Abstract
Objectives. To examine the association between a multibiomarker disease activity (MBDA) score, CRP and clinical disease activity measures among RA patients with and without concomitant FM.
Methods. In an observational cohort of patients with established RA, we performed a cross-sectional analysis comparing MBDA scores with CRP by rank correlation and cross-classification. MBDA scores, CRP and clinical measures of disease activity were compared between patients with RA alone and RA with concomitant FM (RA and FM) by univariate and multivariate analyses.
Results. CRP was ⩽1.0 mg/dl for 184 of 198 patients (93%). MBDA scores correlated with CRP (r = 0.755, P < 0.001), but were often discordant, being moderate or high for 19%, 55% and 87% of patients with CRP ⩽0.1, 0.1 to ⩽0.3, or 0.3 to ⩽1.0 mg/dl, respectively. Among patients with CRP ⩽1.0 mg/dl, swollen joint count (SJC) increased linearly across levels of MBDA score, both with (P = 0.021) and without (P = 0.004) adjustment for CRP, whereas CRP was not associated with SJC. The 28-joint-DAS-CRP, other composite measures, and their non-joint-count component measures were significantly greater for patients with RA and FM (n = 25) versus RA alone (n = 173) (all P ⩽ 0.005). MBDA scores and CRP were similar between groups.
Conclusion. MBDA scores frequently indicated RA disease activity when CRP did not. Neither one was significantly greater among patients with RA and FM versus RA alone. Thus, MBDA score may be a useful objective measure for identifying RA patients with active inflammation when CRP is low (⩽1.0 mg/dl), including RA patients with concomitant FM.
Keywords: biomarkers, C-reactive protein, disease activity, fibromyalgia, multibiomarker, RAPID3, rheumatoid arthritis
Rheumatology key message
Multibiomarker disease activity score frequently indicated elevated RA disease activity when CRP was normal.
Unlike clinically-based measures, multibiomarker disease activity scores were similar between RA patients with FM versus RA alone.
Multibiomarker disease activity score may objectively complement conventional RA disease activity measures when CRP is normal.
Introduction
Regular assessment of disease activity is critical for optimizing treatment outcomes for patients with RA [1, 2]. Measures that use joint counts, patient-reported outcomes and physician global assessment, are partially or entirely subjective and may not detect subclinical synovitis [3]. The acute phase reactants, CRP and ESR, while objective, are often normal for patients with clinically apparent synovitis and can be unreliable for estimating RA disease activity [4–7].
An objective tool is needed for assessing RA patients because their most common symptom, pain, may have inflammatory and non-inflammatory aetiologies. Non-inflammatory pain can confound clinical assessment and treatment decisions in RA [8–10]. Non-inflammatory pain may make RA disease activity appear worse than it really is, potentially leading to overtreatment with DMARDs. Conversely, RA disease activity may be underestimated if physicians incorrectly attribute signs or symptoms of RA to non-inflammatory aetiologies. Hence, an objective measure of RA disease activity that is more reliable than CRP may be useful when assessing RA patients with concomitant FM, a chronic condition of widespread non-inflammatory pain found in 12–21% of patients with RA [11–14].
The multibiomarker disease activity (MBDA) test objectively quantifies disease activity in patients with RA. It measures the serum concentrations of 12 biomarker proteins, including CRP, to produce a score that represents RA disease activity on a scale of 1–100 [15]. The MBDA score correlates with the 28-joint DAS-CRP (DAS28-CRP) and other composite measures of RA disease activity [15–18].
The MBDA test, available to physicians in the USA since 2010, has been validated in multiple RA cohorts, including patients treated with non-biologic and biologic DMARDs and patients who are seropositive or seronegative [15]. A modified version of the MBDA score, with no CRP component, was shown to correlate with the DAS28-CRP, indicating the relevance of non-CRP biomarkers in the MBDA score [15, 17]. The MBDA score reflects pathologically meaningful disease activity, based on its association with risk for progression of radiographic joint damage for patients with established RA [19] and early RA [20, 21]. The MBDA score was a better predictor of risk for radiographic progression than the DAS28-CRP [19], DAS28-ESR, CRP and ESR [20]. It also discriminated risk for progression among patients in DAS28-CRP remission [19].
The primary aim of this study was to evaluate MBDA scores and CRP in patients from the Brigham Rheumatoid Arthritis Sequential Study (BRASS) to compare how they measure RA disease activity. The secondary aim was to understand the utility of the MBDA test in patients with RA and concomitant FM (RA with FM), for whom objective assessment is particularly needed.
Methods
The Brigham Rheumatoid Arthritis Sequential Study
BRASS is a prospective, observational cohort at the Brigham and Women’s Arthritis Center in Boston, MA, that, since 2003, has enrolled patients ⩾18 years old with RA confirmed by a board-certified rheumatologist. Most patients in BRASS had established RA when enrolled; ∼20% had recent-onset RA [22]. From September 2009 to September 2011, 208 of 594 total active participants in BRASS were enrolled in a substudy of the effects of widespread non-joint pain on patients with RA. Each substudy patient provided serum and was assessed for clinical RA disease activity and concomitant FM [23]. The BRASS study and this substudy were approved by the Partners Institutional Review Board, Boston. Informed consent was obtained from all patients at enrolment into the BRASS study and for this substudy, according to the Declaration of Helsinki.
Patient population and clinical assessments
The present cross-sectional analysis included the 198 of 208 BRASS patients in the substudy of non-inflammatory pain in RA who met the following criteria, as applied to the earliest possible substudy visit: non-missing data for DAS28-CRP component scores; non-missing data for the Widespread Pain Index described in the modified version of the preliminary 2010 diagnostic criteria for FM of the ACR [23]; and adequate frozen serum for MBDA testing. The present analysis used data from the first, second or third substudy visit for 177, 20 and 1 patient(s), respectively. Clinical data included: tender joint count (TJC), swollen joint count (SJC), patient global assessment (PGA) of disease activity within the last 24 h, physician global assessment of disease activity, patient assessment of pain within the last week and physical function from the Multidimensional Health Assessment Questionnaire (MDHAQ) and CRP.
The individual physical function score in the MDHAQ and the corresponding Routine Assessment of Patient Index Data 3 (RAPID3) score were excluded if fewer than seven of the 10 physical function questions had been answered. Function scores based on 7, 8 or 9 questions answered were normalized by dividing by the number of questions answered and multiplying by 10. PGA, physician global assessment and pain were based on 10-point numeric rating scales (NRSs). DAS28-CRP, Simple Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI) and RAPID3 were calculated according to established methods, using the same NRS measurement tool for PGA [24–27].
Diagnosis of FM
FM was diagnosed according to the modified ACR 2010 Preliminary Diagnostic Criteria [23], which specify that individuals must have either: Widespread Pain Index ⩾7 and Symptom Severity Score ⩾5, or 2); Widespread Index 3–6 and Symptom Severity Score ⩾9. The Widespread Pain Index sums the number of painful areas (0–19). A modified Symptom Severity Score was calculated using the MDHAQ Fatigue Scale to assess fatigue; the Medical Outcomes Study Sleep Scale to assess waking refreshed; a neurological screen for concentration, memory and word-finding problems to assess cognitive symptoms; and patient self-report of abdominal pain, headaches and depression to assess somatic symptoms. Fatigue, waking refreshed and cognitive symptoms used 0–3-point scales. Abdominal pain, headaches and depression were scored 1 if present and 0 if absent.
CRP assessment
CRP testing (except for the MBDA score) used a high-sensitivity immunoturbidometric assay performed on a Roche P Modular System (Roche Diagnostics, Indianapolis, IN) at Boston Children’s Hospital. The upper limit of normal was 0.5 mg/dl. Blood samples for CRP testing and MBDA testing were obtained at the same time.
MBDA score
One archived, de-identified, frozen serum sample per patient was tested in the clinical laboratory of Crescendo Bioscience (South San Francisco, CA), which is certified under the CMS Clinical Laboratory Improvement Amendments and accredited by the College of American Pathologists for determination of Vectra DA scores. An automated, multiplex, sandwich immunoassay (Meso Scale Discovery, Rockville, MD) measured concentrations of the 12 MBDA biomarkers [vascular cell adhesion molecule-1 (VCAM-1), epidermal growth factor (EGF), VEGF-A, IL-6, TNF receptor type 1 (TNF-R1), matrix metalloproteinase-1 (MMP-1), MMP-3, YKL-40, leptin, resistin, serum amyloid A (SAA) and CRP]. Biomarker concentrations were combined in a validated algorithm to generate an integer score on a scale of 1–100 [16]. The MBDA categories, low (<30), moderate (30–44) and high (>44), were previously determined by translating the DAS28-CRP thresholds to the corresponding MBDA scores based on the linear relationship between the DAS28-CRP and the MBDA score [15]. The CRP measurement in the MBDA panel was used only for determination of the MBDA score.
Statistical analysis
Association between MBDA score and CRP for the entire cohort
A scatter plot of log-transformed CRP versus MBDA scores was constructed, and the Spearman rank correlation (r) was used to characterize strength of association. The relationship between defined categories of MBDA score (described above) and CRP (see below) was estimated by the Spearman rank correlation, using the exact conditional test for the Spearman correlation coefficient [28]. CRP cut-points were: 0.1 mg/dl (the median for all CRP values ⩽0.3 mg/dl in this cohort); 0.3 mg/dl (a threshold for cardiovascular risk [29]); and 1.0 mg/dl (the threshold in the ACR/EULAR Boolean definition of remission [4, 30, 31]).
Association between MBDA score and SJC for patients with CRP ⩽ 1.0 mg/dl
For patients with CRP ⩽1.0 mg/dl, box plots presented SJC within each category of MBDA score. The relationships between SJC and MBDA scores and between SJC and CRP were evaluated separately, in univariate analyses, and jointly, in multivariate analyses, by negative binomial regression [32]. Two models were used, one with MBDA scores and/or CRP included as ordinal scores, and another, with MBDA scores and/or CRP included as continuous scores. Modelling SJC as a function of ordinal scores facilitates testing for linear trend across the three increasing levels of MBDA score or CRP.
Comparison of disease activity between patients with RA and FM and RA alone for the entire cohort
Composite disease activity scores, their component measures, and MBDA scores were compared between patients with RA and FM versus RA alone using: t-tests or Wilcoxon rank sum tests for unadjusted analyses; and multivariate analyses, adjusting for non-redundant variables that differed between patients with RA and FM and RA alone. Multivariate analyses used least-squares linear regression (for log10 CRP), rank-based linear regression (for MBDA score, DAS28-CRP, SDAI, CDAI, RAPID3, PGA, physician global assessment, pain and physical function) [33, 34], or negative binomial regression (for SJC and TJC). Rank-based and negative binomial regressions were employed for multivariate analyses of disease activity measures when the distribution of the disease activity measures did not meet the assumptions (i.e. normally distributed errors) of ordinary least-squares regression [32]. Cumulative probability plots were used to compare the distributions of MBDA scores, disease activity composite scores and component measures for RA patients stratified according to whether they had FM.
No missing data were imputed, except for physical function and RAPID3 scores. All statistical tests were evaluated at the 0.05 two-sided significance level, without adjustments for multiple hypothesis testing.
Results
Patients evaluated
Demographic and clinical characteristics of the 198 patients were consistent with long-standing RA (Table 1). Non-biologic and biologic DMARDs were used by 62% and 61%, respectively, with 34% using both in combination. Significant differences were found between patients with RA and FM (n = 25) versus RA alone (n = 173) only for Body Mass Index (BMI), current methotrexate use, current non-biologic DMARD use and current use of neither a biologic nor non-biologic DMARD (Table 1).
Table 1.
Baseline characteristics
| All patients, N = 198 | RA alone, N = 173 | RA with FM, N = 25 | P values | |
|---|---|---|---|---|
| Age, mean (s.d.), years | 58.1 (11.1) | 57.9 (11.4) | 58.9 (9.0) | 0.691a |
| Female, n (%) | 168 (85) | 146 (84) | 22 (88) | 0.773b |
| Duration of RA, mean (s.d.), years | 15.9 (9.2) | 15.9 (9.2) | 15.8 (9.4) | 0.969a |
| BMI, mean (s.d.), kg/m2 | 26.9 (5.7) | 26.5 (5.5) | 29.4 (6.6) | 0.024a |
| Positive RF, n (%)c,d | 124 (63) | 113 (66) | 11(46) | 0.059b |
| Positive anti-CCP antibody, n (%)c,e | 120 (62) | 106 (63) | 14 (56) | 0.495b |
| RA by 1987 ACR criteria, n (%) | 195 (98) | 171 (99) | 24 (96) | 0.334b |
| Taking biologic DMARD, n (%) | 120 (61) | 106 (61) | 14 (56) | 0.614b |
| Taking MTX, n (%) | 99 (50) | 92 (53) | 7 (28) | 0.019b |
| Taking prednisone, n (%) | 31 (16) | 27 (16) | 4 (16) | 1.000b |
| Taking non-biologic DMARD, n (%) | 122 (62) | 112 (65) | 10 (40) | 0.017b |
| Taking biologic DMARD and MTX, n (%) | 59 (30) | 53 (31) | 6 (24) | 0.498b |
| Taking biologic DMARD and non-biologic DMARD, n (%) | 67 (34) | 60 (35) | 7 (28) | 0.509b |
| Taking neither biologic nor non-biologic DMARD, n (%) | 24 (12) | 16 (9.2) | 8 (32) | 0.004b |
at-test or Wilcoxon rank-sum test.
bPearson chi-squared or Fisher’s exact test.
cMissing values were excluded from analysis.
dAll patients N = 196, RA N = 172, RA and FM N = 24.
eAll patients N = 193, RA N = 168, RA and FM N = 25.
Correlation between MBDA scores and CRP for the entire cohort
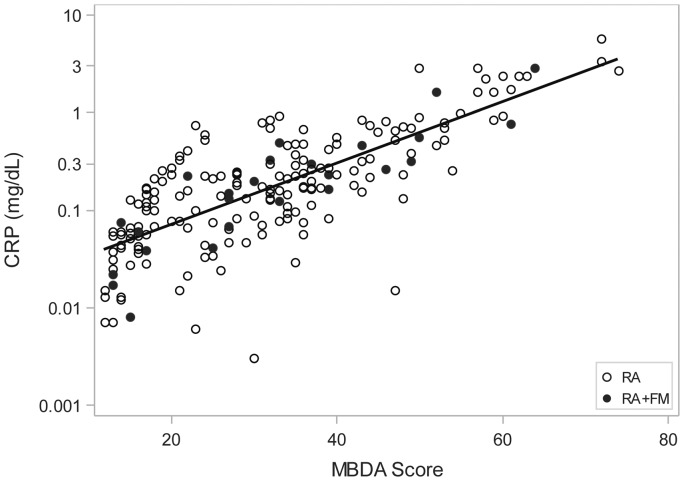
MBDA scores were low, moderate or high in 94 (47%), 67 (34%) and 37 (19%) of all 198 patients, respectively. A strong correlation was observed between MBDA scores and CRP values overall (r = 0.755) and for patients with (r = 0.890) or without concomitant FM (r = 0.734) (Fig. 1). Similar correlations were obtained when analysis was restricted to patients with SJC = 0 (r = 0.792, 0.936, 0.759 for all patients with SJC = 0, those with RA and FM, and those with RA alone, n = 86, 11, 75, respectively). CRP concentrations were ⩽1.0 mg/dl, ⩽0.3 mg/dl, or ⩽0.1 mg/dl in 184 (93%), 139 (70%) and 70 (35%) of 198 patients, respectively.
Fig. 1.
Scatter plot of multibiomarker disease activity scores and CRP values
Linear regression line is shown for 198 patients: 25 with RA and FM (closed circles) and 173 with RA alone (open circles). Spearman rank correlation coefficients were r = 0.755 for all 198 patients; r = 0.890 for patients with RA and FM; and r = 0.734 for patients with RA alone (all P < 0.001). MBDA: multibiomarker disease activity.
Discordance between MBDA scores and CRP in the entire cohort
Despite the strong correlation observed between MBDA score and CRP, MBDA scores spanned broad ranges for every level of CRP (Fig. 1). MBDA scores ranged from 12 to 47 (mean 20.1 ± S.D. 8.2) for patients with CRP ⩽0.1, 15–54 (mean 30.5 ± 9.2) for patients with CRP >0.1 to ⩽0.3 mg/dl and 21–61 (mean 40.9 ± 10.8) for patients with CRP 0.3 to ⩽1.0 mg/dl. MBDA scores were moderate or high in 19%, 55% and 87% of patients in these CRP categories, respectively, and in 49% of all patients with CRP ⩽1.0 mg/dl (Table 2). Patients with CRP >1.0 mg/dl all had high MBDA scores (range 50–74, mean 61.5 ± 7.2). The proportions of low, moderate or high MBDA scores appeared to be similar for patients with RA and FM versus RA alone (Table 2). These results indicate that MBDA scores were frequently elevated when CRP was ⩽1.0 mg/dl.
Table 2.
Patients stratified by MBDA score and CRP
| Complete study cohort: patients with RA and FM or RA alone, N = 198 | |||||
|---|---|---|---|---|---|
| CRP, mg/dl | |||||
| MBDA Score | ≤0.1, (n = 70) | >0.1 to 0.3 (n = 69) | >0.3 to 1.0 (n = 45) | >1.0 (n = 14) | All patients (N = 198) |
| <30 | 57 (81%) | 31 (45%) | 6 (13%) | 0 (0%) | 94 (47%) |
| 30–44 | 12 (17%) | 34 (49%) | 21 (47%) | 0 (0%) | 67 (34%) |
| >44 | 1 (1%) | 4 (6%) | 18 (40%) | 14 (100%) | 37 (19%) |
| Patients with RA and FM (N = 25) | |||||
| CRP, mg/dl | |||||
| MBDA score | ≤0.1 (n = 8) | >0.1 to 0.3 (n = 9) | >0.3 to 1.0 (n = 6) | >1.0 (n = 2) | All patients (N = 25) |
| <30 | 8 (100%) | 3 (33%) | 0 (0%) | 0 (0%) | 11 (44%) |
| 30–44 | 0 (0%) | 5 (56%) | 3 (50%) | 0 (0%) | 8 (32%) |
| >45 | 0 (0%) | 1 (11%) | 3 (50%) | 2 (100%) | 6 (24%) |
| Patients with RA alone (N = 173) | |||||
| CRP, mg/dl | |||||
| MBDA score | ≤0.1 (n = 62) | >0.1 to 0.3 (n = 60) | >0.3 to 1.0 (n = 39) | >1.0 (n = 12) | All patients (N = 173) |
| <30 | 49 (79%) | 28 (47%) | 6 (15%) | 0 (0%) | 83 (48%) |
| 30–44 | 12 (19%) | 29 (48%) | 18 (46%) | 0 (0%) | 59 (34%) |
| >44 | 1 (2%) | 3 (5%) | 15 (38%) | 12 (100%) | 31 (18%) |
Values in each cell, as n (%), represent the numbers and percentages of patients for that column. P < 0.001 for the association between the ordered categories of MBDA score and CRP using the exact conditional test for the Spearman correlation coefficient [29] for all patients (N = 198); for patients with RA and FM (N = 25); and for patients with RA alone (N = 173).
Detection of disease activity by MBDA score when CRP ⩽ 1.0 mg/dl
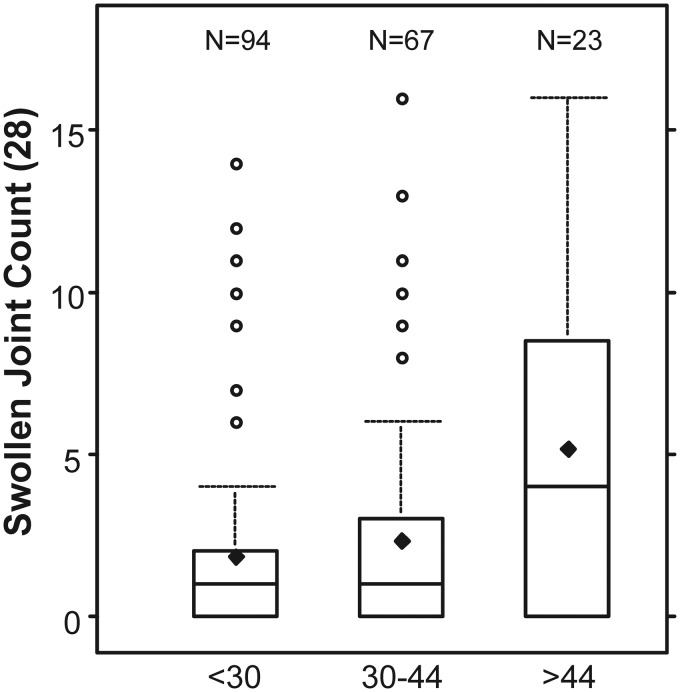
To evaluate whether the MBDA score distinguished between clinically discernable levels of RA disease activity when CRP was low, SJC results were analysed for the 184 patients with CRP ⩽ 1.0 mg/dl. SJC was chosen as a comparator because, lacking imaging data, SJC was the most objective non-blood test indicator of synovitis available. The distributions of SJC increased across the low, moderate and high categories of MBDA score with statistically significant correlation (Fig. 2). Univariate negative binomial regression analyses, using ordinal scores for MBDA score or CRP, demonstrated that SJC was significantly related to MBDA score, but not to CRP (Table 3). The association between SJC and MBDA scores was similar when determined with adjustment for BMI (regression coefficient = 0.52, P = 0.004) or without it (regression coefficient = 0.45, P = 0.004, Table 3). Multivariate negative binomial regression using ordinal scores demonstrated that the MBDA score was significantly associated with SJC after controlling for CRP (P = 0.021), but CRP was not associated with SJC after controlling for MBDA score (P = 0.847). Similar results were obtained with regression analyses using continuous scales for MBDA score and CRP (Table 3). These results indicate that, among patients with CRP ⩽1.0 mg/dl, elevated MBDA scores were independently associated with RA disease activity, as measured with SJC, but CRP was not.
Fig. 2.
Swollen joint counts as a function of multibiomarker disease activity score
SJC are shown for RA patients with CRP ≤1 mg/dl (N = 184), stratified by categories of MBDA score: low (<30), moderate (30–44) and high (>44). Boxes represent IQR. Whiskers extend to the most extreme observed value within 1.5 times the IQR from the median. Horizontal lines within boxes represent medians. Diamonds represent means. SJC increased linearly across the levels of MBDA score (P = 0.021), controlling for CRP in multivariate negative binomial regression analysis with ordinal scores for MBDA score and CRP [32]. MBDA: multibiomarker disease activity; SJC: swollen joint count; IQR: interquartile range.
Table 3.
Univariate and multivariate models of association of MBDA score and CRP with SJC
| Model | Variable | Regression coefficient | S.E. | LR Chi-square | P values |
|---|---|---|---|---|---|
| MBDA score, ordinal | Intercept | 0.10 | 0.29 | — | — |
| MBDA score | 0.45 | 0.16 | 8.11 | 0.004 | |
| CRP, ordinal | Intercept | 0.36 | 0.33 | — | — |
| CRP | 0.27 | 0.16 | 2.83 | 0.092 | |
| Multivariate, ordinal | Intercept | 0.06 | 0.35 | — | — |
| MBDA score | 0.43 | 0.19 | 5.32 | 0.021 | |
| CRP | 0.04 | 0.19 | 0.04 | 0.847 | |
| MBDA score, continuous | Intercept | −0.07 | 0.32 | — | — |
| MBDA score | 0.03 | 0.01 | 9.57 | 0.002 | |
| CRP, continuous | Intercept | 0.74 | 0.18 | — | — |
| CRP | 0.61 | 0.55 | 1.30 | 0.255 | |
| Multivariate, continuous | Intercept | −0.14 | 0.32 | — | — |
| MBDA score | 0.04 | 0.01 | 9.22 | 0.002 | |
| CRP | −0.64 | 0.66 | 0.94 | 0.332 |
Models used negative binomial regression and were for patients with CRP ≤ 1.0 mg/dl (N = 184). LR: likelihood ratio. MBDA: multibiomarker disease activity.
Disease activity measures for patients with RA and FM versus RA alone
In view of the discordances between MBDA scores and CRP, as well as evidence that MBDA score (but not CRP) was associated with SJC, we examined MBDA score, CRP and clinical measures of disease activity in a context where objective measures may be particularly needed by comparing the RA and FM (n = 25) versus RA alone (n = 173) groups. In univariate analyses, median CRP and MBDA scores were similar between the two groups (Supplementary Table S1, available at Rheumatology Online). By contrast, the RA and FM group had significantly greater median values, compared with the RA alone group, for DAS28-CRP (3.4 versus 2.6, P = 0.005), SDAI (17.1 versus 8.0, P < 0.001), CDAI (16.3 versus 8.0, P < 0.001) and RAPID3 (12.9 versus 5.3, P < 0.001).
PGA (P < 0.001), physician global assessment (P < 0.001), pain (P < 0.001) and physical function (P = 0.002) were all statistically significantly greater in the RA and FM group, compared with the RA alone group, in univariate analyses. Median values for each were 2.0-fold to 3.33-fold greater in the RA and FM group (Supplementary Table S1, available at Rheumatology Online). A significant between-group difference was observed for TJC (P = 0.041), but not for SJC (P = 0.378) (Supplementary Table S1, available at Rheumatology Online). Multivariate adjustment for non-redundant factors that were significantly different between the two groups (BMI and non-biologic DMARD use) produced similar results, except the P values for TJC (P = 0.153) were not statistically significant (Table 4). Cumulative probability plots demonstrated that the disease activity differences between groups were widespread and were not due to patients with atypical values (Supplementary Fig. S1, available at Rheumatology Online).
Table 4.
Multivariate analysis of disease activity measures for patients with RA alone or RA with FM
| RA alone |
RA and FM |
||
|---|---|---|---|
| Meana | Meana | P values | |
| MBDA score | 29.8 | 29.8 | 0.988 |
| DAS28-CRP | 2.6 | 3.2 | 0.008 |
| SDAI | 7.9 | 15.2 | <0.001 |
| CDAI | 7.6 | 15.0 | <0.001 |
| RAPID3b | 5.7 | 12.4 | <0.001 |
| Patient global, NRS | 1.9 | 4.7 | <0.001 |
| Physician global, NRS | 2.0 | 3.0 | <0.001 |
| Pain, NRS | 2.0 | 5.0 | <0.001 |
| Physical functionb | 1.3 | 2.2 | <0.001 |
| Tender joint count | 4.1 | 6.7 | 0.153c |
| Swollen joint count | 2.5 | 3.0 | 0.527c |
| CRP, mg/dl | 0.2 | 0.1 | 0.431 |
aMean values adjusted in multivariate analysis for non-redundant factors that were significantly different between the two groups (body mass index and non-biologic disease-modifying anti-rheumatic drug use).
bPhysical function scores were available for all 198 patients; values were normalized for nine patients with RA alone and one patient with RA and FM, based on answers being available for only 8 or 9 of the 10 questions for physical function.
cχ2 test for tender joint count and swollen joint count; all others t-statistic.
NRS: numeric rating scale; RAPID3: Routine Assessment of Patient Index Data 3; MBDA: multibiomarker disease activity; SDAI: Simple Disease Activity Index; CDAI: Clinical Disease Activity Index.
Discussion
In this cross-sectional study of patients with RA, we found that 93% had CRP ⩽1.0 mg/dl, and the MBDA score detected moderate or high disease activity in approximately half of them. Discordance between MBDA score and CRP was observed, even when CRP was ⩽0.3 mg/dl and whether or not patients had concomitant FM. The MBDA score appeared to reflect levels of RA disease activity when CRP was ⩽1.0 mg/dl because SJC values tended to be lowest in these patients when MBDA score was low and greatest when it was high. These results suggest that the non-CRP biomarkers in the MBDA test [16] were able to detect disease activity with greater sensitivity than CRP alone, especially when CRP was low.
Multiple factors may be considered when practitioners assess RA disease activity, including CRP. Our finding that the MBDA score was often elevated when CRP was low is clinically meaningful because low CRP values are common among patients with RA. In a study of patients evaluated at initial presentation of RA, prior to initiation of DMARD therapy, approximately half had normal CRP [6]. In a study of patients with long-standing RA, 75% had CRP ⩽1.0 mg/dl [4]. Among 9135 patients with clinically active RA in a US clinical practice registry, 70% of patients had normal CRP (⩽0.8 mg/dl) [35]. In our study, the high prevalence of low CRP values may reflect that most patients had established RA and were receiving treatment with non-biologic or biologic DMARDs. These studies and ours illustrate that CRP is often too low to be helpful for evaluating RA in clinical practice [35].
Among patients with CRP ⩽1.0 mg/dl, 13% had high MBDA scores. The MBDA score was significantly associated with SJC, with or without adjustment for CRP, whereas CRP was not associated with SJC. In a prior study of patients with established RA receiving DMARD therapy, high MBDA scores were associated with increased radiographic progression over the following year, even among patients in DAS28-CRP remission [19]. In a post hoc analysis of SWEFOT, 30% of patients with clinically active early RA had CRP ⩽1.0 mg/dl at baseline. Among these patients, rapid radiographic progression was restricted to those with a high MBDA score at baseline [20]. These studies suggest that a high MBDA score may reflect joint inflammation and increased risk for subsequent joint damage when conventional measures such as DAS28-CRP or CRP detect low RA disease activity. Other explanations, including comorbidities, such as infections, cancer or, cardiovascular disease, may also warrant consideration when MBDA score is high and clinical disease activity appears to be low, depending on the individual clinical context. Further studies are needed to evaluate the MBDA score in such patients.
We found no association between FM and MBDA score or CRP. By contrast, strong associations were observed between FM and elevation of clinical composite indices, related predominantly to elevations of their subjective component measures. Similar results have been reported for other studies of RA patients with and without FM, although none included MBDA scores [11, 13, 36–38]. Only one of these studies assessed physician global assessment, finding it to be approximately twice as great in patients with RA and FM, as in our study [36].
These findings, and ours, may be interpreted two ways. They may mean that the more subjective, non-laboratory measures overestimated RA disease activity in the RA and FM group, or that MBDA score and CRP underestimated disease activity in the RA and FM group. Prior cross-sectional studies support an overestimation by subjective measures, although none had a gold standard measure of disease activity [12, 14, 36–38]. These studies observed varied relationships between SJC and FM [12, 14, 37, 38]. The largest of the prior studies, like ours, found that SJC was not significantly greater in the RA and FM group [36]. A recent case-control study also found that ultrasound power Doppler and grey scale scores, as well as SJC, ESR and CRP, were not significantly different between patients with RA and FM versus RA alone [39].
A limitation of our study is that only 25 of the 198 patients had FM. This sample size is similar to those of other cross-sectional studies, where the FM subgroups comprised 12–49 patients (12% to 21%) of 100–270 total patients with RA [12, 14, 36–38]. Despite the limited size of the group with RA and FM, statistical significance was achieved for most comparisons of clinical measures between the RA and FM and RA alone groups, even after adjustment in multivariate analysis. By contrast, MBDA scores and CRP were not significantly different and had nearly identical probability distributions between groups.
This study lacked imaging data, precluding use of X-rays, ultrasound or MRI to evaluate discordances between MBDA score and CRP or SJC. Previous studies of patients with established RA [19] and early RA [20] provide evidence that the MBDA score is a better predictor of radiographic progression than DAS28-CRP [19] and CRP [20], including when they are discordant. Thus, the MBDA score may detect subclinical disease activity [19], and patients with low CRP and high MBDA score may have increased risk of progression [20]. Additional research is needed to evaluate these hypotheses.
Non-RA causes of inflammation may have influenced MBDA scores or CRP values, although such effects were probably minor because CRP concentrations were generally low. CRP gene polymorphisms [40] were not studied here. Their relevance in patients with RA is unclear [41, 42] and it seems unlikely that they would alter our findings. We did not examine the relationship between FM and non-CRP biomarkers, because they are not available for clinical use. Evidence regarding non-CRP inflammatory biomarkers in serum of individuals with FM is inconclusive [43, 44].
In summary, for patients with established RA, MBDA scores were frequently elevated when CRP was ⩽1.0 mg/dl. Among patients with CRP ⩽1.0 mg/dl, MBDA scores were significantly associated with greater SJC whereas CRP was not. The subgroup of patients with RA and FM had similar MBDA scores and CRP, compared with the RA alone group, but significantly greater values for DAS28-CRP and other clinical disease activity measures. Thus, the MBDA score may be a more sensitive, objective indicator of RA disease activity than CRP when CRP is low. This finding may have practical implications for RA patients with concomitant FM and low CRP. A high MBDA score in such patients may indicate the presence of inflammation, whereas a low MBDA score may suggest that pain is more likely due to a non-inflammatory cause.
Supplementary Material
Acknowledgements
The authors would like to thank the participants of BRASS. In addition, YL has received grant support from the National Institutes of Health [grant number R01AR064850], and BRASS has received financial support from Crescendo Bioscience, MedImmune and Bristol Myers Squibb.
Funding: Crescendo Bioscience funded the generation of biomarker data, statistical analysis and the formatting of the manuscript (by Arbor Communications, Inc.).
Disclosure statement: Y.C.L. has held stock in Cubist, Perrigo, Merck and Express Scripts within the past two years and has had grant support from Forest Research Institute. N.A.S. has received research grant support from Abbott (Abbvie), Amgen, Crescendo Biosciences, MedImmune, UCB, BMS, Questcor and Genentech. O.G.S. is an employee of Crescendo Bioscience, a wholly-owned subsidiary of Myraid Genetics, Inc. E.H.S. is employed by Crescendo Bioscience, a wholly-owned subsidiary of Myriad Genetics, Inc. and owns stock shares in Myriad Genetics, Inc. J.H. has received consulting fees from Crescendo Bioscience. M.E.W. has received grant support or consulting fees from Bristol Myers Squibb, Crescendo Bioscience and MedImmune. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Smolen JS, Landewé R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying anti-rheumatic drugs. Ann Rheum Dis 2010;69:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh JA, Furst DE, Bharat A. et al. 2012 Update of the 2008 American College of Rheumatology Recommendations for the Use of Disease-Modifying Antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Res Ther 2012;64:625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AK, Conaghan PG, Karim Z. et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 2008;58:2958–67. [DOI] [PubMed] [Google Scholar]
- 4.Graf J, Scherzer R, Grunfeld C, Imboden J. Levels of C-reactive protein associated with high and very high cardiovascular risk are prevalent in patients with rheumatoid arthritis. PLoS One 2009;4:e6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowson CS, Rahman MU, Matteson EL. Which measure of inflammation to use? A comparison of erythrocyte sedimentation rate and C-reactive protein measurements from randomized clinical trials of golimumab in rheumatoid arthritis. J Rheumatol 2009;36:1606–10. [DOI] [PubMed] [Google Scholar]
- 6.Sokka T, Pincus T. Erythrocyte sedimentation rate, C-reactive protein, or rheumatoid factor are normal at presentation in 35%–45% of patients with rheumatoid arthritis seen between 1980 and 2004: analyses from Finland and the United States. J Rheumatol 2009;36:1387–90. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe F. The many myths of erythrocyte sedimentation rate and C-reactive protein. J Rheumatol 2009;36:1568–9. [DOI] [PubMed] [Google Scholar]
- 8.Lee YC. Effect and treatment of chronic pain in inflammatory arthritis. Curr Rheumatol Rep 2013;15:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson ML, Svensson B, Bergman S. Chronic widespread pain in patients with rheumatoid arthritis and the relation between pain and disease activity measures over the first 5 years. J Rheumatol 2013;40:1977–85. [DOI] [PubMed] [Google Scholar]
- 10.Inanc N, Yilmaz-Oner S, Can M, Sokka T, Direskeneli H. The role of depression, anxiety, fatigue, and fibromyalgia on the evaluation of the remission status in patients with rheumatoid arthritis. J Rheumatol 2014;41:1755–60. [DOI] [PubMed] [Google Scholar]
- 11.Pollard LC, Kingsley GH, Choy EH, Scott DL. Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology 2010;49:924–8. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Hauser W, Hassett AL, Katz RS, Walitt BT. The development of fibromyalgia—I: examination of rates and predictors in patients with rheumatoid arthritis (RA). Pain 2011;152:291–9. [DOI] [PubMed] [Google Scholar]
- 13.Toms J, Soukup T, Bradna P, Hrncir Z. Disease activity composite indices in patients with rheumatoid arthritis and concomitant fibromyalgia. J Rheumatol 2010;37:468. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe R, Michaud K, Busch RE. et al. Polysymptomatic distress in patients with rheumatoid arthritis: understanding disproportionate response and its spectrum. Arthritis Care Res 2014; 66:1465–71. [DOI] [PubMed] [Google Scholar]
- 15.Curtis JR, van der Helm-van Mil AH, Knevel R. et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res 2012;64:1794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centola M, Cavet G, Shen Y. et al. Development of a multi-biomarker disease activity test for rheumatoid arthritis. PLoS One 2013;8:e60635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakker MF, Cavet G, Jacobs JWG. et al. Performance of a multi-biomarker score measuring rheumatoid arthritis diesase activity in the CAMERA tight control study. Ann Rheum Dis 2012;71:1692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirata S, Dirven L, Shen Y. et al. A multi-biomarker score measures rheumatoid arthritis disease activity in the BeSt study. Rheumatology 2013; 52:1202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Helm-van Mil AH, Knevel R, Cavet G, Huizinga TW, Haney DJ. An evaluation of molecular and clinical remission in rheumatoid arthritis by assessing radiographic progression. Rheumatology 2013;52:839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hambardzumyan K, Bolce R, Saevarsdottir S. Pretreatment multi-biomarker disease activity score and radiographic progression in early RA: results from the SWEFOT trial. Ann Rheum Dis 2015;74:1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markusse I, Dirven L, van den Broek M. et al. A multibiomarker disease activity score for rheumatoid arthritis predicts radiographic joint damage in the BeSt study. J Rheumatol 2014;41:2114–9. [DOI] [PubMed] [Google Scholar]
- 22.Iannaccone CK, Lee YC, Cui J. et al. Using genetic and clinical data to understand response to disease-modifying anti-rheumatic drug therapy: data from the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study. Rheumatology 2011;50:40–6. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe F, Clauw DJ, Fitzcharles MA. et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38:1113–22. [DOI] [PubMed] [Google Scholar]
- 24.Prevoo ML, van ’t Hof MA, Kuper HH. et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 25.Smolen JS, Breedveld FC, Schiff MH. et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 2003;42:244–57. [DOI] [PubMed] [Google Scholar]
- 26.Aletaha D, Nell VP, Stamm T. et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pincus T, Yazici Y, Bergman M, Swearingen C, Harrington T. A proposed approach to recognise “near-remission” quantitatively without formal joint counts or laboratory tests: a patient self-report questionnaire routine assessment of patient index data (RAPID) score as a guide to a “continuous quality improvement”. Clin Exp Rheumatol 2006;24(Suppl 43):S60–5. quiz S66-S73. [PubMed] [Google Scholar]
- 28.Agresti A. A survey of exact inference for contingency tables. Stat Sci 1992;7:131–77. [Google Scholar]
- 29.Pearson TA, Mensah GA, Alexander RW. et al. Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 30.Felson DT, Smolen JS, Wells G. et al. American College of Rheumatology; European League Against Rheumatism. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011;63:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med 2006;119:166.e17–28. [DOI] [PubMed] [Google Scholar]
- 32.Agresti A. Categorical data analysis, 2nd edn Hoboken (NJ): Wiley, 2002. [Google Scholar]
- 33.Hollander M, Wolfe DA. Nonparametric statistical methods, 2nd edn New York: J. Wiley, 1999. [Google Scholar]
- 34.Hettmansperger TP, McKean JW. Robust nonparametric statistical methods, 2nd edn Boca Raton, FL: CRC Press, 2011. [Google Scholar]
- 35.Kay J, Morgacheva O, Messing SP. et al. Clinical disease activity and acute phase reactant levels are discordant among patients with active rheumatoid arthritis: acute phase reactant levels contribute separately to predicting outcome at one year. Arthritis Res Ther 2014;16:R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranzolin A, Brenol JCT, Bredmeier M. et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, Health Assessment Questionnaire, and Short Form 36 scores in patients with rheumatoid arthritis. Arthritis Care Res 2009;61:794–800. [DOI] [PubMed] [Google Scholar]
- 37.Coury F, Rossat A, Tibeb A. et al. Rheumatoid arthritis and fibromyalgia: a frequent unrelated association complicating disease management. J Rheumatol 2009;36:58–62. [DOI] [PubMed] [Google Scholar]
- 38.Dhir V, Lawrence A, Aggarwal A, Misra R. Fibromyalgia is common and adversely affects pain and fatigure perception in North Indian patients with rheumatoid arthritis. J Rheumatol 2009;36:2443–8. [DOI] [PubMed] [Google Scholar]
- 39.da Silva Chakr RM, Brenol JC, Behar M. et al. Is ultrasound a better target than clinical disease activity scores in rheumatoid arthritis with fibromyalgia? A case-control study. PLoS One;2015;10:e0118620. doi: 10.1371/journal.pone.0118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhodes B, Merriman ME, Harrison A. et al. A genetic association study of serum acute-phase C-reactive protein levels in rheumatoid arthritis: implications for clinical interpretation. PLoS Med 2010;7:e1000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plant D, Ibrahim I, Lunt M. et al. Correlation of C-reactive protein haplotypes with serum C-reactive protein level and response to anti-tumor necrosis factor therapy in UK rheumatoid arthritis patients: results from the Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate cohort. Arthritis Res Ther 2012;14:R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ammitzboll CG, Steffensen R, Bogsted M. et al. CRP genotype and haplotype associations with serum C-reactive protein level and DAS28 in untreated early rheumatoid arthritis patients. Arthritis Res Ther 2014;16:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uceyler N, Hauser W, Sommer C. Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord 2011;12:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Behm FG, Gavin IM, Karpenko O. et al. Unique immunologic patterns in fibromyalgia. BMC Clin Pathol 2012;12:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.