Abstract
A 16-year-old female patient was admitted to our hospital due to progressive renal dysfunction with an increased serum creatinine (sCr) level of 1.7 mg/dL. Her clinical course without any ocular manifestations and results of drug-induced, lymphocyte-stimulating tests, in addition to a renal histological assessment, initially encouraged us to ascribe the patient’s renal abnormalities to drug-induced acute interstitial nephritis (AIN). Four months later, she started to complain about reduced visual acuity when she was found to have anterior bilateral uveitis despite the recovered renal function with almost constant sCr levels around 0.7 mg/dL. Thus, a diagnosis of tubulointerstitial nephritis and uveitis (TINU) syndrome was finally made. Our case illustrates the difficulties in distinguishing late-onset uveitis TINU syndrome from drug-induced AIN at the time of the renal biopsy, thereby suggesting the importance of a longitudinal follow-up to overcome the potential underdiagnosis of the disease. Several diagnostic conundrums that emerged in this case are also discussed.
Keywords: acute interstitial nephritis, uveitis, TINU syndrome, DLST, HLA
Introduction
Tubulointerstitial nephritis and uveitis (TINU) syndrome is a clinical entity characterized by a combination of acute interstitial nephritis (AIN) and uveitis.1 Ocular findings may either precede or concurrently develop with the onset of AIN, although there is often a substantial time interval between the two diseases, with uveitis occurring in more than half of all the cases 1–14 months after presentation with renal abnormalities.2 Thus, prompt recognition of the disease may not be straightforward. In this report, we describe our serendipitous experience with one such case in a female patient whose renal characteristics were initially attributed to drug-induced AIN. Several diagnostic conundrums that emerged in this case are also discussed.
Case Report
A 16-year-old female patient was admitted to our hospital in the middle of October 2013 due to a progressive deterioration in her renal function with an increased serum creatinine (sCr) level of 1.7 mg/dL. Although she had no past history of renal disease, her sCr level was 0.7 mg/dL at the end of September 2013 and increased to 1.4 mg/dL at the beginning of October 2013. Four weeks prior to admission, she demonstrated a spiked fever of more than 38 °C and was empirically treated by a local physician with cefcapene pivoxil hydrochloride hydrate (CFPH) at a dose of 300 mg/day combined with the occasional use of acetaminophen (ACP) for three days; however, her fever persisted and CFPH was changed to co-amoxiclav (CAC) 375 mg/day. Four days later, CAC was further switched to levofloxacin hydrate (LVFX) 500 mg/day, which was used for the next nine days until her temperature fell to ~37 °C. Meanwhile, she started to complain of fatigue and a loss of appetite; thus, she was referred to our hospital for further workup. Her medical history was unremarkable, except the fact that she had been treated with sodium valproate (SV) for a migraine for ~2 years until 15 years of age.
At the time of this admission, the patient had a temperature of 37.4 °C, a pulse of 115 beats/minute, and a blood pressure of 120/80 mmHg. Her bowel sounds were normal. Her abdomen was soft and flat, with lower abdominal tenderness, without rebound pain or palpable masses. The lungs were clear and heart sounds were normal. There was no rash or lymphadenopathy, and no petechiae or joint swellings were observed. Renal sonography showed that the renal dimensions of the right kidney measured 117 × 60 mm, while those of the left kidney measured 105 × 53 mm, and the degree of renal cortex echogenicity was normal. The laboratory data on this admission are shown in Table 1. In addition to renal dysfunction, a serological study revealed an increased level of C-reactive protein (CRP), while antineutrophil cytoplasmic antibody, anti-glomerular basement membrane antibodies, anti-SSA/Ro antibodies, and anti-SSB/La antibodies were all negative. A urinalysis showed active sediments with one four epithelial casts per low-power field and <1 red blood cell per high-power field. Her urine contained 0.175 g of protein in a 24-hour specimen. The fraction of excretion of sodium was 0.11. The urinary excretion of β2-microglobulin (b2MG) and N-acetyl-beta-d-glucosaminidase (NAG) were 3,837 g/L (reference range: <200 μg/L) and 13.4 U/g·Cr (reference range: 0.9–2.4 U/g·Cr), respectively.
Table 1.
The laboratory data on admission.
| White blood cells | 8100/μl | (3900–9800) |
| Neutrophils | 76.1% | (42.0–72.2) |
| Eosinophils | 0.3% | (0.0–5.8) |
| Basophils | 0.6% | (0.0–1.7) |
| Monocytes | 8.4% | (2.5–11.1) |
| Lymphocytes | 14.6% | (19.9–46.1) |
| Hemoglobin | 12.2 g/dl | (13.5–17.6) |
| Platelet count | 32.6 × 104/μl | (13.0–36.9) |
| Blood urea nitrogen | 17 mg/dl | (8–20) |
| Serum creatinine | 1.75 mg/dl | (0.63–1.03) |
| Total protein | 7.7 g/dl | (6.9–8.4) |
| Serum albumin | 3.7 g/dl | (3.9–5.1) |
| Sodium | 140 mmol/l | (136–148) |
| Potassium | 4.1 mmol/l | (3.6–5.0) |
| Chloride | 102 mmol/l | (96–108) |
| Calcium | 9.6 mg/dl | (8.8–10.1) |
| Phosphorus | 3.4 mg/dl | (2.4–4.6) |
| Aspartate aminotransferase | 17 U/l | (11–30) |
| Alanine aminotransferase | 11 U/l | (4–30) |
| CRP | 6.26 mg/dl | (0–0.14) |
| IgG | 1318 mg/dl | (870–1700) |
| IgA | 183 mg/dl | (110–410) |
| IgM | 93 mg/dl | (33–160) |
| C3 | 153 mg/dl | (86–160) |
| C4 | 49 mg/dl | (17–45) |
| Angiotensin-converting enzyme | 10 mU/mL | (8.3–21.4) |
Note: The reference ranges for each parameter used at our institute are indicated in the parentheses.
Abbreviation: Ig, immunoglobulin.
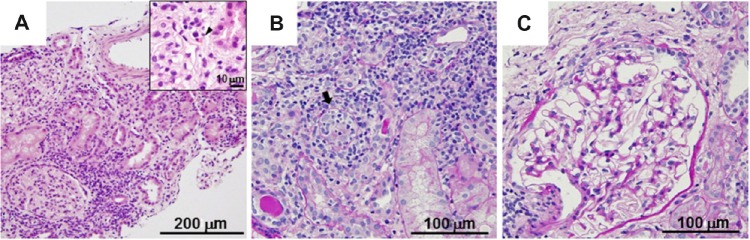
A renal biopsy was performed on the following day of admission. In total, three biopsy cores were obtained and observed carefully to identify the presence of the renal cortex and guide sectioning of the material. Each core was divided, so that the tips were immediately fixed with 2.5% glutaraldehyde for electron microscopy. One half of each remaining core was fixed with 4% phosphate-buffered paraformaldehyde and then evaluated by light microscopy with standard pathological staining, including hematoxylin–eosin (HE), periodic acid–Schiff (PAS), and periodic acid-methenamine silver–Masson’s trichrome stains, and the other half was frozen in liquid nitrogen for an immunofluorescence analysis. Under a light microscope, 10 glomeruli were identified within the renal parenchyma. The glomerular structures were preserved, while there was marked diffuse infiltration with lymphocytes and some eosinophils accompanied by a partial destruction of the tubular structures (Fig. 1). Immunohistochemistry failed to reveal any immune complex deposits among the observed glomeruli. Electron microscopy disclosed focal fusion of the foot processes of the glomerular visceral epithelial cells. A drug lymphocyte stimulation test (DLST), performed by a commercially based clinical diagnostic testing service (SRL, Inc., Tokyo, Japan), confirmed that the patient had a negative stimulation index (SI) score for CFPH, while she had specific high SI scores for CAC of 360%, SV of 312%, ACP of 311%, and LVFX of 252% (the cutoff value for DLST positivity is 180%). According to the lack of suggestive findings of ocular disease by ophthalmological screening and the laboratory and pathological results, as well as her clinical history, drug-induced AIN was suspected. Then, the patient was started on oral prednisolone (PSL) 25 mg daily (0.5 mg/kg/day), which demon strated a favorable response. At the end of December 2013, her sCr had decreased to ~0.7 mg/dL and PSL was tapered to 2.5 mg/day in the beginning of February 2014. However, over the next 17 days, the patient’s fatigue resumed when bilateral red eye and reduced visual acuity became overt. She was then found to have anterior bilateral uveitis without any apparent abnormalities of the fundus oculi by a subsequent ophthalmological review. A diagnosis of TINU syndrome was made thereafter, and she was treated with topical corticosteroids for the eyes when the urinary b2MG excretion levels increased to 11,694 μg/L despite the finding that her sCr levels were nearly constant (Fig. 2). At the follow-up, she was still dependent on topical ophthalmic corticosteroids with successful control of the disease activity and her urinary b2MG excretion levels settled at ~1,000 μg/L, with an increased dosage of oral PSL of 6.25 mg/day. Finally, we determined her human leukocyte antigens (HLAs) and alleles (SRL, Inc., Tokyo, Japan); the phenotypic expressions of HLA were A11/26, B62/48, and DR4/14, while the alleles of DRB1 and DQB1 were 04:06/14:07:01 and 03:02:01/05:02:01, respectively.
Figure 1.
Renal biopsy sections. Light microscopy revealed marked interstitial inflammatory infiltrates consisting of lymphocytes and occasional eosinophils (inset, arrow head) (A). Inflammation extended into the walls of the tubules (arrow) (B), while the glomeruli showed a normal appearance (C). HE stain (A) and PAS stain (B and C); the scale bar is indicated in each panel.
Figure 2.
Changes in the sCr level, urine level of b2MG, and urine level of NAG during the observation period. The number “0” is designated as the point of admission. One week before admission, LVFX was withdrawn and steroid treatment was started on clinical day 11. Note the transient increases in both the urine levels of b2MG and NAG after the onset of uveitis despite the fact that the sCr levels were nearly constant.
Discussion
Our case illustrates the difficulties in distinguishing the late-onset uveitis TINU syndrome from drug-induced AIN at the time of the renal histological assessment, thereby suggesting the importance of a longitudinal follow-up to overcome the potential underdiagnosis of TINU syndrome. Interestingly, transient increases in the urine levels of b2MG and NAG, which can be associated with tubular injuries,1–3 after the onset of anterior uveitis was noted, despite the absence of marked changes in the sCr levels. Relapse episodes of kidney injury have been shown to be accompanied by elevated urine NAG levels, while urine b2MG levels may not necessarily be related to the severity of uveitis.1,3 At present, the diagnostic impact of these parameters on late-onset uveitis in the current patient is unclear.
Numerous types of medications have been implicated in causing AIN, thereby making the clinical diagnosis of drug-induced AIN quite challenging. However, the diagnosis may be assisted by knowledge of the patient’s history and specific laboratory examinations, such as the DLST.4–7 Indeed, the DLST, which measures the proliferation of T cells induced by an agent in vitro, leading to suspicion of previous sensitization in vivo,8,9 has been regarded to be a useful diagnostic procedure for various types of drug hypersensitivity, including drug-induced AIN.6–8 In the current case, the facts that all agents prescribed for our patient before admission have been implicated to be associated with AIN4,10,11 and that our patient showed specific high scores for CAC, SV, ACP, and LVFX, but not CFPH, in the DLST initially encouraged us to ascribe the patient’s renal abnormalities to drug-induced AIN. Although the results of the DLST may not be absolute,6,7,9 we must focus on the point that a T-cell-mediated mechanism has been proposed to play a major pathogenic role in TINU syndrome as well as drug-induced AIN.2,4 Anecdotal information regarding antecedent drug use as a presumable risk for TINU syndrome has continued to accumulate,2,9,12–14 although it should also be noted that similar triggering agents have been identified for both isolated AIN and TINU syndrome.2,4,12 Subsequently, we are of the opinion that the development of TINU syndrome in our patient might be attributed to an additive, synergistic, or cumulative effect of the agents, including CAC, SV, and ACP as well as LVFX, although it is quite difficult to determine the precise contribution of each. Whether TINU syndrome and AIN without uveitis are different phenotypes of the same pathology is of particular interest and a pivotal issue that must be extensively investigated.15 In this regard, a recent study concerning the pathogenic role of modified CRP (mCRP), which can be irreversibly dissociated from native CRP,16 for TINU syndrome should be noted,17 as it may be one of the common target autoantigens in renal and ocular tissues.3,14,17 Interestingly, it has been shown that the serum levels of anti-mCRP autoantibodies may play a role in distinguishing patients with TINU syndrome from those with AIN,3,17 although such a procedure was not applicable in the current patient.
The primary mode of treatment for AIN is withdrawal or discontinuation of the offending agent(s).5,6 There is no clear advantage of corticosteroid therapy following the discontinuation of the offending agent(s); however, there are several anecdotal reports suggesting that the administration of corticosteroids may shorten the course of renal failure.6,7,18 In the current patient, the interval between drug withdrawal and the onset of steroid treatment was approximately three weeks, during which no drastic decrease in the sCr levels was confirmed, while her renal function promptly improved after the commencement of the treatment, leading us to conclude that the discontinuation of the causative agents was insufficient and even a relatively low dose of PSL of 0.5 mg/kg/day had a clinical benefit on her deteriorated renal function, which was similar to our previous experiences.6,7,19 However, the validity of our therapeutic regimen must be carefully assessed as such a protocol might not work well for controlling the ocular inflammatory milieu. Numerous authors agree with a corticosteroid treatment for patients with TINU syndrome complicated by progressive renal insufficiency similar to that of AIN,20,21 although the concurrent use of topical ocular steroid and steroid sparing immunosuppressants, as well as a dose titration of PSL, may be mandatory due to the protracting and/or relapsing nature of uveitis associated with this syndrome.20–22
Polymorphism in the HLA region is associated with a wide spectrum of diseases with an immune etiology,23 and several studies have reported the HLA specificities of patients with TINU syndrome, implying the presence of a genetic predisposition.1,2,14,15 Some of the HLA antigens and alleles of current patients have been included in HLA typing of patients with TINU syndrome1,2,14,15; however, the immunopathogenesis of the disease is likely to be complex and the number of reported cases is not sufficient for a statistical evaluation to determine the relative risks associated with reported HLA specificities.2 Indeed, a previous report on monozygotic twins, one of whom developed AIN without uveitis while the other developed TINU syndrome,24 implied that the same genotype in different individuals may result in discrepant disease manifestations, presumably depending on the nature of environmental factors.2,15 Clearly, further assessments for the role of HLA molecules in TINU syndrome susceptibility and an accumulation of additional cases similar to ours are a matter requiring continuous and careful attention, and such strategies should aid in the establishment of diagnostic and therapeutic managements for TINU syndrome.
Footnotes
ACADEMIC EDITOR: Athavale Nandkishor, Associate Editor
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 793 words, excluding any confidential comments to the academic editor.
FUNDING: This study was supported in part by a Grant-in-Aid for Research on Advanced Chronic Kidney Disease, Practical Research Project for Renal Diseases from Japan Agency for Medical Research and Development, AMED. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
MK, TA drafted the manuscript. TS, NO, T Miki, T Masuda, TK made contributions to the acquisition of the clinical data. ST, SM, DN provided a detailed review of the contents and structure of the manuscript, resulting in significant changes to the original document. All the authors have read and approved the final manuscript.
REFERENCES
- 1.Takemura T, Okada M, Hino S, et al. Course and outcome of tubulointerstitial nephritis and uveitis syndrome. Am J Kidney Dis. 1999;34(6):1016–21. doi: 10.1016/S0272-6386(99)70006-5. [DOI] [PubMed] [Google Scholar]
- 2.Mandeville JT, Levinson RD, Holland GN. The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol. 2001;46(3):195–208. doi: 10.1016/s0039-6257(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Su T, Chu R, Li X, Yang L. Tubulointerstitial nephritis with uveitis in Chinese adults. Clin J Am Soc Nephrol. 2014;9(1):21–8. doi: 10.2215/CJN.02540313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60(2):804–17. doi: 10.1046/j.1523-1755.2001.060002804.x. [DOI] [PubMed] [Google Scholar]
- 5.Praga M, González E. Acute interstitial nephritis. Kidney Int. 2010;77(11):956–61. doi: 10.1038/ki.2010.89. [DOI] [PubMed] [Google Scholar]
- 6.Onishi A, Yamamoto H, Akimoto T, et al. Reversible acute renal failure associated with clomipramine-induced interstitial nephritis. Clin Exp Nephrol. 2007;11(3):241–3. doi: 10.1007/s10157-007-0485-4. [DOI] [PubMed] [Google Scholar]
- 7.Akimoto T, Horikoshi R, Muto S, Kusano E. Low-dose corticosteroid and gallium-67 scintigraphy and acute interstitial nephritis. Saudi J Kidney Dis Transpl. 2014;25(4):864–8. doi: 10.4103/1319-2442.135184. [DOI] [PubMed] [Google Scholar]
- 8.Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59(8):809–20. doi: 10.1111/j.1398-9995.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Yoshioka K, Miyano M, et al. Tubulointerstitial nephritis and uveitis (TINU) syndrome caused by the Chinese herb “Goreisan”. Clin Exp Nephrol. 2009;13(1):73–6. doi: 10.1007/s10157-008-0069-y. [DOI] [PubMed] [Google Scholar]
- 10.Ramalakshmi S, Bastacky S, Johnson JP. Levofloxacin-induced granulomatous interstitial nephritis. Am J Kidney Dis. 2003;41(2):E7. doi: 10.1053/ajkd.2003.50064. [DOI] [PubMed] [Google Scholar]
- 11.Fruchter LL, Alexopoulou I, Lau KK. Acute interstitial nephritis with acetaminophen and alcohol intoxication. Ital J Pediatr. 2011;37:17. doi: 10.1186/1824-7288-37-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cacoub P, Deray G, Le Hoang P, et al. Idiopathic acute interstitial nephritis associated with anterior uveitis in adults. Clin Nephrol. 1989;31(6):307–10. [PubMed] [Google Scholar]
- 13.Kindler J, Kemper R, Helmchen U. Acute tubulointerstitial nephritis and uveitis syndrome (TINU syndrome). Occurrence of uveitis after stopping steroids. Nephrol Dial Transplant. 1998;13(7):1892–3. doi: 10.1093/ndt/13.7.1893. [DOI] [PubMed] [Google Scholar]
- 14.Santoro D, Vita G, Rovito S, et al. Drug-induced TINU syndrome and genetic characterization. Clin Nephrol. 2012;78(3):230–6. doi: 10.5414/cn107119. [DOI] [PubMed] [Google Scholar]
- 15.Levinson RD, Park MS, Rikkers SM, et al. Strong associations between specific HLA-DQ and HLA-DR alleles and the tubulointerstitial nephritis and uveitis syndrome. Invest Ophthalmol Vis Sci. 2003;44(2):653–7. doi: 10.1167/iovs.02-0376. [DOI] [PubMed] [Google Scholar]
- 16.Tan Y, Yu F, Yang H, Chen M, Fang Q, Zhao MH. Autoantibodies against monomeric C-reactive protein in sera from patients with lupus nephritis are associated with disease activity and renal tubulointerstitial lesions. Hum Immunol. 2008;69(12):840–4. doi: 10.1016/j.humimm.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Tan Y, Yu F, Qu Z, et al. Modified C-reactive protein might be a target autoantigen of TINU syndrome. Clin J Am Soc Nephrol. 2011;6(1):93–100. doi: 10.2215/CJN.09051209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toto RD. Acute tubulointerstitial nephritis. Am J Med Sci. 1990;299(6):392–410. doi: 10.1097/00000441-199006000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Inoue M, Akimoto T, Saito O, Ando Y, Muto S, Kusano E. Successful relatively low-dose corticosteroid therapy for diclofenac-induced acute interstitial nephritis with severe renal failure. Clin Exp Nephrol. 2008;12(4):296–9. doi: 10.1007/s10157-008-0039-4. [DOI] [PubMed] [Google Scholar]
- 20.Mackie FE, Rosenberg AR, Kainer G. Tubulointerstitial nephritis: drugs are not always to blame. J Paediatr Child Health. 2008;44(5):305–7. doi: 10.1111/j.1440-1754.2008.01303.x. [DOI] [PubMed] [Google Scholar]
- 21.Vohra S, Eddy A, Levin AV, Taylor G, Laxer RM. Tubulointerstitial nephritis and uveitis in children and adolescents. Four new cases and a review of the literature. Pediatr Nephrol. 1999;13(5):426–32. doi: 10.1007/s004670050634. [DOI] [PubMed] [Google Scholar]
- 22.Goda C, Kotake S, Ichiishi A, Namba K, Kitaichi N, Ohno S. Clinical features in tubulointerstitial nephritis and uveitis (TINU) syndrome. Am J Ophthalmol. 2005;140(4):637–41. doi: 10.1016/j.ajo.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Bodmer WF. The HLA system: structure and function. J Clin Pathol. 1987;40(9):948–58. doi: 10.1136/jcp.40.9.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianviti A, Greco M, Barsotti P, Rizzoni G. Acute tubulointerstitial nephritis occurring with 1-year lapse in identical twins. Pediatr Nephrol. 1994;8(4):427–30. doi: 10.1007/BF00856521. [DOI] [PubMed] [Google Scholar]