Abstract
Background: Transcranial magnetic stimulation (TMS) is a non-invasive tool that is able to modulate the electrical activity of the brain depending upon its protocol of stimulation. Theta burst stimulation (TBS) is a high-frequency TMS protocol that is able to induce prolonged plasticity changes in the brain. The induction of plasticity-like effects by TBS is useful in both experimental and therapeutic settings; however, the underlying neural mechanisms of this modulation remain unclear. The aim of this study was to investigate the effects of continuous TBS (cTBS) on the intrahemispheric and interhemispheric functional connectivity of the resting and active brain.
Methods: A total of 26 healthy humans were randomly divided into two groups that received either real cTBS or sham (control) over the left primary motor cortex. Surface electroencephalogram (EEG) was used to quantify the changes of neural oscillations after cTBS at rest and after a choice reaction time test. The cTBS-induced EEG oscillations were computed using spectral analysis of event-related coherence (ERCoh) of theta (4–7.5 Hz), low alpha (8–9.5 Hz), high alpha (10–12.5 Hz), low beta (13–19.5 Hz), and high beta (20–30 Hz) brain rhythms.
Results: We observed a global decrease in functional connectivity of the brain in the cTBS group when compared to sham in the low beta brain rhythm at rest and high beta brain rhythm during the active state. In particular, EEG spectral analysis revealed that high-frequency beta, a cortically generated brain rhythm, was the most sensitive band that was modulated by cTBS.
Conclusion: Overall, our findings suggest that cTBS, a TMS protocol that mimics the mechanism of long-term depression of synaptic plasticity, modulates motor network oscillations primarily at the cortical level and might interfere with cortical information coding.
Keywords: electroencephalogram, long-term depression, motor cortex, neuromodulation, neuroplasticity
Introduction
Transcranial magnetic stimulation (TMS) is a tool that is able to non-invasively interfere with the activity of the intact human brain directly (1). TMS directs currents into the brain without physical contact, as there are no implanted or surface electrodes. Instead, it works by placing an electromagnetic coil that carries pulses of current near the human scalp. Based upon Faraday’s law of electromagnetic induction—the process by which electrical energy is converted into magnetic fields—the rapidly changing magnetic field will induce an electrical current in the surrounding cortical tissue below the coil (1). As body tissue is electrically conductive, the ionic current will flow, thereby eliciting nerve depolarisation and action potentials and will subsequently stimulate cortical neurons. TMS can be applied as a single pulse or as repetitive pulses. The effects of single-pulse or single-train can add up with repeated stimulation (e.g., as in the rTMS protocol), which results in the modulation of cortical activity beyond the stimulation period (2). This prolonged rTMS aftereffect emulates the pattern of synaptic plasticity (i.e., long term potentiation and long term depression) of the hippocampus (3).
Theta-burst stimulation (TBS) is a variant of high frequency rTMS that is able to prolong the after effects of the induced plastic changes for up to an hour despite its lower stimulus intensity and shorter duration of stimulation (4). This makes TBS particularly useful for neuroplasticity research due to its prolonged residual effects and its relatively safe efficacy (5). In a theta-burst paradigm that involves human subjects, brief trains of pulses are delivered at 5 Hz (i.e., the theta frequency). The term theta derives from the 200 ms main periodicity of the theta rhythm, an oscillatory rhythm that occurs during the periods of increased attention (5). There are two modalities of TBS, continuous TBS (cTBS), which will induce long-lasting, reversible cortical inhibition or long term depression, and intermittent TBS (iTBS), which will induce long-lasting cortical facilitation or long term potentiation (6,7). Although the mechanisms of TBS at the micro- or synaptic level are well understood, it remains unclear how TBS modulates the macro-level neuronal network, such as cortical oscillations (8).
In a previous study, we suggested a probable link between the long-lasting neural synchronisation of electroencephalographic (EEG) oscillations and plasticity-like mechanisms of LTD in humans after continuous TBS (cTBS) (9). Our results showed at least a 30-minute modulation of regional or local neural oscillations in theta and beta brain rhythms (9); however, studies of the effects of cTBS-induced alterations of the remote interregional neural network oscillations remain scarce (9). McAllister et al. (10) explored the modulation of cortical oscillations and the cortical plasticity that is induced by cTBS in M1. They investigated the modulation of cortical oscillatory activity by cTBS of 600 pulses after a visuomotor training task using motor evoked potentials (MEP) and electroencephalogram (EEG) measurements (10). The authors did not observe any significant synchronisation of the baseline EEG power spectra of delta, theta, alpha and beta brain rhythms after the visuomotor training task, when compared to a decrease in MEP sizes (10). They concluded that EEG was not a sensitive index of cortical output to plasticity-inducing paradigms of cTBS (10). Yet, instead of using multichannel EEG that would provide a comprehensive cortical read-out following cTBS, the power spectra in their study was derived from only a single electrode that was placed at C3 (10). Schindler et al. (11) examined EEG network oscillations post-cTBS of 600 pulses on the frontal eye field of four healthy subjects and showed an elevated level of neuronal synchronisation of the cerebral hemisphere ipsilateral to the stimulation site relative to the non-stimulated hemisphere up to one hour (11). The authors concluded that cTBS might interfere with information transfer through its effect on neuronal synchronisation; however, in their study, the authors changed the stimulation parameters (stimulation intensity and number of pulses) from the original TBS protocol on the motor cortex (5), thereby making a direct comparison with the original protocol problematic. This fact was emphasized by Goldsworthy et al. (12), who showed that slight modifications in the stimulation parameters used for the application of cTBS protocol can have a significant impact on its efficacy for inducing human motor cortical neuroplasticity (12).
In the present study, we addressed the limited knowledge of how TBS modulates the macro-level neuronal network of cortical oscillations by applying the original cTBS protocol over the left primary motor cortex in healthy subjects. The aim of this study was to investigate how preconditioning the motor cortex with high-frequency cTBS affects the subsequent patterns of oscillatory activity in the motor cortex and the cortico-cortical areas at rest. EEG spectral analysis was quantified to evaluate the interference by cTBS on the cortical motor network oscillations. We predicted that cTBS would interfere with the connection between the left primary motor cortex and distant brain areas of the same motor neural network.
Methods
Participants
Twenty-six healthy volunteers (13 males, 13 females; mean age, 26.7 years SD 5.8) with no history of neurological disorder were randomly divided into two groups that received either active or sham cTBS as a control. Subjects were right-handed, as assessed by the Edinburgh handedness inventory, and provided written informed consent prior to participation. The experimental procedure was approved by the Local Ethical Committee.
TMS and cTBS
TMS was delivered through a figure-eight magnetic coil (70 mm standard coil, Magstim Co., Whitland, Dyfed, UK) that was connected to a Magstim Super Rapid stimulator (Magstim, Whitland, Dyfed, UK). The coil was oriented so that the induced electric current flowed in a posterior-anterior direction over the left motor cortex. It was placed tangentially to the scalp with the handle pointing backwards and laterally at a 45° angle away from the midline and perpendicular to the central sulcus. The optimal coil position was determined by moving it in 0.5 cm steps around the motor hand area of the left motor cortex where magnetic stimulation produced the largest motor evoked potentials (MEP) from the contralateral right thenar eminence muscle during relaxation (the “motor hot-spot”) (13). Stimulus intensities were expressed as a percentage of the subject’s resting motor threshold (RMT). The active motor threshold (AMT) was the minimum single pulse intensity with an MEP greater than 200 μV in more than 50% trials from the contralateral thenar eminence muscle during a sustained voluntary contraction of 20% maximum strength using visual feedback (13).
In this study, we used the original cTBS protocol (5). The patterns of cTBS consisted of a 20 s train of uninterrupted TBS with bursts of three pulses at 50 Hz being repeated every 200 ms (i.e., 5 Hz) for a total of 300 pulses. cTBS were applied over the left motor cortex and the stimulus intensity was at 80% of individual AMT.
EEG recording
Continuous EEG was recorded with a MRcompatible, EEG amplifier (SD MRI 32, Micromed, Treviso, Italy). Electrode montage and placement was performed according to the 10/10 system (14). The EEG was continuously recorded from 30 Ag/AgCl electrodes sites (Fp1, AF3, AF4, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, T3, C3, Cz, C4, T4, CP5, CP1, CP2, CP6, T5, P3, Pz, P4, T6, PO3, PO4, O1, O2). The reference electrode was placed at the AFz site, whereas the ground electrode was placed at the FCz site. To avoid electrical saturation of EEG channels that were induced by TMS, the EEG amplifier had a resolution of 22 bits with a range of ±25.6 mV. An anti-aliasing hardware bandpass filter was applied with a bandwidth between 0.15 and 269.5 Hz. EEG data were sampled at a frequency of 1024 Hz using the software package System Plus (Micromed, Treviso, Italy).
Experimental design
Subjects were tested in a quiet, dimly light room. They were seated in a comfortable armchair with eyes open, facing a computer screen. Each subject undertook a 40-min recording session that consisted of four blocks of 9’40’’ duration each. Block 0 (i.e., baseline) preceded the application of cTBS while the remaining three blocks followed the cTBS. Each block was comprised of five events: 1) a pause of 1’10”, 2) MEPs recording for 1’10”, 3) EEG recording at rest for 3’00”, where a stationary black fixation cross symbol (0.8° of visual angle) on a grey background was presented at the centre of the screen, 4) a brief pause of 20”, and 5) EEG recording during the execution of a choice reaction time task of 4’00” duration.
Choice reaction time task
In order to investigate the effects of cTBS on the execution of an active motor task, participants were asked to perform a motor choice reaction time task. On each trial, a target stimulus of an arrowhead—pointing either to the left or right—was presented in the centre of the computer screen. The participants were given 1500 ms to respond and were asked to respond as quickly and as accurately as possible. Visual feedback, which occurred over 300 ms, was subsequently provided to indicate whether the participants had achieved a correct response. There were a total of 96 trials in each block of the experiment. Half of the trials displayed a “compatible condition” and another half presented an “incompatible condition”. The duration of the reaction time task was 4’00” in each of the four experimental blocks. The performance was measured at block 0 (baseline) from 6’40” to 1’00” before cTBS, block one from 5’40” to 9’40”, block two from 15’20” to 19’20”, and block three from 25’00” to 29’00” after cTBS.
EEG data analyses
To demonstrate the cTBS-induced oscillations, coherence analyses of EEG data were analysed with commercial software (Vision Analyser, Brain Vision, Munich, Germany) followed by computation of event-related coherence (ERCoh). Coherence values were computed for four blocks of EEG at “rest” and “active” (during a motor reaction time task). A semi-automatic segment inspection-rejection procedure was applied to avoid muscular or ocular activity. Overall, the number of accepted segments for each block ranged between 47 and 81. A discrete Fast Fourier Transform (FFT) of blocks of 2048 data points was computed for all electrodes. Power spectra were estimated for all frequency bins between 0.5 and 40 Hz. Recordings were non-overlapping Hamming-windowed to control spectral leakage.
Coherence was calculated by selecting a combination of the C3 electrode (the nearest channel to the TMS coil position) with nine electrodes, thereby creating the following pairs of electrodes: C3-F3, C3-Fz, C3-F4, C3-C3, C3-Cz, C3-C4, C3-P3, C3-Pz and C3-P4 from the FFT power spectrum. The coherence values were calculated for each frequency bin, λ from 0.5 to 40 Hz (0.5 Hz of maximum bin width) according to equation (1) using commercial software (Vision Analyser, BrainVision, Munich, Germany).
Cohxy (λ) = |Rxy(λ)|2 = [|fxy|2 / (|fxx (λ)| |fyy (λ)|)] Equation (1)
Because coherence is the cross-correlation of two power spectra divided by the respective powers, it is already normalized by power within each subject. Broadband coherence changes were obtained by averaging the coherence values for the theta θ (4.0–7.5 Hz), low alpha α (8.0–9.5 Hz), mu μ (10.0–12.5 Hz), low beta β (13.0–19.5 Hz), and high beta β (20.0–30.0 Hz) frequency bands.
Coherence increments are expressed as positive values and coherence decrements are expressed as negative values. This protocol of ERCoh analyses has been previously used in TMS-EEG studies to assess the modulation of interregional functional connectivity of neural assemblies (15–17).
Statistical analyses
Data were analysed using SPSS for Windows version 21.0. Repeated measures analyses of variances (ANOVA) were used to compare ERCoh effects. Repeated measure ANOVA were performed for EEG at rest for each frequency band of θ (4–7.5 Hz), low α (8–9.5 Hz), μ (10–12.5 Hz), low β (13–19.5 Hz), and high β (20–30 Hz). Each ANOVA had a between-subject factor of group (two levels – active cTBS and sham cTBS), and three within-subject factors, which included block (three levels – block 1, 2 and 3 post cTBS) and the pairs of electrodes (nine levels – C3-F3, C3-Fz, C3-F4, C3-C3, C3-Cz, C3-C4, C3-P3, C3-Pz and C3-P4). For each ANOVA, the sphericity assumption was assessed with Mauchly’s test, and Greenhouse-Geisser epsilon adjustments for non-sphericity were applied where appropriate.
For any significant interaction, increasing or decreasing of functional connectivity between two EEG electrodes was defined to be significantly different if the following criteria were met when performing post-hoc analyses: (1) ERCoh values for each pair of electrodes were significantly different between the two groups using independent samples two-tailed t test, and (2) in order to establish the direction of the coherence effects, ERCoh values of those pairs of electrodes were significantly different from zero using a onesample two-tailed t-test in at least one group. For all statistics, P < 0.05 was considered significant.
Results
We analysed the ERCoh of the ANOVA for the main effects and interaction between the three experimental factors (group, block, and pairs of electrodes) after cTBS. We did not find significant results for θ (4–7.5 Hz), low α (8–9.5 Hz), μ (10–12.5 Hz), low β (13–19.5 Hz) and high β (20–30 Hz) frequency bands. Significant results were obtained for low β (13–19.5 Hz) brain rhythms at rest and high β (20–30 Hz) brain rhythms after the motor choice reaction time task.
Coherence changes in the low β band
The ANOVA of the ERCoh at rest showed significant interactions of Group x Pair of Electrodes [F4.6,109.9 = 4.88; p < .01; hp 2 = .17] and Group x Block x Pair of Electrodes [F7.2,173.5 = 3.06; p < .01; hp 2 = .11].Post-hoc comparisons of the two-way interaction “group x pair of electrodes” showed a significant difference in functional coupling for active cTBS versus sham in C3-Fz (–0.015 vs. 0.004%), C3–Cz (–0.006 vs. 0.016%), C3–P3 (–0.021 vs. 0.019%), and C3-Pz (–0.011 vs. 0.02%). Figure 1 illustrates the percentage of ERCoh modulation of the Group x Pair of Electrodes for low β at rest.
Figure 1.
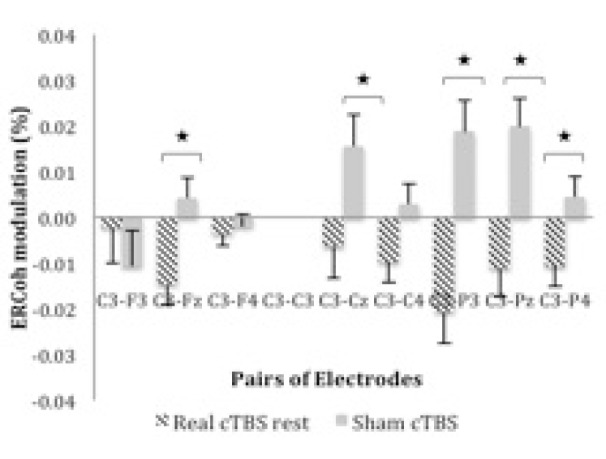
ERCoh low β as a function of Group and pairs of electrodes. The figure illustrates EEG synchronisation of C3-Fz, C3-Cz, C3-P3, C3-Pz, and C3-P4 pairs of electrodes for real cTBS compared to sham at rest.
*significant real cTBS rest vs sham (P < 0.05; Bonferroni corrected; n = 26).
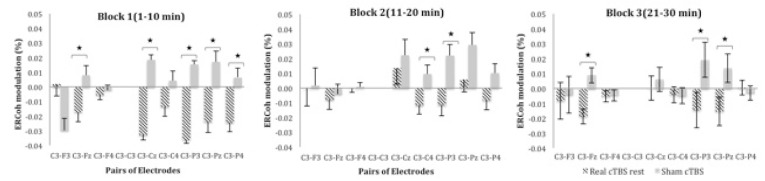
Post-hoc comparisons for the three-way interactions Group x Block x pairs of electrodes demonstrated a decrease in functional coupling for real cTBS when compared to sham cTBS at rest across all blocks of time [block one: C3-Fz (-0.017 vs. 0.008%), C3-Cz (-0.033 vs. 0.019%), C3-P3 (-0.036 vs. 0.015%), C3-Pz (-0.024 vs. 0.017%) and C3-P4 (-0.024 vs. 0.007%); block two: C3-C4 (-0.012 vs. 0.009%), C3-P3 (-0.011 vs. 0.022%); block three: C3-Fz (-0.019 vs. 0.009%), C3-P3 (-0.017 vs. 0.022%), C3-Pz (-0.015 vs. 0.014%)]. Figure 2 illustrates the percentage of ERCoh modulation of Group x Block x Pairs of Electrodes for low β at rest.
Figure 2.
ERCoh low β as a function of Group, Block and pairs of electrodes. The figure illustrates EEG synchronisation of several frontal-central-parietal pairs of electrodes for real cTBS compared to sham at rest across the three blocks of time.
*significant real cTBS rest vs sham (P < 0.05; Bonferroni corrected; n = 26).
Coherence changes in the high β band
The ANOVA of ERCoh at rest indicated non-significant interactions at rest but showed significant interactions of Group x Pair of Electrodes [F3.4,82.5 = 9.88; p < .001; hp 2 = .29] and Group x Block x Pair of Electrodes [F5.2,125.4 = 2.37; p < .05; hp 2 = .09] after the active motor task. Post-hoc comparisons of the two-way interaction Group x Pair of Electrodes showed a decrease in functional coupling for real cTBS versus sham in C3-Cz (-0.018 vs. 0.041%), C3-C4 (-0.009 vs. 0.029%), C3-P3 (-0.018 vs. 0.056%), C3-Pz (-0.029 vs. 0.04%) and C3-P4 (-0.02 vs. 0.034%) pairs of electrodes. Figure 3 illustrates the percentage of ERCoh modulation of Group x Pair of Electrodes for high β during the active motor task.
Figure 3.
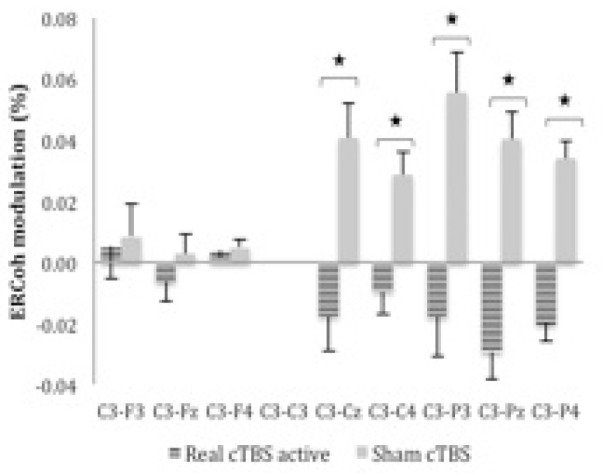
ERCoh high β as a function of Group and pairs of electrodes. The figure illustrates EEG synchronisation of C3-Cz, C3-C4, C3-P3, C3-Pz, and C3-P4 pairs of electrodes for real cTBS compared to sham during the active motor task.
*significant real cTBS rest vs. sham (P < 0 .05; Bonferroni corrected; n = 26).
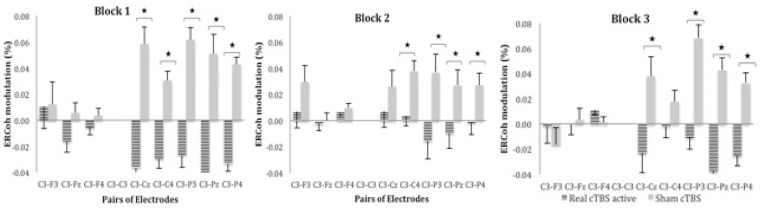
Post-hoc analyses for the significant three way interaction Group x Block x Pair of Electrodes demonstrated a decrease in functional coupling in real cTBS when compared to the sham group across the three blocks in the central-parietal pairs of electrodes: [block one: C3-Cz (-0.036 vs. 0.059%), C3-C4 (-0.03 vs. 0.03%), C3-P3 (-0.027 vs. 0.062%), C3-Pz (-0.041 vs. 0.051%) and C3-P4 (-0.033 vs. 0.043%); block two: C3-C4 (0.004 vs. 0.038%), C3-P3 (-0.016 vs. 0.037%), C3-Pz (-0.01 vs. 0.027%) and C3-P4 (-0.002 vs. 0.027%); block three: C3-Cz (-0.023 vs. 0.038%), C3-P3 (-0.011 vs. 0.068%), C3-Pz (-0.037 vs. 0.043%) and C3-P4 (-0.026 vs. 0.032%)]. Figure 4 illustrates the percentage of ERCoh modulation of Group x Block x Pair of Electrodes for high β during active motor task.
Figure 4.
ERCoh high β as a function of Group, Block and pairs of electrodes. The figure illustrates EEG synchronisation of the central-parietal pairs of electrodes for real cTBS compared to sham across the three blocks during the active motor task.
*significant real cTBS rest vs. sham (P < 0 .05; Bonferroni corrected; n = 26).
Discussion
The proposed mechanisms underlying synaptic plasticity induced by TBS are still debatable (18). Several researchers have highlighted the involvement of N-methyl-Daspartate (NMDA) receptors and brain-derived neurotrophic factor (BDNF) (19,20). Others have proposed the involvement of inhibitory gammaaminobutyric acid (GABA) neurotransmission after TBS perturbation of the motor cortex (21). Alternative mechanisms include TBS modulation on gene expression and protein levels (22). Although the mechanisms of TBS on the microor synaptic level are well understood, it remains unclear how TBS modulates the macro-level neuronal network, such as cortical oscillations (18). Investigations into the mechanisms of the macro-level neuronal network of cortical oscillations is important due to the increasing evidence that patients with neuropsychiatric illnesses have abnormal brain oscillations (23) and that non-invasive transcranial magnetic stimulation, such as the TBS protocol, has the potential to reverse the abnormal brain synchrony (24).
The present experiment was designed as an attempt to investigate how preconditioning the motor cortex with high frequency cTBS affects the oscillatory neural activity of remote cortical regions. We quantified the interregional coupling of remote brain regions using event related coherence (ERCoh), which reflects the spatialtemporal connection between two oscillatory signals (17). The electrodes are referenced to C3, the closest electrode to the left primary motor cortex, in order to investigate how cTBS modulates the cortico-cortical coherence of the motor network. The coherence analyses for the assessment of functional connectivity is a powerful tool to investigate the capability of the human cerebral cortex to integrate and transfer information within and between different areas (17). The coherence analyses of EEG signals within the motor system measures the presence of a correlation in neuronal oscillatory activity across different cortical regions in order to determine their involvement in the same functional motor network (25). In recent years, EEG coherence analysis has provided new insight into the neural basis of cognitive dysfunction in neuropsychiatric illnesses, such as Alzheimer’s disease, which has been shown to be associated with an impairment of functional connectivity between distant cortical areas when compared with healthy subjects (26). Furthermore, research has shown that there is the potential of improving symptoms of Parkinson's disease using TBS and EEG co-registration protocol (27).
The main finding of this study was that intrahemispheric and interhemispheric connectivity changes occur for at least 30 minutes after cTBS. In particular, we found a decrease in functional connectivity for real cTBS between the pre-conditioned left primary motor cortex and the distant areas of the motor network in the β brain rhythm for 30 minutes after the magnetic stimulation. This functional disconnection was mainly in the central-parietal electrodes of the low β rhythm at rest and high β band during the active motor task. In the present experiment, we found that β was the most sensitive frequency band that was modulated by cTBS both during rest and active states.
Physiologically, β oscillations are associated with motor activity and are cortically generated (28). Our results demonstrated a focal enhancement of β oscillations. A focal synchrony suggests a cortical origin (29), whilst a global synchrony indicates the involvement of deeper structures, such as the thalamus, through the thalamocortical networks (29). Previous studies, which analysed the interregional coherence to assess the neurophysiological processes underlying the performance during higher task demands, such as skilled or sequential finger movements, have found an increase of functional coupling mainly in the μ (10-12 Hz) and β frequency range (13-20 Hz) (30,31). Neural synchronization at these frequency bands have been suggested to be of importance for corticocortical and cortico-subcortical motor processing (32). Because β rhythm is mostly represented at the cortical level during awake and alert states of the brain, our findings support the hypothesis that TBS acts more on cortical levels rather than in deeper structures (33). Nevertheless, EEG reflects the activity of a large population of neurons (34), therefore we cannot exclude the influence of the thalamocortical network in generating β oscillations over the motor cortex.
Our results of decreased cortico-cortical coherence are consistent with the findings of previous investigations using high-frequency rTMS, which is commonly used to increase cortical excitability (35,36). We expected that the coherence results would be consistent with the findings from the conventional low frequency 1 Hz rTMS, which demonstrated an increase in EEG coherence after 1 Hz rTMS of subthreshold intensity over the left motor cortex (16,37). Our prediction was based upon the similarity of the cTBS paradigm and the effects of long-term depression in low frequency 1 Hz rTMS (5,6). Instead, our results showed a decrease in interregional functional connectivity or lower EEG coherence in remote cortical areas after stimulation over the motor cortex. Since, in principle, TBS is a high frequency magnetic stimulation (6), the decrease in functional coupling might be an anticipated outcome. Another possible explanation was that we calculated the event related coherence between C3, which was the closest electrode to the site of cTBS stimulation, and the other paired frontal, central and parietal electrodes. Therefore, the interregional decrease of connectivity may be mainly the result of the suppression of the left motor cortex, but not of other regions of the brain.
In the sham cTBS group, we have found a synchronisation of cortico-cortical connectivity between the primary motor cortex and the central-parietal cortex for high β band during the execution of a motor reaction time task. This was in opposition to the decreasing coherence between the same cortical regions after the perturbation that was produced by real cTBS. This result suggests that, in a perfectly functioning brain, the execution of a complex motor task was induced by increased connectivity between functionally connected cortical areas (38). Our coherence results revealed that cTBS could induce long-lasting alterations of interregional cortical oscillations with a functional disconnection between distant brain areas of the same motor neural network in healthy subjects. These changes of a distributed synchronization of interregional networks oscillations suggest that EEG could be used as a direct electrophysiological measure of plasticity of the motor system that is induced after theta burst magnetic stimulation (39).
Conclusion
In conclusion, here we show that the application of cTBS can induce reductions in the functional synchronization and organization of cortico-cortical oscillations, thereby making it a useful tool for understanding brain rhythms and their generation (40). Overall, our present work suggests a probable link between network oscillations and neuroplastic alterations in the human brain after TBS. Although, increased neuronal synchronisation might be associated with mechanisms of long-term depression, it is not possible to conclude this with certainty due to the limitation of inferences of EEG on the micro-level. Surface EEG will only record neural activity if there is synchronicity on a large scale underlying the electrode (41). Therefore, our result can only be interpreted on a macroscopic scale, but not on a micro-level, which cannot be computed with a scalp EEG. In particular, the field spread issue results in a wide representation of sources in many electrodes, which makes the interpretation of functional connectivity measures between pairs or electrodes difficult (41). Nevertheless, due to the rise of a neurotherapeutic protocol that makes use of theta burst stimulation (26,27), it is important to extend previous research using EEG to probe treatment efficacy and the mechanisms of synaptic plasticity post rTMS. Future studies of combined TBS/EEG should investigate the time course of cortical oscillations by cTBS beyond the 30 minutes temporal window.
Acknowledgments
The authors would like to thank University of Leicester for the permission of using the EEG lab.
Footnotes
Funds
None.
Conflict of interest
The authors confirm that they have no actual or potential conflicts of interests that could inappropriately influence or bias this work
Authors’ Contributions
Designed the experiments, contributed reagents/materials/analysis tools: GF, PM
Performed the experiments, analysed the data, wrote the paper: NAN,GF
References
- 1.Nevler N, Ash E. TMS as a Tool for Examining Cognitive Processing. Curr Neurol Neurosci Rep. 2015;15(8):1–11. doi: 10.1007/s11910-015-0575-8. doi: 10.1007/s11910-015-0575-8 . [DOI] [PubMed] [Google Scholar]
- 2.Miniussi C, Ruzzoli M, Walsh V. The mechanism of transcranial magnetic stimulation in cognition. Cortex. 2010;46(1):128–130. doi: 10.1016/j.cortex.2009.03.004. doi: 10.1016/j.cortex.2009.03.004 . [DOI] [PubMed] [Google Scholar]
- 3.Hallett M. Transcranial Magnetic Stimulation: A Primer. Neuron. 2007;55(2):187–199. doi: 10.1016/j.neuron.2007.06.026. doi: http://dx.doi.org/10.1016/j.neuron.2007.06.026 . [DOI] [PubMed] [Google Scholar]
- 4.Vallence AM, Goldsworthy MR, Hodyl NA, Semmler JG, Pitcher JB, Ridding MC. Inter- and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience. 2015;304:266–278. doi: 10.1016/j.neuroscience.2015.07.043. doi: http://dx.doi.org/10.1016/j.neuroscience.2015.07.043 . [DOI] [PubMed] [Google Scholar]
- 5.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. doi: 10.1016/j.neuron.2004.12.033 . [DOI] [PubMed] [Google Scholar]
- 6.Goldsworthy MR, Vallence A-M, Hodyl NA, Semmler JG, Pitcher JB, Ridding MC. Probing changes in corticospinal excitability following theta burst stimulation of the human primary motor cortex. Clin Neurophysiol. (InPress) doi: 10.1016/j.clinph.2015.06.014. doi: http://dx.doi.org/10.1016/j.clinph.2015.06.014 . [DOI] [PubMed] [Google Scholar]
- 7.Paulus W. Toward Establishing a Therapeutic Window for rTMS by Theta Burst Stimulation. Neuron. 2005;45(2):181–183. doi: 10.1016/j.neuron.2005.01.008. doi: http://dx.doi.org/10.1016/j.neuron.2005.01.008 . [DOI] [PubMed] [Google Scholar]
- 8.Wischnewski M, Schutter DJLG. Efficacy and Time Course of Theta Burst Stimulation in Healthy Humans. Brain Stimulation. 2015;8(4):685–92. doi: 10.1016/j.brs.2015.03.004. doi: http://dx.doi.org/10.1016/j.brs.2015.03.004 . [DOI] [PubMed] [Google Scholar]
- 9.Noh NA, Fuggetta G, Manganotti P, Fiaschi A. Long Lasting Modulation of Cortical Oscillations after Continuous Theta Burst Transcranial Magnetic Stimulation. PLoS ONE. 2012;7(4):e35080. doi: 10.1371/journal.pone.0035080. doi: 10.1371/journal.pone.0035080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAllister SM, Rothwell JC, Ridding MC. Cortical oscillatory activity and the induction of plasticity in the human motor cortex. Eur J Neurosci. 2011;33(10):1916–1924. doi: 10.1111/j.1460-9568.2011.07673.x. doi: 10.1111/j.1460-9568.2011.07673.x . [DOI] [PubMed] [Google Scholar]
- 11.Schindler K, Nyffeler T, Wiest R, Hauf M, Mathis J, Hess CW, et al. Theta burst transcranial magnetic stimulation is associated with increased EEG synchronization in the stimulated relative to unstimulated cerebral hemisphere . Neurosci Lett. 2008;436(1):31–34. doi: 10.1016/j.neulet.2008.02.052. doi: http://dx.doi.org/10.1016/j.neulet.2008.02.052 . [DOI] [PubMed] [Google Scholar]
- 12.Goldsworthy MR, Pitcher JB, Ridding MC. A comparison of two different continuous theta burst stimulation paradigms applied to the human primary motor cortex. ClinNeurophysio. 2012;123(11):2256–2263. doi: 10.1016/j.clinph.2012.05.001. doi: http://dx.doi.org/10.1016/j.clinph.2012.05.001 . [DOI] [PubMed] [Google Scholar]
- 13.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. doi: http://dx.doi.org/10.1016/j.clinph.2009.08.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. NeuroImage. 2007;34(4):1600–1611. doi: 10.1016/j.neuroimage.2006.09.024. doi: 10.1016/j.neuroimage.2006.09.024 . [DOI] [PubMed] [Google Scholar]
- 15.Azila Noh N, Fuggetta G. Human cortical theta reactivity to high-frequency repetitive transcranial magnetic stimulation. Hum Brain Mapp. 2012;33(9):2224–37. doi: 10.1002/hbm.21355. doi: 10.1002/hbm.21355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuggetta G, Pavone EF, Fiaschi A, Manganotti P. Acute modulation of cortical oscillatory activities during short trains of high-frequency repetitive transcranial magnetic stimulation of the human motor cortex: A combined EEG and TMS study. Hum Brain Mapp. 2008;29(1):1–13. doi: 10.1002/hbm.20371. doi: 10.1002/hbm.20371 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou W-C, She H-C, Lai K, Gramann K, Jung T-P. Temporal Dynamics and Cortical Networks Engaged in Biological Concepts Encoding. J Neurosci Neuroengineering. 2014;3(1):21–35. doi: 10.1166/jnsne.2014.1093 . [Google Scholar]
- 18.Cárdenas-Morales L, Nowak D, Kammer T, Wolf R, Schönfeldt-Lecuona C. Mechanisms and Applications of Theta-burst rTMS on the Human Motor Cortex. Brain Topogr. 2010;22(4):294–306. doi: 10.1007/s10548-009-0084-7. doi: 10.1007/s10548-009-0084-7 . [DOI] [PubMed] [Google Scholar]
- 19.Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118(5):1028–1032. doi: 10.1016/j.clinph.2007.01.021. doi: 10.1016/j.clinph.2007.01.021 . [DOI] [PubMed] [Google Scholar]
- 20.Edelmann E, Cepeda-Prado E, Franck M, Lichtenecker P , Brigadski T , Leßmann V . Theta Burst Firing Recruits BDNF Release and Signaling in Postsynaptic CA1 Neurons in Spike-Timing-Dependent LTP . Neuron. 2015;86(4):1041–1054. doi: 10.1016/j.neuron.2015.04.007. doi: http://dx.doi.org/10.1016/j.neuron.2015.04.007.v . [DOI] [PubMed] [Google Scholar]
- 21.Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. Theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res. 2009;199(3–4):411–421. doi: 10.1007/s00221-009-1961-8. doi: 10.1007/s00221-009-1961-8 . [DOI] [PubMed] [Google Scholar]
- 22.Aydin-Abidin S, Trippe J, Funke K, Eysel U, Benali A. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp Brain Res. 2008;188(2):249–261. doi: 10.1007/s00221-008-1356-2. doi: 10.1007/s00221-008-1356-2 . [DOI] [PubMed] [Google Scholar]
- 23.Schulman JJ, Cancro R, Lowe S, Lu F, Walton KD, Llinás RR. Imaging of thalamocortical dysrhythmia in neuropsychiatry. Front Hum Neurosci. 2011;5:69. doi: 10.3389/fnhum.2011.00069. doi: 10.3389/fnhum.2011.00069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuggetta G, Noh NA. A neurophysiological insight into the potential link between transcranial magnetic stimulation, thalamocortical dysrhythmia and neuropsychiatric disorders. Exp Neurol. 2013;245(87):95. doi: 10.1016/j.expneurol.2012.10.010. doi: http://dx.doi.org/10.1016/j.expneurol.2012.10.010 . [DOI] [PubMed] [Google Scholar]
- 25.Plewnia C, Rilk AJ, Soekadar SR, Arfeller C, Huber HS, Sauseng P, et al. Enhancement of long-range EEG coherence by synchronous bifocal transcranial magnetic stimulation. Eur J Neuroscience. 2008;27(6):1577–1583. doi: 10.1111/j.1460-9568.2008.06124.x. doi: 10.1111/j.1460-9568.2008.06124.x . [DOI] [PubMed] [Google Scholar]
- 26.Hsiao F-J, Chen W-T, Wang Y-J, Yan S-H, Lin Y-Y. Altered source-based EEG coherence of resting-state sensorimotor network in early-stage Alzheimer's disease compared to mild cognitive impairment. Neurosci Lett. 2014;558:47–52. doi: 10.1016/j.neulet.2013.10.056. doi: http://dx.doi.org/10.1016/j.neulet.2013.10.056 . [DOI] [PubMed] [Google Scholar]
- 27.Eggers C, Günther M, Rothwell J, Timmermann L, Ruge D. Theta burst stimulation over the supplementary motor area in Parkinson’s disease. J Neurol. 2015;262(2):357–364. doi: 10.1007/s00415-014-7572-8. doi: 10.1007/s00415-014-7572-8 . [DOI] [PubMed] [Google Scholar]
- 28.Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neuroscience. 2009;29(24):7679–7685. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thut G, Miniussi C. New insights into rhythmic brain activity from TMS–EEG studies. Trends in Cogn Sci. 2009;13(4):182–189. doi: 10.1016/j.tics.2009.01.004. doi: http://dx.doi.org/10.1016/j.tics.2009.01.004 . [DOI] [PubMed] [Google Scholar]
- 30.Houweling S, van Dijk BW, Beek PJ, Daffertshofer A. Cortico-spinal synchronization reflects changes in performance when learning a complex bimanual task. NeuroImage. 2010;49(4):3269–3275. doi: 10.1016/j.neuroimage.2009.11.017. doi: http://dx.doi.org/10.1016/j.neuroimage.2009.11.017 . [DOI] [PubMed] [Google Scholar]
- 31.Klostermann F, Nikulin VV, Kühn AA, Marzinzik F, Wahl M, Pogosyan A, et al. Task-related differential dynamics of EEG alpha- and beta-band synchronization in cortico-basal motor structures. Eur J Neuroscie. 2007;25(5):1604–1615. doi: 10.1111/j.1460-9568.2007.05417.x. doi: 10.1111/j.1460-9568.2007.05417.x . [DOI] [PubMed] [Google Scholar]
- 32.Sauseng P, Klimesch W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci Biobehav Rev. 2008;32(5):1001–1013. doi: 10.1016/j.neubiorev.2008.03.014. doi: 10.1016/j.neubiorev.2008.03.014 . [DOI] [PubMed] [Google Scholar]
- 33.Grossheinrich N, Rau A, Pogarell O, Hennig-Fast K, Reinl M, Karch S, et al. Theta Burst Stimulation of the Prefrontal Cortex: Safety and Impact on Cognition, Mood, and Resting Electroencephalogram. Biol Psych. 2009;65(9):778–784. doi: 10.1016/j.biopsych.2008.10.029. doi: http://dx.doi.org/10.1016/j.biopsych.2008.10.029 . [DOI] [PubMed] [Google Scholar]
- 34.Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J NeuroEngineering Rehab. 2009;6(1):1–10. doi: 10.1186/1743-0003-6-7. doi: 10.1186/1743-0003-6-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brignani D, Manganotti P, Rossini PM, Miniussi C. Modulation of cortical oscillatory activity during transcranial magnetic stimulation. Hum Brain Mapp. 2008;():–;29(5):603–612. doi: 10.1002/hbm.20423. doi: 10.1002/hbm.20423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuggetta G, Fiaschi A, Manganotti P. Modulation of cortical oscillatory activities induced by varying single-pulse transcranial magnetic stimulation intensity over the left primary motor area: A combined EEG and TMS study. NeuroImage. 2005;27(4):896–908. doi: 10.1016/j.neuroimage.2005.05.013. doi: http://dx.doi.org/10.1016/j.neuroimage.2005.05.013 . [DOI] [PubMed] [Google Scholar]
- 37.Oliviero A, Strens LA, Di Lazzaro V, Tonali P, Brown P. Persistent effects of high frequency repetitive TMS on the coupling between motor areas in the human. Exp Brain Res. 2003;149(1):107–113. doi: 10.1007/s00221-002-1344-x. doi: 10.1007/s00221-002-1344-x . [DOI] [PubMed] [Google Scholar]
- 38.Ilmoniemi RJ, Kicic D. Methodology for combined TMS and EEG. Brain Topogr. 2010;22(4):233–248. doi: 10.1007/s10548-009-0123-4. doi: 10.1007/s10548-009-0123-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung SW, Rogasch NC, Hoy KE, Fitzgerald PB. Measuring Brain Stimulation Induced Changes in Cortical Properties Using TMS-EEG. Brain Stimul. 2015;8(6):1010–1020. doi: 10.1016/j.brs.2015.07.029. doi: http://dx.doi.org/10.1016/j.brs.2015.07.029 . [DOI] [PubMed] [Google Scholar]
- 40.Brownjohn PW, Reynolds JNJ, Matheson N, Fox J, Shemmell JBH. The Effects of Individualized Theta Burst Stimulation on the Excitability of the Human Motor System. Brain Stimul. 2014;7(2):260–268. doi: 10.1016/j.brs.2013.12.007. doi: http://dx.doi.org/10.1016/j.brs.2013.12.007 . [DOI] [PubMed] [Google Scholar]
- 41.Freeman WJ. Origin, structure, and role of background EEG activity. Part 4: Neural frame simulation. Clin Neurophysiol. 2006;117(3):572–589. doi: 10.1016/j.clinph.2005.10.025. doi: 10.1016/j.clinph.2005.10.025 . [DOI] [PubMed] [Google Scholar]