506–8. doi: 10.1212/01.wnl.0000106827.39764.7e. [DOI] [PubMed] [Google Scholar]
- 10.Seelaar H, Kamphorst W, Rosso SM, et al. Distinct genetic forms of frontotemporal dementia. Neurology. 2008;71:1220–6. doi: 10.1212/01.wnl.0000319702.37497.72. [DOI] [PubMed] [Google Scholar]
- 11.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 12.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 13.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–31. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–8. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 16.Urwin H, Josephs KA, Rohrer JD, et al. FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta Neuropathol. 2010;120:33–41. doi: 10.1007/s00401-010-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klassen BT, Ahlskog JE. Normal pressure hydrocephalus: how often does the diagnosis hold water? Neurology. 2011;77:1119–25. doi: 10.1212/WNL.0b013e31822f02f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong M, Shah GV, Adams KM, Turner RS, Foster NL. Spontaneous intracranial hypotension causing reversible frontotemporal dementia. Neurology. 2002;58:1285–7. doi: 10.1212/wnl.58.8.1285. [DOI] [PubMed] [Google Scholar]
- 19.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–35. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman JS, Rademakers R, Huey ED, et al. An algorithm for genetic testing of frontotemporal lobar degeneration. Neurology. 2011;76:475–83. doi: 10.1212/WNL.0b013e31820a0d13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loy CT, Schofield PR, Turner AM, Kwok JB. Genetics of dementia. Lancet. 2014;383:828–40. doi: 10.1016/S0140-6736(13)60630-3. [DOI] [PubMed] [Google Scholar]
- 22.Murrell JR, Spillantini MG, Zolo P, et al. Tau gene mutation G389R causes a tauopathy with abundant pick body-like inclusions and axonal deposits. J Neuropathol Exp Neurol. 1999;58:1207–26. doi: 10.1097/00005072-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Bird TD. Sporadic cases of possible genetic diseases: to test or not to test? Arch Neurol. 2000;57:309–10. doi: 10.1001/archneur.57.3.309. [DOI] [PubMed] [Google Scholar]
- 24.Case Records of the Massachusetts General Hospital (Case 6-1992) N Engl J Med. 1992;326:397–405. doi: 10.1056/NEJM199202063260608. [DOI] [PubMed] [Google Scholar]
- 25.Dickerson BC. Diagnostic tests for Alzheimer disease: judicious use can be helpful in clinical practice. Neurol Clin Pract. 2012;2:154–7. doi: 10.1212/CPJ.0b013e31825a77ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chien DT, Bahri S, Szardenings AK, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34:457–68. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- 27.Wylie MA, Shnall A, Onyike CU, Huey ED. Management of frontotemporal dementia in mental health and multidisciplinary settings. Int Rev Psychiatry. 2013;25:230–6. doi: 10.3109/09540261.2013.776949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardarelli R, Kertesz A, Knebl JA. Frontotemporal dementia: a review for primary care physicians. Am Fam Physician. 2010;82:1372–7. [PubMed] [Google Scholar]
- 29.Jicha GA, Nelson PT. Management of frontotemporal dementia: targeting symptom management in such a heterogeneous disease requires a wide range of therapeutic options. Neurodegener Dis Manag. 2011;1:141–56. doi: 10.2217/nmt.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10:162–72. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- 31.Manoochehri M, Huey ED. Diagnosis and management of behavioral issues in frontotemporal dementia. Curr Neurol Neurosci Rep. 2012;12:528–36. doi: 10.1007/s11910-012-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308:2020–9. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shnall A, Agate A, Grinberg A, Huijbregts M, Nguyen MQ, Chow TW. Development of supportive services for frontotemporal dementias through community engagement. Int Rev Psychiatry. 2013;25:246–52. doi: 10.3109/09540261.2013.767780. [DOI] [PubMed] [Google Scholar]
- 34.Pick A. Uber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prag Med Wochenschr. 1892;17:165–7. [Google Scholar]
- 35.Munoz DG, Morris HR, Rosser M. Pick’s disease. In: Dickson DW, Weller RO, editors. Neurodegeneration: the molecular pathology of dementia and movement disorders. Hoboken, NJ: Wiley-Blackwell; 2011. [Google Scholar]
- 36.Goedert M, Satumtira S, Jakes R, et al. Reduced binding of protein phosphatase 2A to tau protein with frontotemporal dementia and parkinsonism linked to chromosome 17 mutations. J Neurochem. 2000;75:2155–62. doi: 10.1046/j.1471-4159.2000.0752155.x. [DOI] [PubMed] [Google Scholar]
- 37.Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- 38.Probst A, Tolnay M, Langui D, Goedert M, Spillantini MG. Pick’s disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropathol. 1996;92:588–96. doi: 10.1007/s004010050565. [DOI] [PubMed] [Google Scholar]
- 39.Feany MB, Mattiace LA, Dickson DW. Neuropathologic overlap of progressive supranuclear palsy, Pick’s disease and corticobasal degeneration. J Neuropathol Exp Neurol. 1996;55:53–67. doi: 10.1097/00005072-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghetti B, Murrell JR, Zolo P, Spillantini MG, Goedert M. Progress in hereditary tauopathies: a mutation in the Tau gene (G389R) causes a Pick disease-like syndrome. Ann N Y Acad Sci. 2000;920:52–62. doi: 10.1111/j.1749-6632.2000.tb06905.x. [DOI] [PubMed] [Google Scholar]
- 44.Pickering-Brown S, Baker M, Yen SH, et al. Pick’s disease is associated with mutations in the tau gene. Ann Neurol. 2000;48:859–67. [PubMed] [Google Scholar]
- 45.Bermingham N, Cowie TF, Paine M, Storey E, McLean C. Frontotemporal dementia and Parkinsonism linked to chromosome 17 in a young Australian patient with the G389R Tau mutation. Neuropathol Appl Neurobiol. 2008;34:366–70. doi: 10.1111/j.1365-2990.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 46.Case Records of the Massachusetts General Hospital (Case 44131) N Engl J Med. 1958;258:652–61. doi: 10.1056/NEJM195803272581308. [DOI] [PubMed] [Google Scholar]