Abstract
The lifetime prevalence rate for major depressive disorder (MDD) is approximately 17 % for most developed countries around the world. Dietary polyphenols are currently used as an adjuvant therapy to accelerate the therapeutic efficacy on depression. Ferulic acid (FA) or 4-hydroxy-3-methoxy-cinnamic acid (Fig. 1a) is a main polyphenolic component of Chinese herb Radix Angelicae Sinensis, which is found to have antidepressant-like effects through regulating serotonergic and noradrenergic function. The present study examined the synergistic effect of low doses of FA combined with subthreshold dose of piperine, a bioavailability enhancer, on depression-like behaviors in mice, and investigated the possible mechanism. The administration of FA, even in the highest dose tested, reduced immobility time by 60 % in the tail suspension and forced swimming tests (TST and FST) in mice when compared to control. The maximal antidepressant-like effect of FA was obtained with 200 mg/kg. In addition, piperine only produced a weak antidepressant-like effect in the TST and FST. However, the evidence from the interaction analysis suggested a synergistic effect when low doses of FA were combined with a subthreshold dose of piperine. Further neurochemical evidence such as monoamine levels in the frontal cortex, hippocampus, and hypothalamus and measurements of monoamine oxidase activity also supported a synergistic effect of FA and piperine in the enhancement of monoaminergic function. This finding supports the concept that the combination strategy might be an alternative therapy in the treatment of psychiatric disorders with high efficacy and low side effects.
Keywords: Ferulic acid, Piperine, Depression, Monoamines, MAO
Introduction
The lifetime prevalence rate for major depressive disorder (MDD) is approximately 17 % for most developed countries around the world and it causes profound personal suffering and economic loss (Ota et al. 2014). Antidepressant drugs with reasonable efficacy are the most common treatment for depressive disorders, which are among the most prescribed medications in North America (Lopez et al. 2014). However, only half of patients will show some improvement with currently available first-line treatment options, and only one-third will achieve full remission. The low remission rates combined with the weeks-long lag of symptom improvement make it urgent to discover the novel agents for treatment of depression.
Among various herbal medicines, polyphenols have attracted many researchers’ attention because of their anti-oxidant, immunomodulatory and antidepressant-like properties (Awad and El-Sharif 2011; Kwon et al. 2010). Ferulic acid (FA) or 4-hydroxy-3-methoxy-cinnamic acid (Fig. 1a) is a main polyphenolic component of Chinese herb Radix Angelicae Sinensis. It has been widely used as an anti-inflammatory and free radical-scavenging agent (Ogiwara et al. 2002; Wang and Ou-Yang 2005). Recent study suggested that acute treatment with the extract of Chaihu-Sugan-San (containing 19 % ferulic acid) significantly decreased the immobility time in mouse model of despair test, indicating a possible antidepressant-like effect of the extract (Zhang et al. 2011). However, our pilot studies suggested that the efficacy of FA did not increase a lot even if the dosages were increased from 3 mg/kg to 90 mg/kg. To overcome this problem piperine, a major alkaloid of black pepper (Piper nigrum Linn.) and long pepper (P.longum Linn.) has been employed as a combination therapy in the given study since piperine is known to increase the bioavailability of many polyphenols, such as curcumin and trans-resveratrol (Huang et al. 2013; Rinwa et al. 2013). Thus, the current study was focused on the possible synergistic effects of FA and piperine on depression-like behaviors and the underlying mechanisms involving monoaminergic system. Two mouse models of behavioral despair, tail suspension and forced swimming tests (TST and FST) were used for screening the antidepressant-like effects of mice after treatment with FA alone and combined with peperine.
Fig. 1.
Structures of Ferulic Acid (a) and piperine (b)
Materials and methods
Animals
Male ICR mice (20–22 g) were obtained from the Animal Center of Shanghai Branch, Chinese Academy of Sciences. On arrival, the animals were housed 5 per cage and acclimatized to a colony room with controlled ambient temperature (22 ± 1 °C), humidity (50 ± 10 %) and a 12 h natural light/dark cycle. They were fed with standard diet and water ad libitum and were allowed to acclimate 5 days before they were used. The experiments were performed between 10:00 h and 14:00 h. All experiments were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985), and approved by the Wenzhou Medical College Committee on Animal Care and Use.
Drugs and drug treatment
FA, piperine, imipramine hydrochloride, 5-hydroxytryptamine (5-HT), noradrenaline (NE), dopamine (DA), 5-hydroxyindoleacetic acid (5-HIAA), 4-dihydroxy phenylacetic acid (DOPAC), kynuramine dihydrobromide, 4-hydroxyquinoline, clorgyline and selegiline were purchased from Sigma Chemical Co. (USA). FA and piperine were dissolved in redistilled water on the day of testing for oral administration (via gavage, p.o.) and intraperitoneal injection (i.p.), respectively. Various doses of FA (3, 10, 30 and 90 mg/kg, p.o.), piperine (10 mg/kg, i.p.) and imipramine (10 mg/kg, i.p.) were treated 30 min before testing.
Tail suspension test (TST)
The tail suspension test was based on the previous description (Steru et al. 1985) with minor modification (Xu et al. 2005). Each mouse was hung 50 cm above the floor by means of an adhesive tape, placed approximately 1 cm from the tip of the tail. The time during which mice remained immobile was quantified during a test period of 6 min. Mice were considered immobile only when it hung passively and completely motionless. The duration of immobility was recorded during the last 4 min of the 6-min testing period. The inhibition ratio was obtained based on the ratio of the immobility time after treatment with drugs and control groups, i.e. the average immobility time of control group-the average immobility time of drug treatment/the average immobility time of control group.
Forced swimming test (FST)
The forced swimming test was carried out on mice according to the method described previously (Bourin et al. 2004; Xu et al. 2005). Briefly, Twenty-four hours before the formal test, the mice were individually placed in glass cylinder (height: 25 cm, diameter: 10 cm) which contained 19 cm of water at 24 ± 1 °C for 15 min. Following the swimming session, mice were towel dried and returned to their home cage. For the test period, the mice were placed in the same system again for 6 min 24 h later. After the initial 2 min of vigorous activity, the animals showed period of immobility by ceasing struggling and remained floating motionless in the water, making only small movements necessary to keep head above the water. The duration that the mice remained immobile during the last 4 min of the 6 min testing period was recorded. The inhibition ratio was obtained based on the ratio of the immobility time after treatment with drugs and control groups, i.e. the average immobility time of control group-the average immobility time of drug treatment/the average immobility time of control group.
Locomotor activity
Locomotor activity was measured by an ambulometer with five activity chambers (JZZ98, Institute of MateriaMedica, Chinese Academy of Medical Sciences, China) with a minor modification (Xu et al. 2005). When the paws of mice contacted or disconnected the active bars will produce random configurations that were converted into pulses, which were proportional to the locomotor activity of the mice, and were recorded as the cumulative total counts of motor activity automatically. Mice were allowed a period of 5 min to acclimatize to the observation chamber. Locomotion counts were recorded for a period of 10 min for each mouse.
Determination of monoamines and metabolites
Mice were decapitated and the brain regions (cortex, hippocampus and hypothalamus) were rapidly removed and placed on a cold plate. The brain tissues were weighed and stored at −80 °C until homogenization. The protein concentration of brain tissue was calculated by the Bradford assay.
The contents of serotonin, noradrenaline, dopamine, 5-HIAA and DOPAC were measured as described previously by high-performance liquid chromatography (HPLC) (Xu et al. 2010). The frozen tissue samples were homogenized by ultrasonication in 200 μl of 0.4 M perchloric acid (solution A). The homogenate was kept on ice for 1 h and then centrifuged at 12,000 rpm (4 °C) for 20 min. An aliquot of 160 μl of supernatant was added to 80 μl of solution B including 0.2 M potassium citrate, 0.3 M dipotassium hydrogen phosphate and 0.2 M EDTA. The mixture was incubated on ice for 1 h and then centrifuged at 12,000 rpm (4 °C) for 20 min. 20 μl of the resultant supernatant was directly added to the HPLC system equipped with reversed-phase C18 column and an electro-chemical detector (ESA CoulArray, Chelmstord, MA, USA). The detector potential was set at 50, 100, 200, 300, 400, and 500 mV, respectively. The mobile phase consisted of 125 mM citric acid/sodium citrate, 0.1 mM EDTA, 1.2 mM sodium octanesulfonate and 16 % methanol. The flow rate is 1 ml/min. The tissue levels of monoamine were expressed in terms of nanograms per gram of tissue.
Measurements of monoamine oxidase (MAO) activity
Mouse brain monoamine oxidase activity was measured based on the method described previously (Chakrabarti et al. 1998; Xu et al. 2010) with minor modification. Briefly, the brain tissues including cortex, hippocampus and hypothalamus were homogenized with 4 ml of phosphate buffer (pH 7.4, 0.05 M). The activities of monoamine oxidase-A and -B in brain tissues were measured in the presence of either 1 μM deprenyl (type B inhibitor) or clorgyline (type A inhibitor). For lysis of the membranes, the tissue homogenate was treated with 0.4 ml of 20 % Triton X-100, 2.5 ml of phosphate buffer (pH 7.4) was then mixed with 0.2 ml of the tissue homogenate. The mixture was preincubated at 37 °C for 15 min. Then 30 μl of 2.19 mM kynuramine dihydrobromide was added to the reaction mixture (final concentration 22 μM) as substrate. After incubation at 37 °C for 30 min, the reaction was terminated by adding 0.2 ml of 5 M perchloric acid. Then cooling and centrifugation at 1500 Å ~ g for 10 min, an aliquot of 0.5 ml of the supernatant was added to 2.5 ml of 1 M NaOH. The fluorescence intensity was detected with excitation at 315 nm and emission at 380 nm using a fluorescence spectrometer. The concentration of 4-hydroxyquinoline was estimated from a corresponding standard fluorescence curve of 4-hydroxyquinoline. Monoamine oxidase activity was expressed as nmol of 4-hydroxyquinoline formed/30 min/mg protein. Protein concentrations were determined by the method of Bradford.
Statistical analysis
Results were expressed as the mean ± S.E.M., which were analyzed statistically using one-way analysis of variance (ANOVA) followed by a post hoc Dunnett's t-test. p < 0.05 was considered statistically significant. Logit method was used to calculate the median inhibitory dose (defined as the doses at which 50 % of mice achieved maximal anti-immobility response, ID50) values of FA alone, piperine alone, and FA combined with piperine. The isobologram was applied to evaluate the synergistic effect of the combination of FA and piperine (Huang et al. 2013)..
Results
The effects of FA alone and combined with piperine on the immobility time in tail suspension and forced swimming tests
The effects of FA alone and the combination of FA and piperine were evaluated by two mouse models of despair test, tail suspension and forced swimming tests. As shown in Table 1 and 2, FA at the doses of 10, 30, 90 and 180 mg/kg decreased the immobility time in a dose-dependent manner, reaching the maximal effect at the dose of 180 mg/kg both in the FST and TST. The ID50s were 111.1 and 97.7 mg/kg in these two tests. Meanwhile, piperine treated alone from 10 to 540 mg/kg showed the weak anti-immobility response; the immobility time only decreased by 43.6 % and 41.5 % when 270 mg/kg piperine was treated in the FSTand TST, respectively. The ID50s of piperine were 421.6 and 472.3 mg/kg in the FST and TST. However, mice treated with 1/40 of ID50 of piperine used alone, (subthreshold dose) combined with different doses of FA (3, 10, 30 and 90 mg/kg) suggested dose-dependent anti-immobility responses both in the FST and TST. The ID50s of the combination were 77.4 and 51.0 mg/kg, respectively. Similarly, when treatment with a fixed dose of FA (subthreshold dose, about 1/30 of ID50 when FA was used alone) with increasing doses of piperine from 10 to 270 mg/kg, the ID50s were reduced to 264.90 mg/kg and 282.59 mg/kg in the FST and TST, respectively.
Table 1.
The effects of Ferulic acid combined with piperine (10 mg/kg) on TST
| Group | Dose (mg/kg) | Immobility time (s) | Inhibition ratio (%) | ID50 (mg/kg) |
|---|---|---|---|---|
| Control 1 | 158.40 | |||
| 10 | 154.70 | 2.34 | ||
| 30 | 137.58 | 13.14 | ||
| 472.27 | ||||
| Piperine | 90 | 111.62 | 29.54 | |
| 270 | 92.60 | 41.54 | ||
| 540 | 74.44 | 53.01 | ||
| Control 2 | 153.18 | |||
| 3 | 136.19 | 11.09 | ||
| 10 | 121.81 | 20.48 | ||
| 97.71 | ||||
| FA | 30 | 102.91 | 32.82 | |
| 90 | 82.64 | 46.05 | ||
| 180 | 58.45 | 61.84 | ||
| Control 3 | 145.12 | |||
| 3 | 124.53 | 14.19 | ||
| Piperine (10 mg/kg) + FA | 10 | 105.71 | 27.16 | 51.02 |
| 30 | 74.22 | 48.85 | ||
| 90 | 66.40 | 51.28 | ||
| Control 4 | 155.94 | |||
| 10 | 150.21 | 3.67 | ||
| FA (3 mg/kg) + Piperine | 30 | 132.07 | 15.31 | 282.59 |
| 90 | 102.45 | 34.30 | ||
| 270 | 78.33 | 49.77 |
Table 2.
The effects of Ferulic acid combined with piperine (10 mg/kg) on FST
| Group | Dose (mg/kg) | Immobility time (s) | Inhibition ratio (%) | ID50 (mg/kg) |
|---|---|---|---|---|
| Control 1 | 158.75 | |||
| 10 | 154.66 | 2.57 | ||
| 30 | 134.28 | 15.41 | ||
| 421.57 | ||||
| Piperine | 90 | 112.81 | 28.94 | |
| 270 | 89.54 | 43.60 | ||
| 540 | 72.32 | 54.44 | ||
| Control 2 | 154.75 | |||
| 3 | 142.54 | 7.89 | ||
| 10 | 120.09 | 22.40 | ||
| 111.14 | ||||
| FA | 30 | 119.02 | 23.09 | |
| 90 | 88.47 | 42.83 | ||
| 180 | 54.38 | 64.86 | ||
| Control 3 | 166.32 | |||
| 3 | 138.83 | 16.53 | ||
| Piperine (10 mg/kg) + FA | 10 | 115.02 | 30.84 | 77.39 |
| 30 | 106.83 | 35.77 | ||
| 90 | 64.07 | 53.85 | ||
| Control 4 | 155.34 | |||
| 10 | 151.39 | 2.54 | ||
| FA (3 mg/kg) + Piperine | 30 | 131.64 | 15.26 | 264.90 |
| 90 | 106.28 | 31.58 | ||
| 270 | 76.47 | 50.77 |
The further analysis of the interaction between FA and piperine was done by constructing an isobologram. As shown in Fig. 2a and b, the ID50 values for FA and piperine treated alone, as well as the combination of both were blotted on the isobologram. The ID50 values of the combination of FA and piperine fell below the dotted “line of additivity” connecting the ID50s of they used alone, which suggested the nature of the combination was synergistic (the ID50 of the combination falling on that line is additive; while the antagonistic effect is shown by the value of the combination above the line connecting the individual drug).
Fig. 2.
An isobologram for the combination of Ferulic Acid with piperine which generates a synergistic effect. a Isobologram illustration for maximal anti-immobility response which achieved in the forced swim and b in the tail suspension tests. The dose at which 50 % of treated mice achieved a maximal anti-immobility response (ID50) for each drug is indicated on their respective axes. The two points below “the line of additivity” connecting the ID50s of the individual drug represent the ID50s of the combination, which are 10 mg/kg piperine and FA, and 3 mg/kg FA and piperine, respectively. The ID50s of the combination as shown do not cross the line of additivity (a position above the line indicates antagonism)
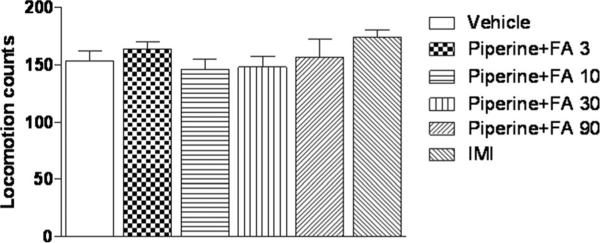
The effects of low doses of FA combined with subthreshold dose of piperine on locomotor activity
The changes of locomotor activity after treatment with suthreshold dose of piperine combined with low doses of FA were shown in Fig. 3. Piperine at 10 mg/kg combined with FA at doses of 3, 10, 30 and 90 mg/kg that included the doses inducing the significant decreases in immobility response in both the FST and TST, did not show any significant effect on the locomotor activity [F (5, 54) =12.10, P > 0.05]. Therefore, in the subsequent study, the subthreshold dose of piperine at 10 mg/kg in combination with FA at 3, 10, 30 and 90 mg/kg were used for investigating the underlying mechanism involving the antidepressant-like effects of the combination.
Fig. 3.
Locomotor activity in mice after subthreshold dose of piperine (10 mg/kg) combined with FA. The locomotor activity was recorded for 10 min. Values are expressed as mean ± S.E.M. with 10 mice in each group
The effects of low doses of FA combined with subthreshold dose of piperine on monoamines and the metabolites
The monoamines and their metabolites were investigated in different brain regions, such as the frontal cortex, hippocampus and hypothalamus, after treatment with the combination of subthreshold dose of piperine (10 mg/kg, i.p.) and increasing doses of FA (3, 10, 30 and 90 mg/kg, p.o.). In the frontal cortex, FA at doses of 10, 30 and 90 mg/kg in combination with piperine increased 5-HT [F (5, 54) =8.10, P's < 0.01; P < 0.001] and NE [F (5, 54) =5.10, P < 0.05] levels as shown in Table 3. Dopamine and its metabolites DOPAC were not showed significant changes following the combination treatment. Furthermore, the ratio of 5-HIAA and 5-HT was decreased after treatment with the combination of high dose of FA at 90 mg/kg and piperine [F (5, 54) =3.36, P < 0.05], which indicated that the turnover of 5-HT was decreased. Similarly, imipramine at 10 mg/kg (i.p.) was found to increase the 5-HT and NE levels in this brain region (P < 0.05; P < 0.001).
Table 3.
The effects of ferulic acid combined with subthreshold dose of piperine (10 mg/kg) on the concentrations of monoamines and their metabolites in the frontal cortex of mice
| Group | Dose (mg/kg) | 5-HT | 5-HIAA | 5-HIAA/5-HT | Noradrenaline | Dopamine | DOPAC |
|---|---|---|---|---|---|---|---|
| Control | 376.9 ± 25.3 | 189.2 ± 16.5 | 0.52 ± 0.06 | 163.0 ± 9.6 | 49.3 ± 4.4 | 47.7 ± 4.4 | |
| Piperine (10 mg/kg) + FA | 313 | 425.2 ± 16.7 | 231.0 ± 10.9 | 0.55 ± 0.03 | 174.8 ± 5.0 | 41.2 ± 7.9 | 48.1 ± 8.8 |
| 10 | 530.4 ± 27.8** | 239.2 ± 26.6 | 0.46 ± 0.06 | 211.5 ± 13.8* | 44.1 ± 4.6 | 45.3 ± 8.2 | |
| 30 | 555.5 ± 21.9** | 221.0 ± 15.8 | 0.40 ± 0.03 | 213.3 ± 11.1** | 49.2 ± 7.1 | 46.3 ± 8.1 | |
| 90 | 610.4 ± 23.1*** | 217.2 ± 15.4 | 0.36 ± 0.03* | 222.9 ± 10.2** | 49.4 ± 3.8 | 43.5 ± 7.1 | |
| Imipramine | 10 | 549.8 ± 47.8** | 212.3 ± 7.2 | 0.41 ± 0.04 | 205.8 ± 11.8* | 58.3 ± 10.7 | 53.7 ± 10.4 |
Values are expressed as mean ± S.E.M. with units of ng/g tissue for 10 mice in each group. Data analysis was performed using one-way ANOVA.
P <0.05
P < 0.01 and
P < 0.001, vs. the control group.
In the hippocampus, piperine combined with increasing doses of FA at 10, 30 and 90 mg/kg was showed to increase the 5-HT [F (5, 54) =6.97, P < 0.05; P's < 0.001] and NE levels significantly [F (5, 54) =14.37, P < 0.01; P < 0.001], with dose-dependent manner. The decreased ratio of 5-HIAA/5-HT was also significant after treatment with the combination of piperine and high doses of FA at 30 and 90 mg/kg [F (5, 54) =3.34, P < 0.01; P < 0.001]. Dopamine and its metabolites DOPAC were not showed significant changes following the combination treatment, although a tendency to increase in Dopamine and decrease in DOPAC was observed in this region. Similar increases in 5-HT and NE levels were found after treatment with the positive drug imipramine at 10 mg/kg (i.p.) (Table 4).
Table 4.
The effects of ferulic acid combined with subthreshold dose of piperine (10 mg/kg) on the concentrations of monoamines and their metabolites in the hippocampus of mice
| Group | Dose (mg/kg) | 5-HT | 5-HIAA | 5-HIAA/5-HT | Noradrenaline | Dopamine | DOPAC |
|---|---|---|---|---|---|---|---|
| Control | 363.6 ± 28.6 | 184.3 ± 8.7 | 0.54 ± 0.05 | 177.2 ± 5.5 | 40.3 ± 3.6 | 60.2 ± 7.2 | |
| Piperine (10 mg/kg) + FA | 3 | 433.2 ± 26.4 | 189.9 ± 13.2 | 0.45 ± 0.03 | 175.6 ± 7.8 | 43.1 ± 8.1 | 47.7 ± 12.3 |
| 10 | 472.8 ± 26.4* | 189.1 ± 38.7 | 0.39 ± 0.07 | 211.5 ± 4.7 | 45.3 ± 8.5 | 48.4 ± 7.0 | |
| 30 | 526.7 ± 23.3*** | 179.2 ± 15.6 | 0.35 ± 0.04* | 233.6 ± 8.3** | 46.1 ± 4.5 | 48.4 ± 8.4 | |
| 90 | 541.1 ± 11.7*** | 180.1 ± 8.2 | 0.33 ± 0.02** | 249.1 ± 7.2*** | 48.2 ± 7.0 | 47.3 ± 10.4 | |
| Imipramine | 10 | 504.3 ± 34.1** | 196.1 ± 16.3 | 0.40 ± 0.04 | 262.8 ± 19.8*** | 53.6 ± 7.2 | 64.4 ± 12.9 |
Values are expressed as mean ± S.E.M. with units of ng/g tissue for 10 mice in each group. Data analysis was performed using one-way ANOVA.
P < 0.05
P < 0.01 and
P < 0.001, vs. the control group
As shown in Table 5, 5-HT levels tended to increase when treatment with piperine and FA (3,10, 30 and 90 mg/kg, p.o.) in the hypothalamus. There is a tendency to decrease in the ratio of 5-HIAA and 5-HT after treatment with the combination, but the change did not show the significance.
Table 5.
The effects of ferulic acid combined with subthreshold dose of piperine (10 mg/kg) on the concentrations of monoamines and their metabolites in the hypothalamus ofmice
| Group | Dose (mg/kg) | 5-HT | 5-HIAA | 5-HIAA/5-HT | Noradrenaline | Dopamine | DOPAC |
|---|---|---|---|---|---|---|---|
| Control | 346.5 ± 16.1 | 242.2 ± 22.3 | 0.72 ± 0.10 | 209.2 ± 7.6 | 446.7 ± 29.5 | 385.9 ± 70.3 | |
| Piperine (10 mg/kg) + FA | 3 | 411.0 ± 27.0 | 179.0 ± 15.8 | 0.45 ± 0.06 | 244.9 ± 27.3 | 580.6 ± 98.5 | 425.9 ± 71.3 |
| 10 | 412.2 ± 47.8 | 189.2 ± 29.4 | 0.47 ± 0.06 | 253.6 ± 26.3 | 519.9 ± 35.5 | 409.3 ± 69.6 | |
| 30 | 433.3 ± 22.8 | 218.1 ± 8.7 | 0.51 ± 0.03 | 284.0 ± 17.9 | 573.0 ± 55.3 | 386.9 ± 63.8 | |
| 90 | 425.7 ± 42.1 | 192.6 ± 25.1 | 0.48 ± 0.08 | 268.9 ± 24.2 | 642.5 ± 69.3 | 367.5 ± 103.0 | |
| Imipramine | 10 | 389.1 ± 27.5 | 240.0 ± 15.3 | 0.64 ± 0.06 | 250.5 ± 14.1 | 537.5 ± 75.2 | 373.8 ± 65.3 |
Values are expressed as mean ± S.E.M. with units of ng/g tissue for 10 mice in each group. Data analysis was performed using one-way ANOVA
The effects of low doses of FA combined with subthreshold dose of piperine on brain MAO-a and -B activities
The MAO-A and -B activities in three brain regions after treatment with low doses of FA and piperine were showed in Table 6. In the frontal cortex, MAO-A activity was inhibited significantly after treatment with high doses of FA at 30 and 90 mg/kg combined with subthreshold dose of piperine at 10 mg/kg [F (5, 54) =4.49, P < 0.05; P < 0.01]. The MAO-B activity was not shown to change after treatment with the combination. The selective MAO-A inhibitor moclobemide at 20 mg/kg (p.o.) inhibited MAO-A activity (P < 0.01), but not MAO-B activity. In the hippocampus, piperine (10 mg/kg) combined with FA (10, 30 and 90 mg/kg) inhibited MAO-A activity significantly [F (5, 54) =8.0, P's < 0.05; P < 0.01]. However, they were not found to inhibit MAO-B activity although it seemed there was a tendency to decrease. However, the classical MAO-A inhibitor moclobemide induced MAO-A inhibition in this region (P < 0.001). In the hypothalamus, there was a tendency to inhibit MAO-A activity with increasing doses of FA combined with piperine. But this difference was not big enough to induce the significant effect. Imipramine, on the other hand, was not found to induce significant inhibition on MAO-A or -B activity in all the three regions.
Table 6.
The inhibitory effect of Ferulic acid combined with subthreshold dose of piperine (10 mg/kg) on type A and type B monoamine oxidase activities in different brain regions
| Group | Does (mg/kg) | Monoamine oxidase-A activity |
Monoamine oxidase-B activity |
||||
|---|---|---|---|---|---|---|---|
| FC | Hippo | Hypo | FC | Hippo | Hypo | ||
| Control | 121.6 ± 13.8 | 119.8 ± 7.4 | 83.3 ± 3.3 | 74.2 ± 6.6 | 87.9 ± 4.6 | 88.5 ± 3.2 | |
| Piperine (10 mg/kg) + FA | 3 | 107.1 ± 11.1 | 106.7 ± 4.9 | 87.0 ± 4.0 | 81.6 ± 5.8 | 95.0 ± 4.3 | 85.6 ± 2.8 |
| 10 | 89.2 ± 12.3 | 94.1 ± 4.8* | 83.0 ± 5.2 | 76.2 ± 5.8 | 93.0 ± 8.2 | 88.2 ± 6.7 | |
| 30 | 84.7 ± 6.6* | 93.1 ± 6.5* | 81.9 ± 4.0 | 79.3 ± 2.3 | 94.4 ± 6.6 | 84.8 ± 4.9 | |
| 90 | 66.8 ± 3.1** | 90.8 ± 5.2** | 76.4 ± 3.9 | 74.1 ± 2.7 | 89.8 ± 6.5 | 82.0 ± 2.1 | |
| Moclobemide | 20 | 73.6 ± 5.3** | 67.9 ± 6.5*** | 72.3 ± 3.5 | 72.5 ± 4.3 | 85.7 ± 9.9 | 74.9 ± 4.6 |
Values are expressed as mean ± S.E.M. with units of ng/g tissue for 10 mice in each group. Data analysis was performed using one-way ANOVA.
P < 0.05
P < 0.01 and
P < 0.001, vs. the control group.
Discussion
Given the large burden imposed by depression on the modern society, it is urgent to discover new approaches or novel agents that can decrease its prevalence as well as reverse its detrimental psychiatric alteration. Growing evidence suggests that combination strategy offers a series of potential advantages including less demoralization of psychiatric effects on depressed patients of therapeutic failure, less withdrawal syndrome and more rapid or effective clinical response (Lam et al. 2002; Rubio et al. 2004). The present study received the effects from the combination of low doses of FA and subthreshold dose of piperine far exceeded those obtained by either compound used alone. The results from locomotor activity suggested that the combination of FA and piperine, at the doses that produced an anti-immobility response, did not show significant locomotor stimulation or inhibition. This interaction between these two compounds was synergistic as shown by an analysis of isobologram. The theoretical basis for this interaction, as suggested in the results, may be involved in the synergistic increases in monoamine transmitters including 5-HT, NE and dopamine, and their metabolic enzymes, MAO-A and -B, in the brain regions that are highly relevant to emotional disorders.
Study came first from the behaviors suggested that the natural polyphenol FA induced significant decreased in immobility time as shown in both TST and FST. However, the antidepressant-like effect of FA was not increased a lot even if the dose was as high as 180 mg/kg. Likewise, piperine was also shown the anti-immobility response, but the effect was weak and the data did not show a significant dose-dependent effect. However, the condition was changed when treatment with mice the low doses of FA combined with subthreshold dose of piperine. The significant anti-immobility response was shown with a supra-additive manner, the maximal efficacies were achieved to 53.85 % when 90 mg/kg FA combined with 10 mg/kg piperine. It is reliable that piperine can potentiate bioavailability of polyphenol including resveratrol and FA by decreasing its metabolism and increasing the effective duration as a bioenhancer of FA (Huang et al. 2013; Johnson et al. 2011). Another likely interpretation is that the enhanced effects of the drug combination are due to the sparing effects of both compounds. The isoboligram analyses seem to also suggest such an interpretation. In our previous study, piperine at 160 mg/kg was found to decrease the spontaneous locomotor activity, however, the combination of FA and piperine did not induce the changes on locomotor counts in the present study, suggesting the specific antidepressant-like effects of the combination.
FA is a natural antioxidant and polyphenol with various therapeutic activities on oxidative stress, immunity dysfunction and neurodegenerative disorders (Das et al. 2014; Muralidhara 2014). The previous studies in our laboratory indicated that FA increased the pain threshold and regulated serotonergic and adrenergic transmission in the pain-depression dyad mouse model induced by reserpine (Xu et al. 2013; Zhang et al. 2013). However, evidence suggests that the bioavailability of natural polyphenols is low and the half-life is short, their poor penetration into the blood–brain barrier curtails their therapeutic utility (Elliott and Jirousek 2008). Piperine, on the other hand, is a major alkaloidal constituent of black pepper, which is also recognized as a potent bioavilability enhancer due to its contribution in increased absorption of some polyphenolic compounds, such as curcumin and resveratrol (Rinwa et al. 2013; Xu et al. 2013). Therefore, the combination therapy is such a reliable strategy to avoid the severe side effect and potentiate the therapeutic utility. Our previous study suggested that resveratrol, a natural polyphenol having similar structure with FA, exerting the antidepressant-like behaviors when combined with piperine through potentiation of monoaminegic system (Zhang et al. 2013). In the present study, we supposed that a superior response should be happened due to the potentiation of serotonergic and noradrenergic system by combined administration with low doses of FA and subthreshold of piperine. The monoamine levels in different brain regions were determined after treatment with the combination of FA and piperine. We focused on three brain regions, such as frontal cortex, hippocampus and hypothalamus, which are related to the emotional and cognitive disorders (Xu et al. 2005). The intriguing finding suggested that 5-HT and NE were increased significantly in the frontal cortex and hippocampus after treatment with the combination. 5-HT is well-known neurotransmitter involving depression and anxiety; decreased 5-HT level is prominent in the different psychiatric diseases. The ratio of 5-HITT to 5-HT reflects neurotransmitter metabolism and utilization. A reduced 5-HT turnover in these two brain regions after administration with the combination of low doses of FA and piperine indicated that the combination prompted the utilization of 5-HT and led to enhancing the serotonergic function.
The frontal cortex, hippocampus and hypothalamus are involved in emotion and motivation, which may be related to the expression of depression (Butterweck et al. 2002). Central noradrenergic dysfunction with autonomic nervous system dysregulation in these brain regions is reported in major depressive disorder (Hamon and Blier 2013). The classical antidepressants, such as the tricyclic antidepressants, monoamine oxidase inhibitors and noradrenaline uptake inhibitors, increase serotonin or noradrenaline levels by either preventing neurotransmitter reuptake or inhibiting its degradation. Measure of noradrenaline after treatment with low doses of FA and subthreshold dose of piperine suggested that the combination of FA at 10, 30 and 90 mg/kg and piperine at 10 mg/kg increased in noradrenaline levels both in the frontal cortex and hippocampus. The positive drug imipramine also affected the 5-HT and noradrenaline levels in these two brain regions. Judging from the results from both the behaviors and HPLC detection, the antidepressant-like effects of this combination may be related to, at least in part, the regulation of noradrenaline levels in the brain.
In addition to noradrenaline and 5-HT, recent studies suggested that dopamine pathway also participates the antidepressant-like behaviors of some polyphenols, such as curcumin and resveratrol (Xu et al. 2005; Yu et al. 2013). In our present study, a tendency to increase in dopamine and decrease in DOPAC levels was found after treatment with the combination of FA and piperine in the hippocampus. This suggests that the combination also modulates dopaminergic function by influencing the synthesis or metabolism of dopamine.
The classical antidepressants including desipramine and fluoxetine are able to increase in brain monoamines by preventing their reuptake or inhibiting MAO enzyme activity (Bhutani et alBhutani et al. 2009). MAO plays a major role in the pathogenesis of psychiatric disorders, particularly depression and anxiety. MAO-A is involved in metabolism of 5-HT, noradrenaline and dopamine, while MAO-B preferentially metabolizes the dopamine neurotransmitter. To further address the mechanisms underlying the increase in the monoamines after administration of FA and piperine, we evaluated the MAO activity in all the frontal cortex, hippocampus and hypothalamous. The dose-dependent inhibition of MAO-A activity was found with the increasing doses of FA combined with subthreshold piperine in these three brain regions, mainly in the hippocampus and frontal cortex. There was a tendency to inhibit the MAO-A activity in the hypothalamus after treatment with the combination. These results were similar to those observed after treatment with the positive drug, which suggested moclobemide induced a significant MAO-A inhibition, but did not affect MAO-B activity in the above brain regions. These observations indicated that the increased the monoamines following the combination of FA and piperine treatment is involved in the inhibition of MAO enzyme activity, particularly MAO-A enzyme activity.
Polyphenols have the poor oral bioavailability due to the rapid metabolism that curtails their penetration into the brain (Atal et al. 1985). Indeed, to improve the systemic absorption of FA is becoming a real challenge. Piperine is a potent inhibitor of hepatic and intestinal glucuronidation. It is possible to increase in the free form of FA when they are combined to use. The present study provides evidence that oral administration of low doses of FA combined with subthreshold of piperine can confer synergistic effect on depression-like behaviors and might be a natural alternative in the prevention of psychiatric disorders with high efficacy and low side effects.
Acknowledgments
G.W. Li, L.N. Ruan, R.J. Chen and R.Y. Wang contributed equally to this work. This work was funded by the Natural Science Foundation of Ningbo (No. 2012A610256; 2014A610256), 2015 Zhejiang provincial Public Welfare Technology Application Research Plan (2015C33297), and Ministry of Science and Technology Spark Plan (2014GA701011) for G.W. Li.; The Natural Science Foundation of Changzhou (No. ZD2O1413) for Dr. W. Huang; The National Institute of General Medical Sciences (Reed - U54GM104942) for Dr. M. Reed. Zhejiang Province Extremely Key Subject Building Funding “Pharmacology and Biochemical Pharmaceutics 2008”, Latitudinal project of Wenzhou Medical University (No. 95012011) and Natural Science Foundation of Zhejiang Province (No. Y14H310034) for Professor J. C. Pan.
References
- Atal CK, Dubey RK, Singh JJ. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. Pharmacol Exp Ther. 1985;232:258–262. [PubMed] [Google Scholar]
- Awad AS, El-Sharif AA. Curcumin immune-mediated and anti-apoptotic mechanisms protect against renal ischemia/reperfusion and distant organ induced injuries. Int Immunopharmacol. 2011;11:992–996. doi: 10.1016/j.intimp.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Bhutani MK1, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Bourin M, Mocaër E, Porsolt R. Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors. J Psychiatr Neurosci. 2004;29:126–133. [PMC free article] [PubMed] [Google Scholar]
- Butterweck V, Böckers T, Korte B, et al. Long-term effects of St. John's wort and hypericin on monoamine levels in rat hypothalamus and hippocampus. Brain Res. 2002;930:21–29. doi: 10.1016/s0006-8993(01)03394-7. [DOI] [PubMed] [Google Scholar]
- Chakrabarti SK, Loua KM, Bai CJ, Durham H, Panisset JC. Modulation of monoamine oxidase activity in different brain regions and platelets following exposure of rats to methylmercury. Neurotoxicol Teratol. 1998;20:161–168. doi: 10.1016/s0892-0362(97)00104-9. [DOI] [PubMed] [Google Scholar]
- Das U, Manna K, Sinha M, Datta S, Das DK, Chakraborty A, Ghosh M, Saha KD, Dey S. Role of ferulic acid in the amelioration of ionizing radiation induced inflammation: a murine model. PLoS One. 2014;9:e97599. doi: 10.1371/journal.pone.0097599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9:371–378. [PubMed] [Google Scholar]
- Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;45:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Huang W, Chen Z, Wang Q, Lin M, Wu S, Yan Q, Wu F, Yu X, Xie X, Li G, Xu Y, Pan J. Piperine potentiates the antidepressant-like effect of trans-resveratrol: involvement of monoaminergic system. Metab Brain Dis. 2013;28:585–595. doi: 10.1007/s11011-013-9426-y. [DOI] [PubMed] [Google Scholar]
- Johnson JJ, Nihal M, Siddiqui IA, Scariett CO, Bailey HH, Mukhtar H, Ahmad N. Enhancing the bioavailability of resveratrol by combining it with piperine. Nutr Food Res. 2011;55:169–176. doi: 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S, Lee B, KimM LH, Park HJ, Hahm DH. Antidepressant like effect of the methanolic extract from bupleurum falcatum in the tail suspension test. Progr Neuro Psychopharmacol Biol Psychiatr. 2010;34:265–270. doi: 10.1016/j.pnpbp.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Lam RW, Wan DD, Cohen NL, Kennedy SH. Cobining antidepressants for treatment-resistant depression: a review. J Clin Psychiatr. 2002;63:685–693. doi: 10.4088/jcp.v63n0805. [DOI] [PubMed] [Google Scholar]
- Lopez JP, Lim R, Cruceanu C, Crapper L, Fasano C, Labonte B, Maussion G, Yang JP, Yerko V, Vigneault E, El Mestikawy S, Mechawar N, Pavlidis P, Turecki G. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression andantidepressant treatment. Nat Med. 2014 doi: 10.1038/nm.3582. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidhara KMDJ. Neuroprotective efficacy of a combination of fish oil and ferulic acid against 3-nitropropionic acid-induced oxidative stress and neurotoxicity in rats: behavioural and biochemical evidence. Appl Physiol Nutr Metab. 2014;39:487–496. doi: 10.1139/apnm-2013-0262. [DOI] [PubMed] [Google Scholar]
- Ogiwara T, Satoh K, Kadoma Y, Murakami Y, Unten S, Atsumi T, Sakagami H, Fujisawa S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002;22:2711–2717. [PubMed] [Google Scholar]
- Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M, Dutheil S, Duman C, Boikess S, Lewis DA, Stockmeier CA, Dileone RJ, Rex C, Aghajanian GK, Duman RS. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014;20:531–535. doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinwa P, Kumar A, Garg S. Suppression of neuroinflammatory andapoptotic signaling cascade by curcumin alone and in combination with piperine in rat model of olfactory bulbectomy induced depression. PLoS One. 2013;8:e61052. doi: 10.1371/journal.pone.0061052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rubio G, San L, Lopez-Munoz F, Alamo C. Reboxetine adjunct for partial or nonresponders to antidepressant treatment. J Affect Disord. 2004;81:67–72. doi: 10.1016/j.jad.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Wang BH, Ou-Yang JP. Pharmacological actions of sodium ferulate in cardiovascular system. Cardiovasc Drug Rev. 2005;23:161–172. doi: 10.1111/j.1527-3466.2005.tb00163.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ. Antidepressant effects of curcumin in the forced swim test and ol-factory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005;82:200–206. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li S, Chen RJ, Li GW, Barish PA, You WT, Chen L, Lin MM, Ku BS, Pan JC, Ogle W. Antidepressant-like effect of low molecular proanthocyanidin in mice: involvement of monoaminergic system. Pharm. Biochem. Behav. 2010;94:447–453. doi: 10.1016/j.pbb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang L, Shao T, Ruan L, Wang L, Sun J, Li JX, Zhu X, O'Donnell JM, Pan JC. Ferulic acid increases pain threshold and ameliorates depression-like behaviors in reserpine-treated mice: behavioral and neurobiological analyses. Metab Brain Dis. 2013;28:571–583. doi: 10.1007/s11011-013-9404-4. [DOI] [PubMed] [Google Scholar]
- Yu YL, Wang R, Chen C, Du X, Ruan L, Sun J, Li JX, Zhang L, O'Donnell JM, Pan JC, Xu Y. Antidepressant-like effect of trans-resveratrol in chronic stress model: behavioral and neurochemical evidences. J. Psychia. Res. 2013;47:315–322. doi: 10.1016/j.jpsychires.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang QD, Shi HM, Pan JC. Influence of ferulic acid on the pain-depression dyad induced by reserpine. Yao Xue Xue Bao. 2013;48:32–37. [PubMed] [Google Scholar]
- Zhang Y, Huang X, Wang Y, Xie Y, Qiu X. Ferulic acid-induced anti-depression and prokinetics similar to chaihu-shugan-San via polypharmacology. Brain Res Bull. 2011;86:222–228. doi: 10.1016/j.brainresbull.2011.07.002. [DOI] [PubMed] [Google Scholar]