Abstract
Background
Although adding plasma exchange (PLEX) to steroids in severe neuromyelitis optica (NMO) attacks is common practice in steroid-resistant cases, the benefit of this strategy has not been previously quantified.
Objective
The objective of this paper is to compare the efficacy of high-dose intravenous methylprednisolone (IVMP) versus IVMP+PLEX in treatment of acute NMO relapses.
Methods
We conducted a retrospective review of the last 83 NMO admissions to the Johns Hopkins Hospital treated with IVMP alone versus IVMP+PLEX (for steroid-resistant cases). Extended Disability Status Scale (EDSS) score was calculated at baseline, at presentation, at discharge, and on follow-up.
Results
Eighteen NMO relapses (16 patients, 87% female, mean age at relapse: 33.9±23.8, median baseline EDSS 2.5) were treated with IVMP alone and 65 relapses (43 patients, 95% female, mean age at relapse: 43.8±15.7, median baseline EDSS 5.75) were treated with IVMP + PLEX. Sixty-five percent of IVMP + PLEX patients achieved an EDSS equal or below their baseline at follow-up while only 35% of the IVMP-only patients achieved their baseline EDSS on follow-up (odds ratio=3.36, 95% CI 1.0657 to 10.6004, p = 0.0386). PLEX was more effective in improving EDSS in patients on preventive immunosuppressive medications at time of relapse.
Conclusions
PLEX+IVMP are more likely to improve EDSS after NMO relapses compared to IVMP alone, especially in patients taking preventive medications.
Keywords: Neuromyelitis optica, plasma exchange, acute relapse, steroids, preventive medications, EDSS
Background
Neuromyelitis optica (NMO) is a severe autoimmune inflammatory disease with a special predilection to the spinal cord and optic nerves, and strong association with antibodies to aquaporin 4 (AQP4) water channels on astrocytes.1 In contrast to multiple sclerosis (MS), NMO relapses tend to be more severe and less responsive to high-dose-steroid therapy.2,3 Significant motor and visual disability often results from the cumulative effect of repeated relapses rather than ongoing neuro-degeneration as seen in MS.4 Effective relapse treatment followed by relapse prevention is the key for disability reduction in NMO.5
Although the use of plasma exchange (PLEX) is a common practice when steroid therapy is insufficient,6 the degree of benefit of adding PLEX to steroid therapy for treatment of acute relapses of NMO has not been quantified in an adequate sample size. Three previous studies have reported on the efficacy of PLEX in acute NMO relapses,7–9 and two studies compared PLEX to steroid-only treatment in either transverse myelitis10 or optic neuritis11 associated with NMO. In this study we compared the clinical efficacy of high-dose intravenous methylprednisolone (IVMP) versus high-dose IVMP + PLEX in the treatment of acute NMO relapses in a large cohort of anti-AQP4 seropositive and seronegative NMO patients, including a mixture of spinal cord, optic nerve, and brain relapses with varying degrees of severity.
Methods
We retrospectively analyzed the last 83 admissions to the Johns Hopkins Hospital from 2005 to 2013 in which patients with NMO were treated for an acute relapse of transverse myelitis and/or optic neuritis. The criteria for NMO required history of both acute optic neuritis and acute transverse myelitis with two of three supporting criteria among a longitudinally extensive spinal cord lesion, normal brain magnetic resonance imaging (MRI) and anti-AQP4 seropositivity (by enzyme-linked immunosorbent assay (ELISA) at Quest/LabCorp/Mayo or cell-based assay at Mayo Labs).1 The diagnosis of NMO spectrum disorder (NMOSD) was applied to patients who exhibited limited forms of disease with either transverse myelitis, optic neuritis or a brainstem lesion plus anti-AQP4 seropositivity.12–14 A relapse was defined as an immune-mediated attack of the central nervous system (CNS) manifesting as new or worsening symptoms attributable to a new T2 or contrast-enhancing lesion by MRI. We collected demographic data and disease characteristics for all patients. Extended Disability Status Scale (EDSS) score was calculated retrospectively from available records for the following time periods: prior to admission (baseline), at presentation, at discharge, and on follow-up (when available).
Relapses were classified according to the type of acute treatment provided: either IVMP only or IVMP + PLEX. All relapses were treated with high-dose IVMP at a dose of 1000 mg daily for five days starting on day 1 of admission. Patients were considered steroid responders if they showed improvement in strength or sensation of the affected limb, improvement in visual acuity or improvement in bowel/bladder function. Those who did not respond were considered candidates for PLEX if there were no contraindications such as active infection or coagulopathy. In the IVMP + PLEX group, all patients received five to seven sessions of PLEX in the second week of presentation following a five-day trial of IVMP. One to 1.5 volumes of plasma were exchanged at each session. Although we occasionally treat acute relapses with two rounds of IVMP or other immunosuppressive therapies, these cases were not included in this study with the purpose of having a more uniform group for comparison.
The primary assessment was to compare the number of patients whose neurological function returned to baseline EDSS on discharge and on follow-up. The secondary assessment was to examine the effect of being on preventive medications at the time of PLEX treatment on the change in EDSS at follow-up. For the primary analysis, we calculated odds ratio and confidence intervals (CIs). Fischer’s exact test was used to test statistical significance. For the secondary analysis, Mann-Whitney U test was used to compare outcomes between PLEX patients on preventive medications and those who were not on preventive medications at the time of relapse.
This study was approved by the Johns Hopkins Institutional Review Board.
Results
We studied 83 NMO relapses from 59 patients, of whom 55 (93.2%) were female, 36 patients (61%) were African American, 16 (27%) were Caucasian, and six (10%) were Latin American. The mean age at relapse was 41.6 (±18.2) years. Based on the 2006 NMO diagnostic criteria,1 33 patients (55.9%) had a diagnosis of seropositive NMO, eight (13.5%) patients had seronegative NMO, and 18 (30.5%) patients had seropositive NMOSD. In total there were 51 seropositive patients in the studied individuals, representing 86.4% of the group. Forty-five relapses involved the spinal cord, 30 involved the optic nerves, and 12 relapses occurred in the brain or brainstem. Eleven relapses involved more than one of the three locations.
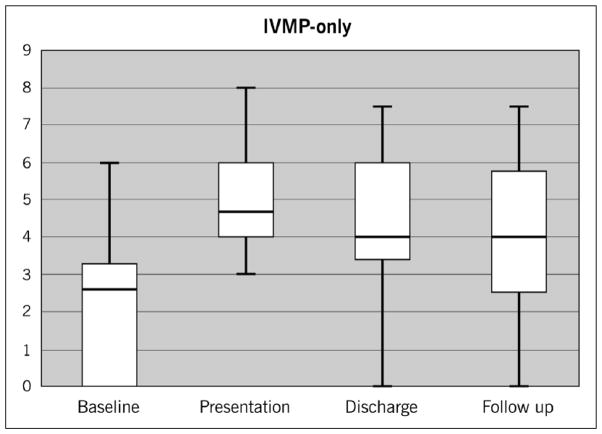
Eighteen NMO relapses were treated with IVMP alone and 65 relapses were treated with IVMP + PLEX. The demographics of each group are listed in Table 1. In the IVMP-only group, the median baseline EDSS was 2.5, which increased to 4.5 at presentation. The degree of improvement at discharge dropped to a median of 4, reflecting a clinical response to steroids. The median EDSS remained at 4 on follow-up one year later (Figure 1).
Table 1.
Patient characteristics.
| Characteristics | IVMP alone | IVMP + PLEX |
|---|---|---|
| Number of relapses | 18 | 65 |
| Number of patients | 16 | 43 |
| Age at relapse, mean (years) | 33.9 | 43.8 |
| Female | 14 | 41 |
| Duration of disease, median (years) | 2 | 6 |
| Race | ||
| African American | 12 | 24 |
| Caucasian | 3 | 3 |
| Latino-American | 1 | 5 |
| Diagnosis | ||
| NMO, AQP4 seropositive | 6 | 27 |
| NMO, AQP4 seronegative | 5 | 3 |
| NMO spectrum disorder | 5 | 13 |
| Lesion location | ||
| Spinal cord | 9 | 33 |
| Cervical | 1 | 13 |
| Thoracic | 3 | 8 |
| Cervical+thoracic | 5 | 7 |
| Unspecified | 0 | 5 |
| Optic nerve | 8 | 22 |
| Unilateral | 7 | 16 |
| Bilateral | 1 | 6 |
| Corticalmedullary junction | 0 | 3 |
| Brain | 1 | 5 |
| Brainstem/cerebellar peduncles | 1 | 5 |
IVMP: intravenous methylprednisolone; PLEX: plasma exchange; NMO: neuromyelitis optica; AQP4: aquaporin 4.
Figure 1.
Median EDSS changes in the IVMP-only group: Baseline EDSS of 2.5 rose to 4.5 on presentation and declined to 4 on discharge and remained at 4 at follow-up.
EDSS: Expanded Disability Status Scale; IVMP: intravenous methylprednisolone.
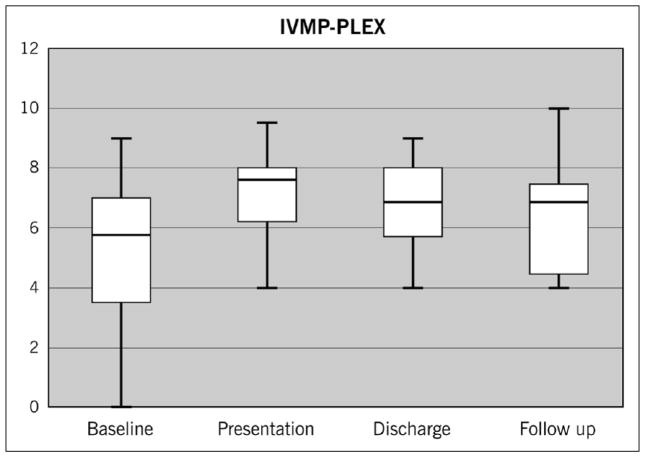
In the IVMP + PLEX group, the median age was 43.8 with a strong trend toward older age (p = 0.059) that is not due to duration of disease (mean of five years in the IVMP + PLEX group versus four years in the IVMP-alone group, p = 0.38). The IVMP + PLEX group was also significantly more disabled at baseline prior to relapse with a median baseline EDSS of 5.75 compared to 2.5 for the IVMP-alone group (p < 0.0001). Similar to the IVMP-alone group, the median EDSS increased two points on presentation of the IVMP+PLEX group to 7.75, which decreased to a median of 6.5 at discharge after PLEX and remained at a median of 6.5 at follow-up one year later (Figure 2). Overall, the median EDSS improved from presentation by 1.25 points in the IVMP + PLEX group compared to a 0.5-point improvement in the IVMP-only group.
Figure 2.
Median EDSS changes in the IVMP + PLEX group: Baseline EDSS of 5.75 rose to 7.75 on presentation and declined to 6.5 on discharge and remained at 6.5 at follow-up.
EDSS: Expanded Disability Status Scale; IVMP: intravenous methylprednisolone; PLEX: plasma exchange.
Fifty-one percent (51%) of the IVMP + PLEX group improved to an EDSS that was equal to or below their baseline EDSS at the time of discharge while only 16.6% of the IVMP-only group improved to their baseline EDSS at the time of discharge (odds ratio = 5.1667, 95% CI: 1.3564 to 19.6803, p = 0.0161) (Table 2). Sixty-five percent (65%) of the IVMP + PLEX group improved to an EDSS that was equal to or below their baseline EDSS at approximately one year follow-up (range six to 18 months) while only 35% of the IVMP-only group improved to their baseline EDSS at follow up (odds ratio= 3.36, 95% CI 1.0657 to 10.6004, p = 0.0386) (Table 2). Twenty-nine (29) relapses in the IVMP + PLEX group showed no improvement by EDSS although mild benefits not captured by EDSS were reported in 26 (89.6%) of those cases. There were 22 relapses within the IVMP + PLEX group that involved one or both optic nerves. Significant improvement in vision was reported in 12 (54.5%) of those cases although the changes were not high enough to be reflected in the EDSS score.
Table 2.
Number of relapses improving to baseline EDSS at discharge and follow-up.
| Treatment group | At or below baseline EDSS at discharge | At or below baseline EDSS within one year follow-up |
|---|---|---|
| IVMP only | 3 (16.6%) | 6 (35%) |
| IVMP+ PLEX | 31 (51%) | 33 (65%) |
There were 14 relapses (11 patients) in the IVMP + PLEX group that occurred within less than a year from the previous relapse in the same patient (median three months). In these cases, any available follow-up visit between the two consecutive relapses was used for follow-up analysis. Relapses without available outpatient follow-up prior to the subsequent relapse in the same patient were excluded from the follow-up endpoint analysis. Out of the 65 relapses in the IVMP + PLEX group, 50 relapses had available follow-up data at approximately one year (range six to 18 months). There was only one relapse in the IVMP-only group that occurred within less than a year from the previous relapse in the same patient and was handled in a manner similar to the IVMP + PLEX group.
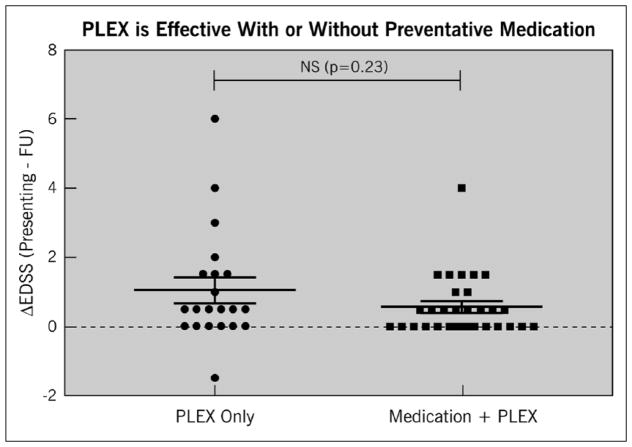
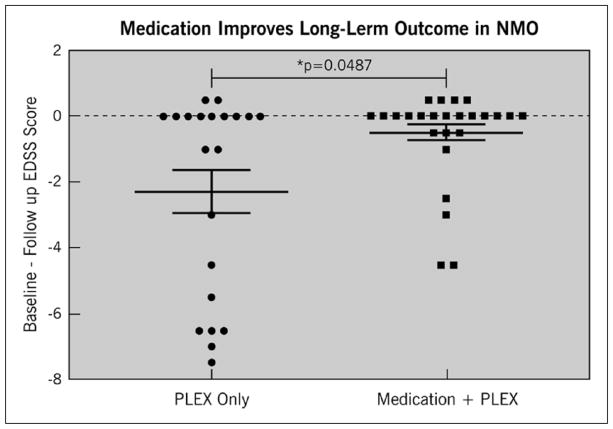
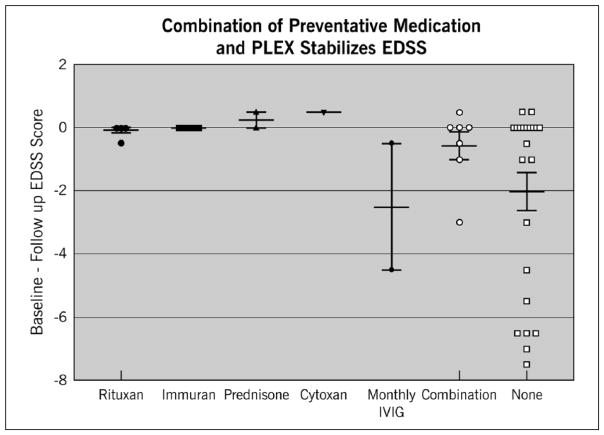
Among patients in the IVMP+PLEX group, PLEX significantly reduced disability from presentation to discharge regardless of whether patients were on preventive medication at the time of relapse (Figure 3). However, patients on preventive medications (mycophenolate, rituximab, azathioprine, cyclophosphamide, or prednisone) at the time of relapse were more likely to recover to an EDSS near their baseline within one year of follow-up compared to patients who were not on preventive medications at the time of relapse onset (p = 0.0487) (Figure 4). This held true regardless of the type of preventive medication used with the exception of monthly intravenous immunoglobulin (IVIG) (Figure 5). Effectiveness of PLEX did not correlate with patients’ age, sex, location of the lesion (cord versus optic nerve versus brain/brainstem), length of cord lesion, or duration of illness.
Figure 3.
Effect of IVMP + PLEX or IVMP + PLEX plus preventive medications on EDSS after relapse compared to presenting EDSS at the time of relapse. The y-axis represents the difference between presenting EDSS and follow-up EDSS within one year. A value higher than zero indicates improvement of follow-up EDSS compared to presenting EDSS. A value of 0 or lower indicates no improvement or worsening EDSS score on follow-up compared to presenting EDSS.
EDSS: Expanded Disability Status Scale; IVMP: intravenous methylprednisolone; PLEX: plasma exchange.
Figure 4.
Effect of IVMP+PLEX or IVMP + PLEX plus preventive medications on EDSS after relapse compared to baseline. The y-axis represents the difference between baseline EDSS (before relapse) and follow-up EDSS within one year of relapse. A value of 0 or higher indicates improvement of EDSS to baseline or better. A value below 0 indicates worse EDSS score on follow-up compared to baseline.
EDSS: Expanded Disability Status Scale; IVMP: intravenous methylprednisolone; PLEX: plasma exchange.
Figure 5.
Effect of individual preventive immunosuppressive medications with IVMP + PLEX on EDSS after relapse compared to baseline. The y-axis represents the difference between baseline EDSS (before relapse) and follow-up EDSS within one year of relapse. A value of 0 or higher indicates improvement of EDSS to baseline or better. A value below 0 indicates worse EDSS score on follow-up compared to baseline.
EDSS: Expanded Disability Status Scale; IVMP: intravenous methylprednisolone; PLEX: plasma exchange.
Discussion
This study of a large cohort of NMO/NMOSD patients with acute relapses of transverse myelitis and/or optic neuritis confirms that the combination of PLEX and high-dose steroid therapy is superior to high-dose steroid therapy alone for recovery of baseline neurological function. This conclusion was based on a majority of anti-AQP4 seropositive cases and a combination of spinal, optic, and brain/brainstem attacks of varying degrees of severity. This study also demonstrated the benefit of being on preventive immunosuppressive medications at the time of relapse on the long-term response to PLEX.
Following a seminal prospective PLEX study in acute demyelinating diseases that first demonstrated the benefit of PLEX in a small number of NMO patients,15 all future studies have been retrospective.16,17 In 2009, Bonnan and colleagues reported their experience with 29 relapses treated with PLEX + IVMP for acute longitudinally extensive spinal attacks either isolated or as part of NMO versus 67 relapses treated with IVMP alone.10 PLEX + IVMP was significantly more effective in lowering EDSS on discharge and at follow-up but only in relapses with a baseline EDSS of 0. Their cohort of 43 patients had a majority of seronegative cases as well as patients who did not meet criteria for NMO including idiopathic longitudinally extensive transverse myelitis. Only six patients (26%) of their cohort were seropositive; on the contrary, our study included a majority of seropositive patients (86.4%) with a more reliable diagnosis of NMO/NMOSD. In addition to involving all types of relapses (spinal, optic, and brain/brainstem), our cohort also included patients with more severe disease and higher baseline median EDSS. Unlike the results of Bonnan et al., our results showed that the combination of PLEX + IVMP is also effective in patients with high baseline EDSS.
In 2012, Merle and colleagues11 compared the combination of PLEX + IVMP in 16 patients to IVMP alone in 36 patients with optic neuritis relapses in NMO. They showed that the combination PLEX+IVMP was significantly more effective in improving visual acuity at six months’ follow-up. Although their cohort contained a minority of seropositive patients (36%), their results were similar to ours in that significant visual improvement as documented by clinicians was reported in more than 50% of patients treated with PLEX + IVMP for optic neuritis relapses.
To our knowledge, the effect of preventive medications on the response to acute treatment in NMO has not been previously studied. We have shown for the first time that patients on preventive immunosuppressive medications at the time of relapse have a better chance of recovering to baseline than those who are not on immunosuppressive therapy at onset of relapse. This beneficial effect was observed for the four most commonly used immunosuppressive medications in NMO including rituximab, mycophenolate, azathioprine, and prednisone. This may indicate that in addition to the role of continuous immunosuppressive therapy in preventing NMO relapses,18–20 these medications may play a role in ameliorating relapse severity or promoting recovery from acute relapses.
There are several limitations to our study. First, this is a retrospective review based on extraction of data from medical records. A randomized, blinded study is the ideal way of comparing two different therapeutic modalities in any given disease. Secondly, selection bias to a tertiary NMO referral center led to higher numbers in the IVMP + PLEX group compared to the IVMP-alone group. However, we do believe that the selection bias in this particular situation worked in our favor. Patients in the IVMP + PLEX group had generally more severe disease than those in the IVMP-only group (baseline EDSS of 5.75 versus 2.5), yet they had a greater chance of improvement to baseline. Lastly, we used retrospectively applied EDSS scores as the primary outcome measures but the EDSS is limited in sensitivity to both visual acuity changes and improvements in neurological function among patients who cannot walk. In addition, investigators were not blinded to the type of acute treatment used in each patient when they calculated the EDSS values, which might have resulted in some degree of investigator bias.
Conclusion
High-dose IVMP provides only a moderate degree of neurologic recovery from acute NMO relapses. Adding plasma exchange to high-dose IVMP improves the outcome at discharge and on follow-up in cases where IVMP alone is insufficient. PLEX is effective in improving neurological function back to baseline among the majority of patients who were steroid non-responsive and is even more effective in patients on preventive immunosuppressive medications at the time of relapse. The benefit of PLEX in NMO relapses was seen across a wide spectrum of severity, including those with severe disease at baseline regardless of age, sex, lesion location (spinal, optic, brain/brainstem), lesion size, and duration of illness.
Acknowledgments
The authors’ contributions are as follows: HA: drafting/revising the manuscript, study concept or design, acquisition of data, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. AP: analysis or interpretation of data, creation of the graphs, revising the manuscript. MM: revising the manuscript, study concept or design, analysis or interpretation of data. SS: revising the manuscript, study concept or design, analysis or interpretation of data. LS: revising the manuscript, study concept or design, analysis or interpretation of data. ML: drafting/revising the manuscript, study concept or design, acquisition of data, analysis or interpretation of data, accepts responsibility for conduct of research and final approval.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Conflicts of interest
Hesham Abboud has received an educational grant from Teva Pharmaceuticals unrelated to this study. Dr Levy receives research support from the National Institutes of Health (NIH), Guthy Jackson Charitable Foundation, Viropharma, Acorda, Sanofi, NeuralStem and Genentech, and serves as a consultant for Chugai Pharmaceuticals, GlaxoSmithKline and MedImmune. The other authors have nothing to declare.
Contributor Information
Hesham Abboud, Department of Neurology, Cleveland Clinic, USA/Department of Neurology, University of Alexandria, Egypt.
Alex Petrak, Department of Neuroscience, Ohio State University, USA.
Maureen Mealy, Department of Neurology, Johns Hopkins University, USA.
Sarana Sasidharan, Department of Neurology, Johns Hopkins University, USA.
Laila Siddique, Department of Neurology, Johns Hopkins University, USA.
Michael Levy, Department of Neurology, Johns Hopkins University, USA.
References
- 1.Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Hogancamp WF, O’Brien PC, et al. The clinical course of neuromyelitis optica (Devic’s syndrome) Neurology. 1999;53:1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 3.Bichuetti DB, Oliveira EM, Souza NA, et al. Patients with neuromyelitis optica have a more severe disease than patients with relapsing remitting multiple sclerosis, including higher risk of dying of a demyelinating disease. Arq Neuropsiquiatr. 2013;71:275–279. doi: 10.1590/0004-282x20130020. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Pittock SJ, Lucchinetti CF, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68:603–605. doi: 10.1212/01.wnl.0000254502.87233.9a. [DOI] [PubMed] [Google Scholar]
- 5.Jacob A, McKeon A, Nakashima I, et al. Current concept of neuromyelitis optica (NMO) and NMO spectrum disorders. J Neurol Neurosurg Psychiatr. 2012;84:922–930. doi: 10.1136/jnnp-2012-302310. [DOI] [PubMed] [Google Scholar]
- 6.Bonnan M, Cabre P. Plasma exchange in severe attacks of neuromyelitis optica. Mult Scler Int. 2012;2012:787–630. doi: 10.1155/2012/787630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SH, Kim W, Huh SY, et al. Clinical efficacy of plasmapheresis in patients with neuromyelitis optica spectrum disorder and effects on circulating anti-aquaporin-4 antibody levels. J Clin Neurol. 2013;9:36–42. doi: 10.3988/jcn.2013.9.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan SM, Zantek ND, Carpenter AF. Therapeutic plasma exchange in neuromyelitis optica: A case series. J Clin Apher. 2014;29:171–177. doi: 10.1002/jca.21304. [DOI] [PubMed] [Google Scholar]
- 9.Lim YM, Pyun SY, Kang BH, et al. Factors associated with the effectiveness of plasma exchange for the treatment of NMO-IgG-positive neuromyelitis optica spectrum disorders. Mult Scler. 2013;19:1216–1218. doi: 10.1177/1352458512471875. [DOI] [PubMed] [Google Scholar]
- 10.Bonnan M, Valentino R, Olindo S, et al. Plasma exchange in severe spinal attacks associated with neuromyelitis optica spectrum disorder. Mult Scler. 2009;15:487–492. doi: 10.1177/1352458508100837. [DOI] [PubMed] [Google Scholar]
- 11.Merle H, Olindo S, Jeannin S, et al. Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch Ophthalmol. 2012;130:858–862. doi: 10.1001/archophthalmol.2012.1126. [DOI] [PubMed] [Google Scholar]
- 12.Weinshenker BG, Wingerchuk DM, Vukusic S, et al. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol. 2006;59:566–569. doi: 10.1002/ana.20770. [DOI] [PubMed] [Google Scholar]
- 13.Jarius S, Frederikson J, Waters P, et al. Frequency and prognostic impact of antibodies to aquaporin-4 in patients with optic neuritis. J Neurol Sci. 2010;298:158–162. doi: 10.1016/j.jns.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Popescu BFG, Lennon VA, Parisi JE, et al. Neuromyelitis optica unique area postrema lesions: Nausea, vomiting, and pathogenic implications. Neurology. 2011;76:1229–1237. doi: 10.1212/WNL.0b013e318214332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinshenker BG, OBrien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46:878–886. doi: 10.1002/1531-8249(199912)46:6<878::aid-ana10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe S, Nakashima I, Misu T, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128–132. doi: 10.1177/1352458506071174. [DOI] [PubMed] [Google Scholar]
- 17.Wang KC, Wang SJ, Lee CL, et al. The rescue effect of plasma exchange for neuromyelitis optica. J Clin Neurosci. 2011;18:43–46. doi: 10.1016/j.jocn.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Elsone L, Kitley J, Luppe S, et al. Long-term efficacy, tolerability and retention rate of azathioprine in 103 aquaporin-4 antibody- positive neuromyelitis optica spectrum disorder patients: A multicentre retrospective observational study from the UK. Mult Scler. 2014;20:1533–1540. doi: 10.1177/1352458514525870. [DOI] [PubMed] [Google Scholar]
- 19.Mealy MA, Wingerchuk DM, Palace J, et al. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: Multicenter study of treatment efficacy. JAMA Neurol. 2014;71:324–330. doi: 10.1001/jamaneurol.2013.5699. [DOI] [PubMed] [Google Scholar]
- 20.Kimbrough DJ, Fujihara K, Jacob A, et al. Treatment of neuromyelitis optica: Review and recommendations. Mult Scler Relat Disord. 2012;1:180–187. doi: 10.1016/j.msard.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]