Abstract
CYP3A enzymes metabolize endogenous hormones and chemotherapeutic agents used to treat cancer, thereby potentially impacting drug effectiveness. Here we refined the genetic basis underlying the functional effects of a CYP3A haplotype on urinary estrone glucuronide (E1G) levels and tested for an association between CYP3A genotype and outcome in patients with chronic lymphocytic leukemia (CLL), breast, or lung cancers. The most significantly associated single nucleotide polymorphism (SNP) was rs45446698, a SNP that tags the CYP3A7*1C allele; this SNP was associated with a 54% decrease in urinary E1G levels. Genotyping this SNP in 1,008 breast cancer, 1,128 lung cancer, and 347 CLL patients, we found that rs45446698 was associated with breast cancer mortality (hazard ratio [HR]=1.74, P=0.03), all-cause mortality in lung cancer patients (HR=1.43, P=0.009), and CLL progression (HR=1.62, P=0.03). We also found borderline evidence of a statistical interaction between the CYP3A7*1C allele, treatment of patients with a cytotoxic agent that is a CYP3A substrate and clinical outcome (Pinteraction=0.06). The CYP3A7*1C allele, which results in adult expression of the fetal CYP3A7 gene, is likely to be the functional allele influencing levels of circulating endogenous sex hormones and outcome in these various malignancies. Further studies confirming these associations and determining the mechanism by which CYP3A7*1C influences outcome are required. One possibility is that standard chemotherapy regimens that include CYP3A substrates may not be optimal for the approximately 8% of cancer patients who are CYP3A7*1C carriers.
Keywords: CYP3A7, breast cancer, lung cancer, chronic lymphocytic leukemia
INTRODUCTION
The CYP3A5, CYP3A7 and CYP3A4 genes, which form the cytochrome P450 3A (CYP3A) gene cluster at 7q22.1, encode enzymes that metabolise a diverse range of substrates (1). Specifically, in addition to a role in the oxidative metabolism of endogenous hormones, CYP3A enzymes metabolise around 50% of all clinically used drugs including many of the agents used in treating cancer (2). Of particular relevance to breast cancer, the hormonal agent tamoxifen, the alkylating agent cyclophosphamide, the taxanes, paclitaxel and docetaxel and the topoisomerase II inhibitor, mitoxantrone are all CYP3A substrates (1, 3). CYP3A genes are differentially regulated and substantial inter-individual differences in expression have been reported for all three genes (4). CYP3A4, the major isoform in adults, is predominantly expressed in the liver, where it is the most abundant P450, accounting for 30% of total CYP450 protein (5). CYP3A5 is “polymorphically” expressed, with approximately 33% of Europeans and 60% of African Americans expressing detectable levels in the adult liver (4). CYP3A7, the major isoform in the fetus, is generally silenced shortly after birth (6).
We have previously screened 642 single nucleotide polymorphisms (SNPs) tagging 42 genes involved in sex steroid synthesis or metabolism, and tested for association with premenopausal urinary estrone glucuronide (E1G) levels, measured in serial urine samples collected at pre-specified days of the woman’s menstrual cycle (7). We demonstrated that a rare haplotype, defined by two SNPs spanning the CYP3A gene cluster (rs10273424 and rs680055), was associated with a highly significant 32% difference in urinary E1G (7). Predicated on the assumption that genetically-determined effects on metabolism may impact on patient outcome, we have (i) refined the genetic basis for this association and (ii) examined the association between genotype and outcome in three cancers - breast and lung cancer and chronic lymphocytic leukaemia (CLL).
MATERIALS AND METHODS
Ethics
The study was conducted in accordance with the tenets of the Declaration of Helsinki and all patients provided written informed consent. Ethical approval for the study was obtained from the Royal Marsden NHS Trust.
Study Subjects - Fine mapping of the CYP3A locus
Full details of the 371 women from the British Breast Cancer (BBC (8)) and the 358 women from the Mammography Oestrogens and Growth Factors (MOG (9)) studies genotyped for this analysis have been published previously (7). Briefly they comprised premenopausal women who were first-degree relatives and friends of breast cancer cases (BBC study) or participants in the intervention arm of a trial of annual mammographic screening in young women (10) conducted in Britain (MOG study). To be eligible women had to be having regular menstrual cycles, not using hormone replacement therapy or oral contraceptives and not to have been diagnosed with breast cancer at recruitment to the study. All women had self-reported Northern European ancestry. To be included in the original analysis and this fine-mapping analysis women had to have provided serial urine samples, at pre-specified days of their menstrual cycle for measurement of creatinine adjusted urinary E1G. E1G was measured using an in-house enzyme-linked immunosorbent assay (ELISA; (7)).
Study Subjects - Risk analysis
Royal Marsden Hospital (RMH) Lifestyle and Family History study
The RMH Lifestyle and Family History study comprises 1,786 consecutive breast cancer patients who attended the RMH Breast Unit (Royal Marsden Hospital, Chelsea, London, UK) between June 2000 and January 2007. Women were invited to participate in the study by completing a questionnaire, consenting to access to medical records and providing a blood sample. We identified all women with self-reported White ethnicity for whom DNA was available (n=1536). We excluded: women born outside the British Isles (n=173, 11.3%), women not followed up at the RMH (n=96, 6.3%), secondary referrals where the pathology report from the referring hospital was missing or incomplete (n=97, 6.3%), cases presenting with metastatic disease (n=13, 0.8%), complex disease histories (n=8), atypical histology (n=6), women who had a prophylactic mastectomy (n=2) and cases unable to be traced (n=13). Finally we excluded 118 (7.7%) cases with non-invasive cancer leaving 1,010 cases for analysis. Two hundred and twenty-one patients died during follow up; for 159 (71.9%) cause of death was recorded as breast cancer.
GELCAPS lung cancer cases
Patients with lung cancer were ascertained through the Genetic Lung Cancer Predisposition Study (GELCAPS), a population-based study of lung cancer. Full details of the design and conduct of the study have been described previously (11). The current analysis is based on 1,142 patients from whom detailed clinico-pathological data and follow-up information had been collected using a standardised proforma. All cases were of self-reported White ethnicity and were United Kingdom residents.
UK Leukemia Research Fund CLL4 trial
We studied CLL patients entered in the UK Leukemia Research Fund CLL4 trial. Comprehensive details about the design and conduct of the trial have been published elsewhere (12). Briefly, CLL4 was a randomized phase III trial established to compare the efficacy of fludarabine, chlorambucil, and the combination of fludarabine plus cyclophosphamide as a first-line treatment for Binet stages B, C and A-progressive CLL. Age was not a criterion for entry into the study. Of the 777 patients entered into the trial the current analysis is based on a random subset of 356 patients of White Caucasian ethnicity who had blood samples taken for clinical diagnostic purposes and cell marker studies at participating centres.
SNP selection, imputation and genotyping
To fine map the 7q22.1 association signal for urinary estrone glucuronide levels(7) we used SNAP (13) to identify 184 SNPs that were correlated (r2 ≥ 0.1) with rs10273424 and rs680055, based on the CEU 1000 genomes (1KG) pilot data. We were able to design Sequenom plexes (Sequenom Inc, San Diego, USA) for 154 of these; we also included 7 SNPs genotyped as part of the original study(7) for monitoring of QC. Post genotyping we excluded 19 SNPs that failed genotyping and 4 SNPs for which the call rate was <95%, leaving 138 SNPs for analysis. The mean call rate for these 138 SNPs was 99.4% and genotypes of all SNPS were in accordance with Hardy-Weinberg equilibrium (i.e. P>0.05). Based on 25 (3.4%) duplicate samples concordance was 100% across the 138 SNPs.
To increase the density of our fine mapping, we imputed untyped genotypes using IMPUTE version 2.2 software with 1KG as the reference. Thresholding at an INFO score of ≥0.8, 725 additional SNPs and indels were successfully imputed resulting in a total of 863 variants for analysis in 727 samples.
To confirm imputed genotypes for the most significant SNP (rs45446698) and to test for association with patient outcome we genotyped rs45446698 by Taqman (Life Technologies, Paisley, UK). Call rates were 96.9% (fine-mapping and RMH Lifestyle and Family History study), 98.8% (GELCAPS) and 97.5% (CLL4). Concordance with imputed genotypes (fine-mapping) and between duplicate samples (cancer cases) was 100%.
Sequencing
We confirmed that rs45446698 was serving as a proxy for the CYP3A7*1C allele by Sanger sequencing a 370 bp PCR fragment including the 60 bp region that defines this allele in four common homozygotes, three heterozygotes and one rare homozygote (primer sequences available on request).
Statistical analysis
Fine-mapping
The percent change in hormone level per allele of each SNP was estimated by linear regression models of loge-transformed hormone levels. We used t tests of the regression coefficient to calculate P values for linear trend.
Risk analysis
We used Cox regression to test for an association between rs45446698 genotype and breast cancer specific survival (BCSS; RMH Lifestyle and Family History study) or progression-free survival (PFS; CLL4). For lung cancer (GELCAPS), where the prognosis is poor (5 year survival <10%), we used overall survival as a proxy for a disease-specific outcome. In the breast cancer series, to allow for differences in ascertainment between incident and prevalent cases, the time at risk began on the date of diagnosis but the time under observation began on the date of entry to the study (defined as receipt of blood sample) (14, 15). Time at risk ended on the date of death from breast cancer, or censoring (defined as date of last follow up or death from other causes). All cases were censored at 15 years after diagnosis on the basis that follow up was likely to be unreliable after this period. For GELCAPS cases, time at risk began on the date of diagnosis and ended on the date of death from any cause, last follow up or censoring. For the CLL4 cases, time at risk began at randomization to the trial and ended on date of progression, date of last follow up or censoring. Since rs45446698 has a minor allele frequency of 0.04, we combined rare homozygotes with heterozygotes and used a one degree of freedom (1df) test. To test for statistical interaction between genotype and each stratifying variable we compared models with and without interaction terms using likelihood ratio tests. The proportional hazards assumption was tested using Schoenfeld residuals. Statistical analyses were performed using R software, version 2.11 (R Foundation for Statistical Computing, Vienna, Austria) and STATA software, version 11.0 (College Station, TX, USA). All reported P-values are two-sided.
RESULTS
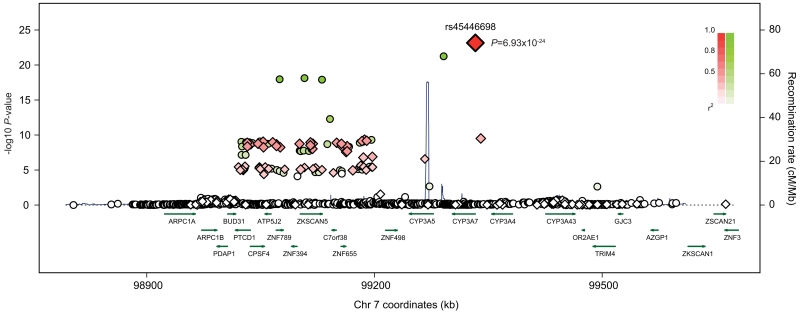
The most significantly associated SNP was rs45446698, which was associated with a 54% reduction in urinary estrone glucuronide (95% CI −61.0% to −47.7%, P=6.9x10−24; Figure 1, Supplementary Table S1). rs45446698 is one of seven highly correlated SNPs (rs11568824, rs45494802, rs45575938, rs45467892, rs11568825, rs11568826 and rs45446698) that cluster within the CYP3A7 promoter and comprise the CYP3A7*1C allele (4). Sequencing of the CYP3A7 promoter in four carriers of the rare rs45446698-C allele and four rs45446698-A common homozygotes confirmed that rs45446698 tags all seven base changes that define the CYP3A7*1C allele in these Northern European women (data not shown).
Figure 1. Regional association plot of the CYP3A locus at 7q22.1 (98,803,430-99,662,733).
Chromosome position is indicated on the x-axis, and -log10 P-value on the y-axis. Directly genotyped SNPs are represented as red diamonds, with the most significant SNP (rs45446698) indicated by a large red diamond. Imputed SNPs/indels are represented as green circles. The colour intensity of each diamond/circle reflects the extent of linkage disequilibrium with rs45446698 – red/green (r2 > 0.8) through to white (r2 < 0.2). The local recombination rate is plotted in blue. Physical positions are based on hg19.
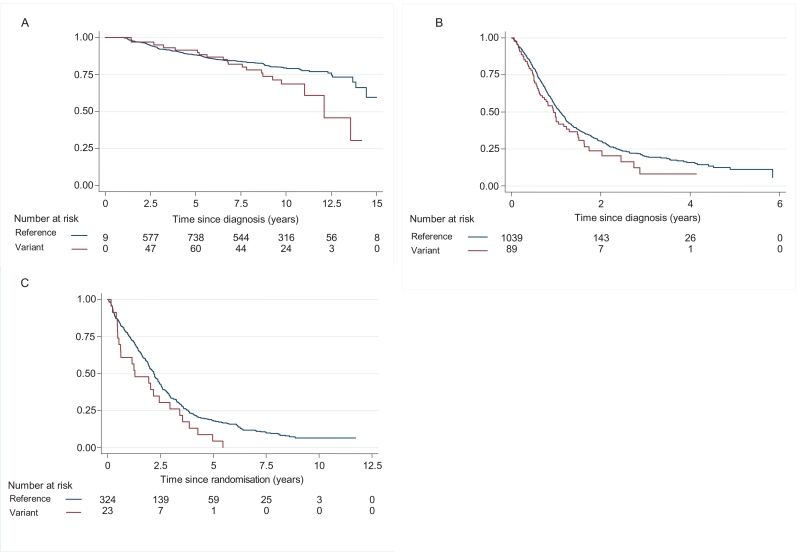
To assess rs45446698 as a potential marker of disease outcome, we genotyped 1,008 breast cancer patients participating in the RMH Lifestyle and Family History study (Methods; Table 1). For the majority of patient and tumour characteristics, there was no association with rs45446698 genotype. The exception was the number of positive lymph nodes; rs45446698-C carriers were more likely to have four or more positive nodes than rs45446698-A homozygotes (Table 1; P=0.003). In an unadjusted analysis, carrier status for rs45446698-C was associated with a 74% increase in breast cancer mortality (HR=1.74, 95% CI 1.08–2.82, P=0.02; Figure 2A). Restricting the analysis to the 889 individuals for whom we had complete data, and adjusting for established prognostic factors (age, tumour size, grade, positive nodes, vascular invasion) and radiotherapy did not alter this result (HR=1.74, 95% CI 1.05–2.90, P=0.03). Stratifying on estrogen receptor (ER) status showed no evidence that the association differed by ER-status (HR=1.86, 95% CI 1.03–3.34, P=0.04 and HR=1.70, 95% CI 0.55-5.24, P=0.35 for ER-positive and ER-negative disease respectively; Pheterogeneity=0.92).
Table 1. Characteristics of 1,008* breast cancer cases according to rs45446698 genotype.
| rs45446698 A:C + C:C n = 73 |
rs45446698 A:A n = 935 |
P-value# | ||
|---|---|---|---|---|
| Mean age | 55.0 | 56.1 | ||
| Age range | 27 - 82 | 24 - 89 | 0.44 | |
|
| ||||
| Tumor size group (cm) | < 2 | 44 (60.3) | 521 (55.7) | |
| 2-5 | 23 (31.5) | 363 (38.8) | ||
| 5+ | 6 (8.2) | 51 (5.5) | 0.35 | |
|
| ||||
| Grade | 1 | 11 (15.1) | 155 (16.6) | |
| 2 | 36 (49.3) | 435 (46.5) | ||
| 3 | 26 (35.6) | 345 (36.9) | 0.89 | |
|
| ||||
| ER status | Positive | 64 (87.7) | 789 (84.4) | |
| Negative | 9 (12.3) | 146 (15.6) | 0.45 | |
|
| ||||
| Positive nodes | 0 | 30 (41.1) | 498 (53.3) | |
| 1-3 | 17 (23.3) | 239 (25.6) | ||
| 4+ | 19 (26.0) | 115 (12.3) | ||
| N/A | 7 (9.6) | 83 (8.9) | 0.003 | |
|
| ||||
| Vascular invasion | Positive | 29 (39.7) | 315 (33.7) | |
| Negative | 41 (56.2) | 598 (64.0) | ||
| N/A | 3 (4.1) | 22 (2.3) | 0.24 | |
|
| ||||
| Surgery | Yes | 72 (98.6) | 923 (98.7) | |
| No | 1 (1.4) | 12 (1.3) | 0.95 | |
|
| ||||
| Radiotherapy | Yes | 68 (93.1) | 808 (86.4) | |
| No | 5 (6.9) | 124 (13.3) | ||
| N/A | 0 (0) | 3 (0.3) | 0.11 | |
|
| ||||
| Chemotherapy | Yes | 43 (58.9) | 481 (51.4) | |
| No | 30 (41.1) | 452 (48.3) | ||
| N/A | 0 (0) | 2 (0.2) | 0.23 | |
|
| ||||
| Tamoxifen | Yes | 58 (79.5) | 736 (78.7) | |
| No | 15 (20.5) | 195 (20.9) | ||
| N/A | 0 (0) | 4 (0.4) | 0.94 | |
|
| ||||
| Pyrs | 447 | 5625 | ||
| Events | 19 | 140 | ||
| Rate (per 1000 pyrs) | 42.5 (27.1-66.6) | 24.9 (21.1-29.4) | 0.02 | |
Excludes two women with missing genotype;
two-sided t test (age), chi-squared test (size, grade, ER status, positive nodes, vascular invasion, surgery, radiotherapy, chemotherapy, tamoxifen), log rank test (events).
Figure 2. Kaplan-Meier survival estimates based on (A) breast cancer specific mortality (1,008 breast cancer cases) (B) all cause mortality (1,128 lung cancer cases) and (C) progression (347 CLL cases), according to rs45446698 genotype.
Estimated survivor function (y-axis) is plotted against the time at risk (x-axis).The number of carriers of the reference (A) and the variant (C) alleles under observation at each time point are shown beneath the x-axis.
In this cohort of breast cancer patients, median time from diagnosis to enrolment was 1.7 years but the range was wide (0-12.3); excluding the 65 (7.3%) cases diagnosed more than 5 years prior to enrolment did not alter the HR estimate (HR=1.82, 95% CI 1.09-3.04, P=0.02) and stratifying on time from diagnosis to enrolment we found no evidence that the HR was biased by the inclusion of prevalent cases (HR=1.72, 95% CI 0.72-4.08; HR=2.03, 95% CI 1.06-3.89, for cases diagnosed <1 or ≥1 year before entry to the study, Pheterogeneity=0.76). There was, however, some evidence that the risk associated with rs45446698-C carrier status varied with time since diagnosis (test of the assumption of proportional hazards, P=0.01); stratifying the analysis into two time periods the hazard ratios were HR=1.14 (95% CI 0.53–2.47, P=0.74) and HR=4.46 (95% CI 1.98–10.04, P=0.0003) for the first (t < 7.5 year since diagnosis) and second (t ≥ 7.5 years since diagnosis) halves of the study, respectively.
To determine whether rs45446698 was associated with outcome for other site-specific cancers we genotyped the GELCAPS lung cancer cases (11) (Table 2) and the CLL4 trial series (12) (Table 3). There was no evidence that rs45446698 was associated with patient or disease characteristics. In GELCAPS, rs45446698-C carrier status was associated with a 26% increase in all causes mortality (HR=1.26, 95% CI 0.96-1.64, P=0.09; Figure 2B). After adjusting for standard prognostic factors (age, gender, stage and smoking status), surgery and radiotherapy the HR was 1.43 (95% CI 1.09-1.87, P=0.009) with no evidence that the association differed between small cell lung cancer (SCLC) and non-small cell lung cancer (NCLSC) (HR=1.93, 95% CI 1.18-3.16, P=0.009 and HR=1.25, 95% CI 0.90-1.74, P=0.19 for SCLC and NSCLC respectively, Pheterogeneity=0.16). In this cohort of lung cancer cases there was no evidence that the association varied with time since diagnosis (test of non-proportional hazards P=0.93). In the CLL series carrying the variant allele of rs45446698-C was associated with a 54% increased risk of progression (HR=1.54, 95% CI 1.00-2.36, P=0.05; Figure 2C). Adjusting for standard prognostic factors (age, gender and stage) altered this result only marginally (HR=1.62, 95% CI 1.05-2.50, P=0.03). In the 283 cases for whom 13q deletion and IGHV mutation status were available (HR=1.46, 95% CI 0.89-2.39, P=0.13), adjusting for 13q deletion (HR=1.46, 95% CI 0.89-2.38, P=0.13) or mutation status (HR=1.36, 95% CI 0.83-2.22, P=0.23) altered the result only marginally. In this cohort of CLL cases, there was no evidence that the association between genotype and disease free progression varied with time since diagnosis (test of non-proportional hazards P=0.78).
Table 2. Characteristics of 1,128* lung cancer cases according to rs45446698 genotype.
| rs45446698 A:C + C:C n = 89 |
rs45446698 A:A n = 1039 |
P-value# | ||
|---|---|---|---|---|
| Mean age | 62.8 | 64.8 | ||
| Age range | 32 - 81 | 26 - 88 | 0.08 | |
|
| ||||
| Gender | Male | 41 (46.1) | 435 (41.9) | |
| Female | 48 (53.9) | 604 (58.1) | 0.44 | |
|
| ||||
| Smoking | Never | 7 (7.9) | 72 (6.9) | |
| status | Ever | 82 (92.1) | 967 (93.1) | 0.74 |
|
| ||||
| Diagnosis | SCLC | 28 (31.5) | 245 (23.6) | |
| NSCLC (squamous) | 30 (33.7) | 383 (36.9) | ||
| NSCLC (adeno) | 23 (25.8) | 242 (23.3) | ||
| NCSLC (other) | 8 (9.0) | 169 (16.3) | 0.15 | |
|
| ||||
| Stage | 1 | 16 (18.0) | 223 (21.5) | |
| 2 | 10 (11.2) | 110 (10.6) | ||
| 3 | 32 (36.0) | 335 (32.2) | ||
| 4 | 31 (34.8) | 371 (35.7) | 0.83 | |
|
| ||||
| Surgery | Yes | 14 (15.7) | 179 (17.2) | |
| No | 75 (84.3) | 860 (82.8) | 0.72 | |
|
| ||||
| Radiotherapy | Yes | 24 (27.0) | 265 (25.5) | |
| No | 65 (73.0) | 774 (74.5) | 0.76 | |
|
| ||||
| Chemotherapy | Yes | 67 (75.3) | 745 (71.7) | |
| No | 22 (24.7) | 294 (28.3) | 0.47 | |
|
| ||||
| Pyrs follow up | 824 | 1151 | ||
| Events | 59 | 644 | ||
| Rate (per 1000 pyrs) | 715.8 (554.6-923.8) | 559.7 (518.1-604.7) | 0.09 | |
Excludes 14 women with missing genotype;
two-sided t test (age), chi-squared test (gender, smoking status, diagnosis, stage, surgery, radiotherapy, chemotherapy), log rank test (events).
Table 3. Characteristics of 347* CLL cases according to rs45446698 genotype.
| rs45446698 A:C + C:C n = 23 |
rs45446698 A:A n = 324 |
P-value# | ||
|---|---|---|---|---|
| Median age | 63.4 | 64.8 | ||
| Age range | 42 - 83 | 46 - 84 | 0.44 | |
|
| ||||
| Gender | Male | 18 (78.3) | 239 (73.8) | |
| Female | 5 (21.7) | 85 (26.3) | 0.64 | |
|
| ||||
| Stage | A | 2 (8.7) | 88 (27.2) | |
| B | 12 (52.2) | 139 (42.9) | ||
| C | 9 (39.1) | 97 (29.9) | 0.15 | |
|
| ||||
| 13q deletion | Deletion | 12 (52.2) | 186 (57.4) | |
| No deletion | 10 (43.5) | 115 (35.5) | ||
| N/A | 1 (4.4) | 23 (7.10) | 0.50 | |
|
| ||||
| IGHV Mutation status | Mutation | 6 (26.1) | 111 (34.3) | |
| No mutation | 12 (52.2) | 169 (52.2) | ||
| N/A | 5 (21.8) | 44 (13.6) | 0.60 | |
|
| ||||
| Pyrs follow up | 45 | 933 | ||
| Events | 23 | 298 | ||
| Rate (per 1000 pyrs) | 514.3 (341.8-773.9) | 319.4 (285.1-357.8) | 0.05 | |
Excludes 9 with missing genotype;
two-sided t test (age), chi-squared test (gender, stage, 13q deletion, mutation), log rank test (events).
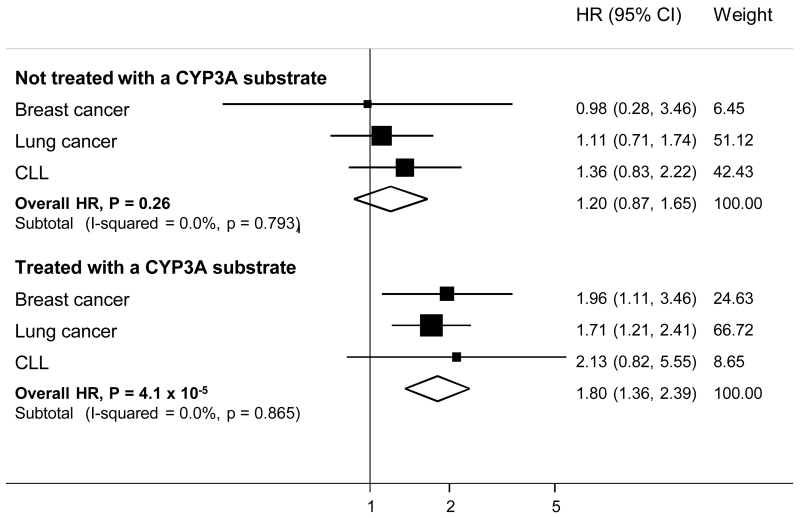
To determine whether the association between rs45446698 genotype and outcome was influenced by chemotherapy, specifically treatment with an agent that is a CYP3A substrate (Table 4), we carried out stratified analyses and tested for statistical interaction. Stratifying on treatment with tamoxifen, in the breast cancer series, there was no evidence that the association differed between strata; HRs were 1.68 (95% CI 0.90-3.13, P=0.10) and 1.89 (95% CI 0.69-5.17, P=0.21) for treated and non-treated, respectively (Pheterogeneity=0.89). Stratifying on treatment with a cytotoxic agent that is metabolised by a CYP3A enzyme (Table 4), the association between genotype and outcome appeared to be specific to patients who were treated with a CYP3A substrate (HR=1.96, 95% CI 1.11-3.45, P=0.02) compared to those who were not (HR=0.98, 95% CI 0.28-3.49, P=0.98) but this difference was not statistically significant (Pheterogeneity=0.57). Similarly, in the lung cancer study, the HR in cases who were treated with a CYP3A substrate was more extreme (HR=1.71, 95% CI 1.21-2.41, P=0.003) than in those who were not (HR=1.11, 95% CI 0.71-1.74, P=0.64) but with no evidence of statistical interaction (Pheterogeneity=0.24). In the CLL trial, the only chemotherapeutic agent that was a CYP3A substrate was cyclophosphamide (Table 4). Comparing cases who were or were not treated with cyclophosphamide, respective HRs for rs45446698-C carriers were HR=2.15 (95% CI 0.82-5.64, P=0.12) and HR=1.36 (95% CI 0.83-2.22, P=0.22; Pheterogeneity=0.52). Combining data across all three studies, the HR for rs45446698-C carriers who were treated with a cytotoxic agent that is a CYP3A substrate was 1.80 (95% CI 1.36-2.39; P=4.1 × 10−5) compared to an HR of 1.20 (95% CI 0.87-1.65; P=0.26; Figure 3) for those who were not, with borderline evidence of statistical interaction between treated and non-treated (Pheterogeneity=0.06) but no evidence of heterogeneity between studies (Pheterogeneity=0.79 and 0.87 for non-treated and treated, respectively).
Table 4. Chemotherapy regimens used in the treatment of breast cancer, lung cancer and CLL cases.
| Breast cancer (cytotoxic treatment)1 | N treated (%) |
|---|---|
| Doxorubicin, cyclophosphamide | 173 (17.2)2 |
| Cyclophosphamide, methotrexate, fluorouracil | 18 (1.8) |
| Epirubicin, cisplatin, fluorouracil | 13 (1.3)3 |
| Fluorouracil, epirubicin, cyclophosphamide | 202 (20.0)4 |
| Methotrexate, mitoxantrone | 93 (9.2)5 |
| Vinorelbine, epirubicin | 21 (2.1) |
| Other/not known | 6 (0.6) |
| No cytotoxic treatment | 482 (47.8) |
|
| |
| Total | 1,008 (100) |
|
| |
| Breast cancer (hormonal treatment)1 | |
|
| |
| Tamoxifen | 495 (49.1) |
| Tamoxifen, anastrozole | 128 (12.7) |
| Tamoxifen, letrozole | 171 (17.0) |
| Other/not known | 75 (7.4)6 |
| No hormonal treatment | 139 (13.8) |
|
| |
| Total | 1,008 (100) |
|
| |
| Lung cancer | |
|
| |
| Cisplatin/carboplatin, etoposide | 206 (18.3)7 |
| Cisplatin/carboplatin, vinorelbine/vincristin | 180 (16.0)8 |
| Cisplatin/carboplatin, vinorelbine/vincristin, mitomycin | 133 (11.8) |
| Cisplatin/carboplatin, gemcitabine | 194 (17.2) |
| Cisplatin/carboplatin, taxane | 28 (2.5) |
| Doxorubicin, cyclophosphamide, vinorelbine/vincristin | 33 (2.9) |
| Doxorubicin, cyclophosphamide, etoposide | 11 (1.0) |
| Other/not known | 27 (2.4) |
| No cytotoxic treatment | 316 (28.0) |
|
| |
| Total | 1,128 (100) |
|
| |
| CLL | |
|
| |
| Chlorambucil | 166 (47.8) |
| Fludarabine | 88 (25.4) |
| Fludarabine, cyclophosphamide | 93 (26.8) |
| No cytotoxic treatment | 0 (0) |
|
| |
| Total | 347 (100) |
Agents that are metabolised by a CYP3A enzyme are in bold.
for breast cancer, treatment with a cytotoxic agent and a hormonal agent were not mutually exclusive; 415 (41.2%) of the 1,008 cases were treated with both a cytotoxic agent and a hormonal agent.
includes 19 women also treated with a taxane,
includes one woman also treated with a taxane,
includes 20 women also treated with a taxane,
includes 14 women also treated with a taxane,
comprises 41 women who were treated with anastrozole, 29 who were treated with letrozole, 1 who was treated with both and 4 for whom treatment details are not known.
includes 4 individuals also treated with ifosfamide, and 1 who was also treated with both ifosfamide and vincristine,
includes 2 individuals also treated with ifosfamide.
Figure 3. Association of rs45446698 with disease specific mortality (breast cancer), all cause mortality (lung cancer) and progression (CLL), stratified by whether the patient’s treatment regimen included a cytotoxic agent that is metabolised by a CYP3A enzyme.
Horizontal lines represent 95% CIs. Square boxes represent cancer specific fixed-effects estimates. Diamonds represent the combined, fixed-effects estimates of the HRs and 95% CIs in each stratum. The vertical line represents the null effect (HR = 1.0).
DISCUSSION
Our data supports the CYP3A7*1C allele, tagged by rs45446698 as being the likely genetic basis for the association between the rs10273424-A rs680055-G haplotype and urinary estrone glucuronide levels (7). This allele has previously been associated with significantly reduced serum dehydroepiandrosterone sulfate (DHEAS) and estrone (E1) levels in men, providing independent support for our findings (16).
The CYP3A7*1C allele arose from a gene conversion event in which an approximately 60 bp region within the fetal CYP3A7 promoter was replaced with the equivalent region from the adult CYP3A4 gene (17). Comparing the variant CYP3A7*1C allele with the reference CYP3A7 allele, there are seven, highly correlated, single base changes all of which map to the CYP3A7 promoter and which result in expression of CYP3A7 in adult carriers of the CYP3A7*1C allele. Functional analyses demonstrated that two of these SNPs (rs11568825 and rs11568826) are necessary and sufficient for determining pregnane-X-receptor (PXR) dependent activation of CYP3A7 and four of the other five SNPs (rs11568824, rs45494802, rs45575938, rs45467892) influence constitutively activated receptor (CAR) mediated activation (18).
To our knowledge this is the first study to test for an association between the CYP3A7*1C allele and outcome in cancer patients. Genome-wide association studies of breast and lung cancer survival have been published (19-30); while none has reported an association with variants at the CYP3A locus only one, a recent meta-analysis from the Breast Cancer Association Consortium (BCAC), that combined data from nine breast cancer studies, has had power to detect moderate effects for variants with MAF<0.05 at genome-wide significance. The lack of association between rs45446698 and outcome in the BCAC meta-analysis (30) may reflect a relatively short mean duration of follow up with censoring of cases at 10 years after diagnosis. In our breast cancer data we found evidence of non-proportional hazards such that the association between the CYP3A7*1C allele and outcome varied with time since diagnosis with HRs of 1.14 and 4.46 at < 7.5 and ≥ 7.5 years after diagnosis, respectively. Replication of this finding in additional studies will be needed to determine whether this time-dependence is a chance finding, whether any such effect is specific to breast cancer, and whether it is influenced by the doses and combinations of chemotherapeutic agents that the patients received.
Due to the wide diversity of exogenous and endogenous substrates that are metabolized by the CYP3A enzymes, there are many different potential mechanisms by which CYP3A expression could influence disease outcome and we cannot at this juncture confirm or refute any particular mechanism. Possibilities include the CYP3A7*1C allele (i) associating with markers of disease prognosis (eg stage, grade or lymph node involvement) dependent on, or independent of, endogenous hormone levels or (ii) by influencing plasma clearance of chemotherapeutic agents that are CYP3A substrates. In support of a CYP3A allele influencing outcome through association with prognostic markers, association of the CYP3A4*1B allele with higher tumor-lymph node-metastasis and Gleason score has been reported for prostate cancer (31) and in a study of Ewing’s sarcomas, high expression of CYP3A4 was significantly associated with distant metastases (32). In this analysis we observed an association between the CYP3A7*1C allele and lymph node metastasis (≥4 positive nodes) in breast cancer cases. There was, however, no association between carrier status and disease stage for lung cancer or CLL and the association of CYP3A7*1C with adverse outcome for all three cancers remained after adjusting for established prognostic markers.
In support of the CYP3A7*1C allele influencing plasma clearance of chemotherapeutic agents, this allele was associated with adverse outcome across three site-specific cancers and, while these cancers have differing aetiologies and prognoses, the treatment regimens for all three include CYP3A substrates (Table 4). Consistent with an extensive body of evidence demonstrating that the efficacy of tamoxifen therapy cannot be predicted by CYP2D6 genotype (33, 34), we found no evidence of statistical interaction between the CYP3A7*1C allele, outcome and treatment with tamoxifen. For cytotoxic cancer drugs, there was consistency across the three studies; HRs in CYP3A7*1C carriers were more extreme in patients treated with a drug that was a CYP3A substrate and in the combined data there was some evidence of statistical interaction between the CYP3A7*1C allele, treatment with a cytotoxic agent that was a CYP3A substrate and outcome (Pheterogeneity=0.06). Further studies confirming the association of the CYP3A7*1C allele with adverse outcome and investigating the mechanism by which this allele may influence outcome are required.
There are several limitations to this analysis; the endpoints that we analyzed varied across the three different malignancies (disease-specific mortality (breast cancer), all cause mortality (lung cancer) and disease progression (CLL). While genotypes are effectively randomized at birth (35), and there was no association between being a carrier of the CYP3A7*1C allele and receiving chemotherapy (Table 1, P=0.23 and Table 2, P=0.47 for breast and lung cancers respectively), treatment was not randomized in the two observational studies. The frequency of CYP3A7*1C allele is just 4% (8% carriers); accordingly we were unable to analyze cases who were heterozygous or homozygous for the variant allele separately. The relative rarity of this allele and the lack of detailed information on drug doses and numbers of treatment cycles limited our ability to carry out meaningful subgroup analyses. We could not investigate whether the time-dependence of the association between the CYP3A7*1C allele and outcome that we observed in the breast cancer study depended on particular combinations of cancer drugs and we were unable to test for statistically significant interaction between outcome, the CYP3A7*1C allele and individual chemotherapeutic agents or specific treatment regimens. The pooled estimate of the increased risk in carriers of the CYP3A7*1C allele, treated with one or more cytotoxic agents that are CYP3A substrates (HR=1.80, P=4.1×10−5) represents a “weighted average” which may vary substantially between different treatment regimens and across cancer types. Finally, even in the combined data from all three of these retrospective studies, the evidence for statistical interaction between outcome, the CYP3A7*1C allele and the cytotoxic agents that are CYP3A substrates was weak (P=0.06). While this may reflect the heterogeneity of treatment regimens and a lack of power, we cannot exclude the possibility that the association between CYP3A7*1C and disease outcome is mediated by some other mechanism.
In conclusion, we have shown that the CYP3A7*1C allele, which results in the adult expression of the fetal CYP3A7 gene, is likely to be the functional allele that is associated with both lower levels of circulating endogenous sex hormones and adverse outcome in breast cancer, lung cancer and CLL. Our results require independent replication in larger studies, preferably with more detailed information on chemotherapy schedules and dosages, and across other cancer sites. However, one implication of our findings is that the doses and regimens of chemotherapeutic agents that provide optimal benefit for the average patient may not be optimal for the approximately 8% of cancer patients who are CYP3A7*1C carriers.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all the patients and control subjects for their participation. We thank the clinicians and other hospital staff, cancer registries, and study staff who contributed to the blood sample and data collection for the British Breast Cancer study, CLL4, GELCAPS, the Royal Marsden Lifestyle and Family History study and the Mammography Oestrogens and Growth Factors study.
Financial support: O. Fletcher, N. Orr and A. Ashworth received funding from Breakthrough Breast Cancer (recently merged with Breast Cancer Campaign forming Breast Cancer Now); R.S. Houlston and D. Catovsky received funding from Leukaemia and Lymphoma Research (LRF05001, LRF06002 and LRF13044) and Cancer Research UK (C1298/A8780 and C1298/A8362). R.S. Houlston, A. Matakidou and T. Eisen received funding from HEAL; T. Eisen received funding from Sanofi-Avensis; J. Peto and I. dos-Santos-Silva received funding from Cancer Research UK (C150/A5660 and C1178/A3947); F. Dudbridge received funding from the MRC (G1000718 and K006215); G. Ross received funding from the Cridlan Trust. All the authors acknowledge National Health Service funding to the NIHR Biomedical Research Centre and the National Cancer Research Network (NCRN).
Footnotes
The authors disclose no potential conflicts of interest.
REFERENCES
- 1.Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–91. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 2.Perera MA. The missing linkage: what pharmacogenetic associations are left to find in CYP3A? Expert Opin Drug Metab Toxicol. 2010;6:17–28. doi: 10.1517/17425250903379546. [DOI] [PubMed] [Google Scholar]
- 3.Westbrook K, Stearns V. Pharmacogenomics of breast cancer therapy: an update. Pharmacology & therapeutics. 2013;139:1–11. doi: 10.1016/j.pharmthera.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nature genetics. 2001;27:383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 5.Keshava C, McCanlies EC, Weston A. CYP3A4 polymorphisms--potential risk factors for breast and prostate cancer: a HuGE review. American journal of epidemiology. 2004;160:825–41. doi: 10.1093/aje/kwh294. [DOI] [PubMed] [Google Scholar]
- 6.Schuetz JD, Beach DL, Guzelian PS. Selective expression of cytochrome P450 CYP3A mRNAs in embryonic and adult human liver. Pharmacogenetics. 1994;4:11–20. doi: 10.1097/00008571-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Johnson N, Walker K, Gibson LJ, Orr N, Folkerd E, Haynes B, et al. CYP3A variation, premenopausal estrone levels, and breast cancer risk. Journal of the National Cancer Institute. 2012;104:657–69. doi: 10.1093/jnci/djs156. [DOI] [PubMed] [Google Scholar]
- 8.Johnson N, Fletcher O, Naceur-Lombardelli C, dos Santos Silva I, Ashworth A, Peto J. Interaction between CHEK2*1100delC and other low-penetrance breast-cancer susceptibility genes: a familial study. Lancet. 2005;366:1554–7. doi: 10.1016/S0140-6736(05)67627-1. [DOI] [PubMed] [Google Scholar]
- 9.Walker K, Fletcher O, Johnson N, Coupland B, McCormack VA, Folkerd E, et al. Premenopausal mammographic density in relation to cyclic variations in endogenous sex hormone levels, prolactin, and insulin-like growth factors. Cancer research. 2009;69:6490–9. doi: 10.1158/0008-5472.CAN-09-0280. [DOI] [PubMed] [Google Scholar]
- 10.Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L, et al. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomised controlled trial. Lancet. 2006;368:2053–60. doi: 10.1016/S0140-6736(06)69834-6. [DOI] [PubMed] [Google Scholar]
- 11.Eisen T, Matakidou A, Houlston R, Consortium G. Identification of low penetrance alleles for lung cancer: the GEnetic Lung CAncer Predisposition Study (GELCAPS) BMC cancer. 2008;8:244. doi: 10.1186/1471-2407-8-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catovsky D, Richards S, Matutes E, Oscier D, Dyer MJ, Bezares RF, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370:230–9. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 13.Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O’Donnell CJ, de Bakker PI. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–9. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham JE, Maranian MJ, Driver KE, Platte R, Kalmyrzaev B, Baynes C, et al. CYP2D6 gene variants: association with breast cancer specific survival in a cohort of breast cancer patients from the United Kingdom treated with adjuvant tamoxifen. Breast cancer research : BCR. 2010;12:R64. doi: 10.1186/bcr2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azzato EM, Greenberg D, Shah M, Blows F, Driver KE, Caporaso NE, et al. Prevalent cases in observational studies of cancer survival: do they bias hazard ratio estimates? British journal of cancer. 2009;100:1806–11. doi: 10.1038/sj.bjc.6605062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smit P, van Schaik RH, van der Werf M, van den Beld AW, Koper JW, Lindemans J, et al. A common polymorphism in the CYP3A7 gene is associated with a nearly 50% reduction in serum dehydroepiandrosterone sulfate levels. J Clin Endocrinol Metab. 2005;90:5313–6. doi: 10.1210/jc.2005-0307. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez FJ. The molecular biology of cytochrome P450s. Pharmacological reviews. 1988;40:243–88. [PubMed] [Google Scholar]
- 18.Burk O, Tegude H, Koch I, Hustert E, Wolbold R, Glaeser H, et al. Molecular mechanisms of polymorphic CYP3A7 expression in adult human liver and intestine. J Biol Chem. 2002;277:24280–8. doi: 10.1074/jbc.M202345200. [DOI] [PubMed] [Google Scholar]
- 19.Azzato EM, Pharoah PD, Harrington P, Easton DF, Greenberg D, Caporaso NE, et al. A genome-wide association study of prognosis in breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:1140–3. doi: 10.1158/1055-9965.EPI-10-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu XO, Long J, Lu W, Li C, Chen WY, Delahanty R, et al. Novel genetic markers of breast cancer survival identified by a genome-wide association study. Cancer research. 2012;72:1182–9. doi: 10.1158/0008-5472.CAN-11-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafiq S, Khan S, Tapper W, Collins A, Upstill-Goddard R, Gerty S, et al. A genome wide meta-analysis study for identification of common variation associated with breast cancer prognosis. PloS one. 2014;9:e101488. doi: 10.1371/journal.pone.0101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, Xu B, Yuan P, Miao X, Liu Y, Guan Y, et al. Genome-wide interrogation identifies YAP1 variants associated with survival of small-cell lung cancer patients. Cancer research. 2010;70:9721–9. doi: 10.1158/0008-5472.CAN-10-1493. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Ye Y, Rosell R, Amos CI, Stewart DJ, Hildebrandt MA, et al. Genome-wide association study of survival in non-small cell lung cancer patients receiving platinum-based chemotherapy. Journal of the National Cancer Institute. 2011;103:817–25. doi: 10.1093/jnci/djr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu L, Wu C, Zhao X, Heist R, Su L, Zhao Y, et al. Genome-wide association study of prognosis in advanced non-small cell lung cancer patients receiving platinum-based chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:5507–14. doi: 10.1158/1078-0432.CCR-12-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y, Yoon KA, Joo J, Lee D, Bae K, Han JY, et al. Prognostic implications of genetic variants in advanced non-small cell lung cancer: a genome-wide association study. Carcinogenesis. 2013;34:307–13. doi: 10.1093/carcin/bgs356. [DOI] [PubMed] [Google Scholar]
- 26.Han JY, Lee YS, Shin ES, Hwang JA, Nam S, Hong SH, et al. A genome-wide association study of survival in small-cell lung cancer patients treated with irinotecan plus cisplatin chemotherapy. The pharmacogenomics journal. 2014;14:20–7. doi: 10.1038/tpj.2013.7. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Wang L, Ye Y, Aakre JA, Pu X, Chang GC, et al. Genome-wide association study of genetic predictors of overall survival for non-small cell lung cancer in never smokers. Cancer research. 2013;73:4028–38. doi: 10.1158/0008-5472.CAN-12-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang S, Pan Y, Wang Y, Hu L, Cao S, Chu M, et al. Genome-wide association study of survival in early-stage non-small cell lung cancer. Annals of surgical oncology. 2015;22:630–5. doi: 10.1245/s10434-014-3983-0. [DOI] [PubMed] [Google Scholar]
- 29.Galvan A, Colombo F, Frullanti E, Dassano A, Noci S, Wang Y, et al. Germline polymorphisms and survival of lung adenocarcinoma patients: a genome-wide study in two European patient series. Int J Cancer. 2015;136:E262–71. doi: 10.1002/ijc.29195. [DOI] [PubMed] [Google Scholar]
- 30.Guo Q, Schmidt MK, Kraft P, Canisius S, Chen C, Khan S, et al. Identification of novel genetic markers of breast cancer survival. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebbeck TR, Jaffe JM, Walker AH, Wein AJ, Malkowicz SB. Modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. Journal of the National Cancer Institute. 1998;90:1225–9. doi: 10.1093/jnci/90.16.1225. [DOI] [PubMed] [Google Scholar]
- 32.Zia H, Murray GI, Vyhlidal CA, Leeder JS, Anwar AE, Bui MM, et al. CYP3A isoforms in Ewing’s sarcoma tumours: an immunohistochemical study with clinical correlation. International journal of experimental pathology. 2015 doi: 10.1111/iep.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lash TL, Lien EA, Sorensen HT, Hamilton-Dutoit S. Genotype-guided tamoxifen therapy: time to pause for reflection? The Lancet Oncology. 2009;10:825–33. doi: 10.1016/S1470-2045(09)70030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Binkhorst L, Mathijssen RH, Jager A, van Gelder T. Individualization of tamoxifen therapy: much more than just CYP2D6 genotyping. Cancer treatment reviews. 2015;41:289–99. doi: 10.1016/j.ctrv.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Davey Smith G. Capitalizing on Mendelian randomization to assess the effects of treatments. Journal of the Royal Society of Medicine. 2007;100:432–5. doi: 10.1258/jrsm.100.9.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.