Abstract
Objectives. This trial aimed to test the effectiveness of a wearable pulsed electromagnetic fields (PEMF) device in the management of pain in knee OA patients.
Methods. In this randomized [with equal randomization (1:1)], double-blind, placebo-controlled clinical trial, patients with radiographic evidence of knee OA and persistent pain higher than 40 mm on the visual analog scale (VAS) were recruited. The trial consisted of 12 h daily treatment for 1 month in 60 knee OA patients. The primary outcome measure was the reduction in pain intensity, assessed through VAS and WOMAC scores. Secondary outcomes included quality of life assessment through the 36-item Medical Outcomes Study Short-Form version 2 (SF-36 v2), pressure pain threshold (PPT) and changes in intake of NSAIDs/analgesics.
Results. Sixty-six patients were included, and 60 completed the study. After 1 month, PEMF induced a significant reduction in VAS pain and WOMAC scores compared with placebo. Additionally, pain tolerance, as expressed by PPT changes, and physical health improved in PEMF-treated patients. A mean treatment effect of −0.73 (95% CI − 1.24 to − 0.19) was seen in VAS score, while the effect size was −0.34 (95% CI − 0.85 to 0.17) for WOMAC score. Twenty-six per cent of patients in the PEMF group stopped NSAID/analgesic therapy. No adverse events were detected.
Conclusion. These results suggest that PEMF therapy is effective for pain management in knee OA patients and also affects pain threshold and physical functioning. Future larger studies, including head-to-head studies comparing PEMF therapy with standard pharmacological approaches in OA, are warranted.
Trial registration: ClinicalTrials.gov, http://www.clinicaltrials.gov, NCT01877278
Keywords: OA, pain, pain threshold, knee, clinical trial
Rheumatology key messages
Pulsed electromagnetic fields therapy is safe and effective in improving knee osteoarthritis symptoms.
Pain threshold increases after pulsed electromagnetic field therapy in knee osteoarthritis patients compared with placebo.
Introduction
OA affects a large proportion of the population, especially the elderly, leading to pain and disability [1]. Knee OA is the most common form of joint disease [2] and the major cause of pain and physical disability among middle-aged and elderly people [3]. To relieve pain, many patients, in order to avoid the side effects of long-term use of conventional therapies, are turning towards non-pharmacological therapies [4]. Several non-pharmacological interventions for OA are in different stages of development, investigation and application. Conservative and effective approaches for relieving pain are needed for knee OA patients and, among these, pulsed electromagnetic fields (PEMF) are emerging with promising results. In vitro studies have demonstrated that PEMF therapy is effective in reducing chondrocyte apoptosis and MMP-13 expression of knee cartilage in ovariectomized rats [5] and in favourably affecting cartilage homeostasis [6].
Nonetheless, data from human studies are contradictory [7–9], suggesting that further studies using different types of electromagnetic devices, treatment protocols and patient populations are warranted to confirm the efficacy of PEMF therapy in OA. A recent review, comprising 482 patients in the treatment group and 448 patients in the placebo group, highlighted that in trials employing high-quality methodology PEMF therapy was effective in reducing pain and improving function [10]. When the efficacy of PEMF was evaluated for function, a significant improvement was observed 8 weeks after initiation of treatment, and no significant association was found between the use of PEMF and the occurrence of adverse events.
The aim of the present study, therefore, was to evaluate the efficacy for reduction of pain intensity, measured by visual analog scale (VAS) and WOMAC, in patients affected by knee OA treated for 1 month with a wearable device using PEMF. The secondary aim was to evaluate the pain threshold, measured by an algometre, the improvements in quality of life and the changes in intake of NSAIDs/analgesics.
Methods
Patients
This randomized, with equal randomization (1:1), double-blind, placebo-controlled clinical trial, parallel group study, was approved by the ethics committee of the Faculty of Medicine at the University of Messina. The trial was performed in compliance with the Declaration of Helsinki and ICH-GCP. All patients provided their written informed consent. This trial was registered on clinicalTrials.gov (NCT01877278).
Eligibility criteria were: a diagnosis of primary OA of the knee according to the ACR criteria, including radiological evidence of OA [11]; age >40 years; symptomatic disease for at least 6 months prior to enrolment; persistent pain despite receiving the maximal tolerated doses of conventional medical therapy, including acetaminophen and/or an NSAID, with persistent pain defined as a minimal mean score of 40 mm on the VAS for global pain (0–100 mm range for each); daily pain during the month prior to study enrolment; ability to attend follow-up appointments; and no change in pain medication during the last month. Patients affected by secondary causes of OA, DIP joint OA, local or systemic infection, secondary FM, diabetes mellitus, systemic arthritis, coagulopathy, patients on anticoagulant therapy and patients who had received previous intra-articular steroid injection or with avascular necrosis of bone were excluded. The study took place at the rheumatology outpatient clinic of AOU G. Martino Policlinico Universitario of the University of Messina from June 2013 to December 2014.
Randomization and blinding
Both the placebo and the PEMF devices were provided by Bioelectronics Corporation. Before the randomization and blinding procedures, every device was tested through an electromagnetic field detector in order to allocate each device to the proper group.
Randomization and blinding of treatment was conducted by the research coordinator, which ensured similarity between preparations. Devices were consecutively numbered for each patient according to the randomization schedule. For allocation of the participants, a computer-generated list of random numbers was used.
An outcome assessor maintained the randomization codes in sealed envelopes, while another assessor, blinded to the randomization codes, dispensed the devices. Each patient was assigned an order number and received the device in the corresponding pre-packed envelope. Patients continued to remain blinded to the original treatment allocation. Outcome assessors and data analysts were kept blinded to group allocation of patients.
Treatment groups
Patients were randomly allocated to one of two treatment groups, either placebo or PEMF wearable device. Patients in the treatment group were given a PEMF wearable device (PEMF group). Patients in the placebo group were given a device with no electromagnetic properties (placebo group).
The device is manufactured by Bioelectronics Corporation, MD, USA (www.bielcorp.com), and is commercially available. The device used in the present study is a pulsed radiofrequency energy device (ActiPatch) that emits a safe form of non-ionizing electromagnetic radiation. The carrier frequency is 27.12 MHz, the assigned Federal Communications Commission medical frequency, and it has a pulse rate of 1000 Hz and a 100 µs burst width. The peak burst output power of the 12 cm antenna is ∼0.0098 W and covers a surface area of ∼103 cm2. The circuitry consists of low-voltage (3 V) digital/analog electronics that control all timing functions to produce the therapeutic radiofrequency field, with the antenna field placed directly above the therapeutic site. This closed-loop system of the antenna, low-energy signal generator circuit and battery power supply transfers the radiofrequency energy to the tissue. The placebo devices do not emit a radiofrequency electromagnetic field but are identical to the active devices, including a light-emitting diode light showing operation. The energy from the active device is not felt by the user, and the active device cannot be distinguished in any way from the placebo device.
Study procedures and assessments
Patients were trained in the use of the PEMF device, which was worn consecutively for a minimum of 12 h, mainly at night, with the antenna placed over the knee. The device was kept in place with a wrap and switched off when not in use. Patients were asked, during the enrolment phase, to record wear/hours per day and to report, at the end of the study, the hours per day of device use.
Study end points and outcome measures
Each patient was re-evaluated at 4 weeks, to assess the safety and efficacy of treatment, by an assessor who was blinded to the treatment. The primary end point for assessment of efficacy was set at 1 month. The primary outcome measure was the pain score improvement response to treatment from baseline to 1 month in the VAS and in WOMAC. In addition, in order to complete the core set of three primary efficacy variables, recommended by the Outcome Measures in Rheumatology Clinical Trials group [12], quality-of-life assessment [36-item Medical Outcomes Study Short-Form 36 version 2 (SF-36 v2)] was performed.
The secondary end point was to evaluate pain threshold measured by a pressure algometre applied on the anterior aspect of the thigh and at the DIP joint. The algometre consists of a mechanical digital pressure component carrying a sharp section, which could evoke a major painful stimulus. The device, powered by electricity, has a pressure-sensitive terminal connected to an electronic converter that records on a display in real time the amount of pressure in Bar as units of measurement (Wagner FPX 25 Algometer; Wagner Instruments; http://wagnerforce.com/). One rheumatologist, trained in quantitative sensory testing, performed all testing.
The pain threshold test was performed twice on the same day, with 2–5 min separating tests. The first test was designated as a trial run, to accustom participants to the testing procedures. The second test was designated as the test run, from which all data were obtained. The tests were performed on the same day to minimize heterogeneity caused by daily changes in environment, disease activity and mental status. Previous studies have indicated that pressure pain thresholds (PPTs) are highly reproducible when testing is done on the same day [13]. The pain threshold is defined as the pressure at which the participant first feels pain. The pain threshold was measured in two distinct anatomical areas, namely the DIP joint of the second finger and the anterior portion of the quadriceps muscle.
Another secondary outcome measure was to analyse the change in daily intake of NSAIDs per week at baseline and after 4 weeks of treatment. Patients reported analgesic and anti-inflammatory medications taken in the last week prior to each assessment.
Statistical analysis
Statistical analyses were performed using SPSS version 21. We used analysis of covariance on the post-intervention values to assess the group differences with P-values, mean difference and 95% CI. Baseline values were included as covariates. A value of P < 0.05 was considered to be statistically significant. It was calculated that a sample size of 66 (33 in each group, allowing for 10% withdrawals) was sufficient, with a power of 80% using a two-tailed test with α level of 0.05, to detect a 10-point difference in VAS, WOMAC total score and SF-36, set as primary outcomes of the study. Calculations were based on standard deviations data from Nelson et al. [14] (Pain VAS), Pipitone and Scott [15] (WOMAC total score) and Iannitti et al. [16] (SF-36).
Results
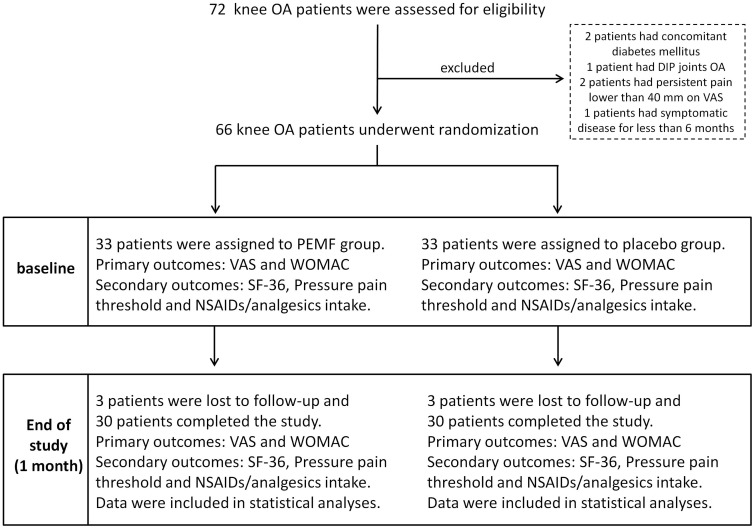
A total of 72 patients affected by knee OA were assessed for eligibility. Two patients with concomitant diabetes mellitus, one patient with concomitant DIP joint OA, two patients with persistent pain lower than 40 mm on the VAS and one patient with symptomatic disease for <6 months were not enrolled. Sixty-six patients were recruited into this study. Participants attended clinic visits at the time of randomization (baseline) and at 1 month for a total period of 1 month. Three patients from the PEMF group and three patients from the placebo group were lost to follow-up. Thus, each group comprised 30 (in the PEFM group) and 30 (in the placebo group) completers (see Fig. 1 for flow chart of participants). Baseline characteristics, such as, sex, age, BMI, duration of disease and outcome parameters are reported in Table 1. During the study, the rates of compliance with the different devices were similar. Patients from PEMF group reported an average use of 11.3 ± 0.8 h/day, whereas patients treated with the placebo device reported 11 ± 0.7 h/day. No statistically significant difference was observed in daily use of the devices between the two groups. No adverse events were detected during the study.
Fig. 1.
Flow chart of knee OA patients recruited in the trial
Among 72 eligible patients, 6 were excluded (diabetes mellitus, OA of DIP joints, pain duration <6 months, persistent pain lower than 40 on VAS). A total of 66 patients underwent randomization and 6 patients (3 for each group) were lost to follow-up. A total of 60 subjects, 30 for each group, completed the study. The primary outcomes, VAS and WOMAC, and the secondary outcomes, quality of life measured through the 36-item Medical Outcomes Study Short-Form version 2 (SF-36), pain pressure threshold, measured through a pressure algometer and intake of NSAIDs/analgesics, were assessed at baseline and after 1 month for statistical analysis.
Table 1.
Baseline demographic and clinical characteristics of patients affected by knee OA treated with pulsed electromagnetic fields or placebo device
| Characteristic | All patients (n = 60) | PEMF (n = 30) | Placebo (n = 30) |
|---|---|---|---|
| Age, mean (s.d.), years | 67.7 (10.9) | 68.6 (11.9) | 66.9 (10) |
| Gender (female/male) | 43/17 | 21/9 | 22/8 |
| BMI, mean (s.d.), kg/m2 | 27.4 (4.3) | 27.7 (4.6) | 27.1 (4.1) |
| Disease duration, mean (s.d.), years | 12.1 (8.2) | 12.4 (9.1) | 11.9 (7.4) |
| Pain score (100 mm VAS), mean (s.d.), mm | 65.3 (15.8) | 67 (16.6) | 63.6 (15.1) |
| WOMAC total score, mean (s.d.) | 132.9 (45.2) | 136.6 (49.6) | 129.2 (40.8) |
| SF-36 v2 physical health, mean (s.d.) | 52.1 (6.8) | 52 (7.4) | 52.2 (6.2) |
| SF-36 v2 mental health, mean (s.d.) | 41.1 (5.9) | 40.4 (5.8) | 41.8 (5.6) |
| DIP PPT, mean (s.d.) | 3.3 (1.3) | 3.4 (1.4) | 3.3 (1.2) |
| QDR PPT, mean (s.d.) | 12.3 (5.8) | 12.4 (6) | 12.4 (5.8) |
| NSAIDs, n (%) | 21 (35) | 10 (33) | 11 (36) |
| Analgesics, n (%) | 26 (43) | 12 (40) | 14 (46) |
PEMF: pulsed electromagnetic fields; PPT: pressure pain threshold; QDR: quadriceps femoris; SF-36 v2: 36-item Medical Outcomes Study Short-Form version 2; VAS: visual analog scale.
PEMF treatment reduced pain intensity and improved physical functioning
In the initial analysis, we sought to compare the primary outcome in the PEMF and placebo groups. In patients treated with the PEMF device, we found that VAS pain and WOMAC pain scores decreased significantly after 1 month of treatment compared with placebo. Consistently, WOMAC stiffness and function scores improved after PEMF treatment (Table 2).
Table 2.
Effect of electromagnetic field device therapy on pain and clinical status
| PEMF (n = 30) |
Placebo (n = 30) |
Estimated mean group difference (95% CI) | P-values | |||
|---|---|---|---|---|---|---|
| Outcomes | Baseline | 1 month | Baseline | 1 month | ||
| VAS, mean (s.d.) | 67 (16.6) | 50 (16.1) | 63.6 (15.1) | 61.3 (15) | −13.6 (−19.3 to − 7.9) | 0.0005 |
| WOMAC pain, mean (s.d.) | 28.2 (9.9) | 21.6 (9.6) | 27.6 (7.4) | 26.8 (8.2) | −5.6 (−8.4 to − 2.9) | 0.0005 |
| WOMAC function, mean (s.d.) | 97.6 (39.9) | 81.7 (37.9) | 91.2 (36.7) | 89.7 (34.4) | −13 (−23.3 to − 2.8) | 0.013 |
| WOMAC stiffness, mean (s.d.) | 10.8 (4.2) | 8.1 (3.8) | 10.4 (2.9) | 9.6 (3.1) | −1.7 (−2.9 to − 0.6) | 0.004 |
| WOMAC total, mean (s.d.) | 136.6 (49) | 111.5 (48) | 129.2 (40) | 126.2 (39) | −20.8 (−32.6 to − 8.9) | 0.001 |
| SF-36 v2, physical health, mean (s.d.) | 52 (7.4) | 55.8 (6.1) | 52.2 (6.2) | 53.1 (6.2) | 2.7 (0.3 to 5.2) | 0.024 |
| SF-36 v2, mental health, mean (s.d.) | 40.4 (5.8) | 43.8 (3.6) | 41.8 (6.0) | 43.6 (4.7) | 0.5 (−1.5 to 2.6) | 0.6 |
| DIP PPT, mean (s.d.) | 3.4 (1.4) | 4 (1.6) | 3.3 (1.2) | 3.4 (1.2) | 0.6 (0.1 to 1) | 0.015 |
| QDR PPT, mean (s.d.) | 12.4 (6) | 13.5 (6.2) | 12.3 (5.8) | 12 (5.3) | 1.4 (0.7 to 2.1) | 0.0005 |
Differences between the groups in post-intervention (1 month) values were evaluated with analysis of covariance, with baseline values as covariates.
PEMF: pulsed electromagnetic fields; PPT: pressure pain threshold; QDR: quadriceps femoris; SF-36 v2: 36-item Medical Outcomes Study Short-Form version 2; VAS: visual analog scale.
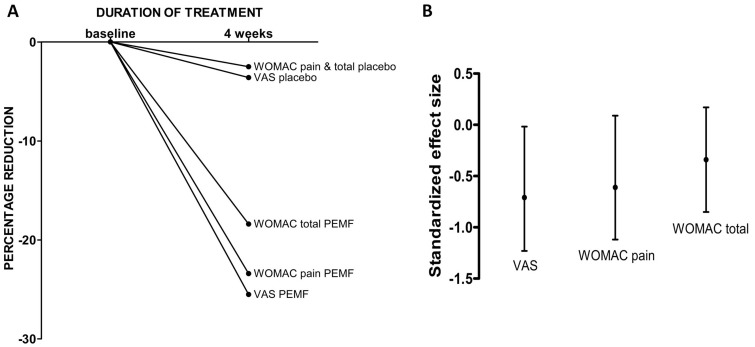
After 1 month of treatment, there was a 25.5% reduction in VAS pain scores for subjects treated with the PEMF device and a 3.6% reduction in those who received placebo, with a standardized effect size of −0.73 (95% CI −1.24 to −0.19) in VAS score.
There was a 23.4% reduction in WOMAC pain subscale and 18.4% reduction in WOMAC total score compared with 2.3% reduction for both WOMAC pain and total in the placebo group. The standardized effect size was −0.61 (95% CI −1.12 to −0.09) for WOMAC pain and −0.34 (95% CI −0.85 to 0.17) for WOMAC total score (Fig. 2).
Fig. 2.
Changes over time and standardized effect size of VAS pain, WOMAC pain and WOMAC total score
(A) The percentage reduction in VAS pain, WOMAC pain and WOMAC total in knee OA participants according to the group of treatment. (B) The standardized size effect induced by PEMF treatment is higher for the parameters evaluating pain (VAS score: −0.73 (95% CI −1.24 to −0.19); WOMAC pain: 0.61, 95% CI − 1.12 to − 0.09), while the effect size associated with an improvement in WOMAC, considering all the subscales, is −0.34 (95% CI − 0.85 to −0.17). PEMF: pulsed electromagnetic fields; VAS: visual analog scale.
Pain threshold and physical health improved during electromagnetic treatment
Our results showed that both measurements of PPT improved in OA patients after 1 month of treatment with the PEMF device compared with placebo. Next, we assessed whether quality of life, measured through the SF-36 questionnaire, was modified by the treatment. Only physical health scores improved in the PEMF group (Table 2).
PEMF treatment reduced intake of NSAIDs/analgesics
Given that recruited patients continued to take prescribed analgesic therapy as needed, we analysed the changes in intake of NSAIDs/analgesics. Among the patients from the PEMF group, eight patients (26%) stopped previously prescribed medications, whereas in the placebo group one patient (3%) stopped and 3 (10%) started a new therapy for chronic pain (Table 3).
Table 3.
Changes in intake of NSAIDs/analgesics
| NSAID/analgesic intake | PEMF (n = 30) | Placebo (n = 30) |
|---|---|---|
| Subject’s daily drug intake at 1 months | ||
| NSAIDs, n (%) | 6 (20) | 12 (40) |
| Analgesics, n (%) | 8 (26) | 15 (50) |
| Changes in drug intake at 1 month follow-up | ||
| Started NSAIDs/ analgesics, n (%) | - (0) | 3 (10) |
| Stopped NSAIDs/ analgesics, n (%) | 8 (26) | 1 (3) |
At the end of the trial, 46% subjects from the PEMF group and 90% patients from the placebo group were under treatment with NSAIDs/analgesics. In the PEMF group, 26% (n = 8) stopped the pharmacological therapy compared with baseline, whereas in the placebo group 10% (n = 3) started a new therapy with NSAIDs/analgesics and 3% (n = 1) stopped previous treatment. PEMF: pulsed electromagnetic fields.
Discussion
In this randomized clinical trial, PEMF therapy improved pain and dysfunction in knee OA patients. Although previous studies have reported contradictory results on the efficacy of this non-pharmacological approach, our results support previous high-quality randomized clinical trials. In our study, the electromagnetic therapy was applied for 12 h each day for a treatment duration of 4 weeks, whereas previous studies ranged from 20 min in nine sessions for 3 weeks [17] to 2 h a day in 30 sessions for 6 weeks [18]. Thus, the absence of a standardized treatment protocol limits the comparison with previous studies.
Additionally, the pulse frequency and duration were different among the randomized clinical trials available, further limiting the possibility of comparing efficacy and safety. Significant pain reduction has been observed in trials using both low pulse frequency and duration (3–7.8 Hz and 10 µs) [15] and relatively high pulse frequency and duration (145 Hz and 400 µs) [19].
In order to explore pain perception, in addition to the self-reported pain scores, such as the VAS and WOMAC scores, we measured pain threshold using pressure algometry, which is the most commonly used quantitative and objective sensory testing method used in rheumatic diseases [20]. It has been clearly shown that patients with rheumatic disease, including OA, have decreased pain thresholds [21–26]. We compared quantitative sensory testing scores, performed on an osseous anatomical surface, the DIP joint, and a muscular anatomical site, the quadriceps muscle, between baseline and 4 weeks of treatment, and we found that pain threshold increased in the PEMF group compared with placebo. The induction of changes in the neuronal sensory mechanism underlying pain perception and threshold remains debated and complex. Exposure to PEMF can increase pain thresholds toward an analgesic response, without affecting thermal sensory threshold, in healthy subjects [27, 28]. Recently, it has been demonstrated that exposure to PEMF can reduce the pain threshold in lateral epicondylitis [29] and also in refractory carpal tunnel syndrome [30]. Neuromodulation could be related to nociceptive C and large A-fibre activity, probably through ion–ligand binding modifications or through changes in the excitability of cell membranes [31]. Another interesting aspect of the interaction between electromagnetic fields and pain is related to opioid function; it has been demonstrated in mice that the induction of analgesia by electromagnetic exposure was equivalent to a moderate dose of morphine [32].
Patients with knee OA have significantly poorer quality of life compared with healthy controls, and this is related to functional disability and chronic pain [33]. We assessed quality of life using the SF-36 v2 questionnaire, as a sensitive health status measure for clinical evaluation, and we found that physical health improved after the exposure to PEMF.
OA is the most prevalent form of joint disease, and the incidence is rising because of the ageing population [1]. Although NSAIDs remain the gold standard for the treatment of pain in OA, there is an increasing need to find conservative and alternative approaches, in order to avoid the toxicity associated with the chronic use of the analgesics, mostly in the elderly population [34]. In our study, OA patients treated with the PEMF device significantly reduced their intake of NSAIDs compared with the placebo group. Given that the factors influencing pain perception in each individual patient remain complex, an attempt to define the mechanisms of pain modulation of this form of therapy in relationship to previously described biological effects remains speculative. Our data on the evidence for the regulation of pain threshold at two different anatomical sites indicates the need for specific studies designed to explore neuronal adaptation in a pulsed electromagnetic environment.
Given that our data are limited to a low number of participants, and the long-term efficacy of the wearable device is unknown, the generalizability of the results needs to be confirmed in a larger clinical trial with a longer duration of treatment. However, the use of a wearable PEMF therapy in knee OA can be considered as an alternative safe and effective therapy in knee OA, providing the possibility for home-based management of pain compared with previous studies.
Taken together, these results suggest that PEMF therapy is a plausible option for the treatment of chronic pain in knee OA. The possibility that some of the effects of this therapeutic approach might be derived from neuromodulation of the pain mechanism needs to be explored further in order to identify the interactions between cartilage function, pain perception and electromagnetic fields.
Acknowledgements
We acknowledge Bioelectronics Corporation for providing both the pulsed electromagnetic fields and the placebo devices.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–26 [DOI] [PubMed] [Google Scholar]
- 2.Felson DT. The epidemiology of knee and hip osteoarthritis. Epidemiol Rev 1998;10:1–28 [DOI] [PubMed] [Google Scholar]
- 3.Elders MJ. The increasing impact of arthritis on public health. J Rheumatol 2000;60:6–8 [PubMed] [Google Scholar]
- 4.Lee YC, Shmerling RH. The benefit of nonpharmacologic therapy to treat symptomatic osteoarthritis. Curr Rheumatol Rep 2008;10:5–10 [DOI] [PubMed] [Google Scholar]
- 5.Luo Q, Li SS, He C. et al. Pulse electromagnetic fields effects on serum E2 levels, chondrocyte apoptosis, and matrix metalloproteinase-13 expression in ovariectomized rats. Rheumatol Int 2009;29:927–35 [DOI] [PubMed] [Google Scholar]
- 6.Ciombor DM, Aaron RK, Wang S, Simon B. Modification of osteoarthritis by pulsed electromagnetic field–a morphological study. Osteoarthritis Cartilage 2003;11:455–62 [DOI] [PubMed] [Google Scholar]
- 7.McCarthy CJ, Callaghan MJ, Oldham JA. Pulsed electromagnetic energy treatment offers no clinical benefit in reducing the pain of knee osteoarthritis: a systematic review. BMC Musculoskelet Disord 2006;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjordal JM, Johnson MI, Lopes-Martins RA. et al. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord 2007;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vavken P, Arrich F, Schuhfried O, Dorotka R. Effectiveness of pulsed electromagnetic field therapy in the management of osteoarthritis of the knee: a meta-analysis of randomized controlled trials. J Rehabil Med 2009;41:406–11 [DOI] [PubMed] [Google Scholar]
- 10.Ryang We S, Koog YH, Jeong KI, Wi H. Effects of pulsed electromagnetic field on knee osteoarthritis: a systematic review. Rheumatology 2013;52:815–24 [DOI] [PubMed] [Google Scholar]
- 11.Altman R, Asch E, Bloch D. et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039–49 [DOI] [PubMed] [Google Scholar]
- 12.Bellamy N, Kirwan J, Boers M. et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip and hand osteoarthritis. Consensus development at OMERACT III. J Rheumatol 1997;24:799–802 [PubMed] [Google Scholar]
- 13.Lee YC, Chibnik LB, Fossel AH. et al. The reproducibility of presure pain thresholds in RA patients. In: American College of Rheumatology Annual Scientific Meeting. CA: San Francisco, 2008
- 14.Nelson FR, Zvirbulis R, Pilla AA. Non-invasive electromagnetic field therapy produces rapid and substantial pain reduction in early knee osteoarthritis: a randomized double-blind pilot study. Rheumatol Int 2013;33:2169–73 [DOI] [PubMed] [Google Scholar]
- 15.Pipitone N, Scott DL. Magnetic pulse treatment for knee osteoarthritis: a randomised, double-blind, placebo-controlled study. Curr Med Res Opin 2001;17:190–6 [DOI] [PubMed] [Google Scholar]
- 16.Iannitti T, Fistetto G, Esposito A, Rottigni V, Palmieri B. Pulsed electromagnetic field therapy for management of osteoarthritis-related pain, stiffness and physical function: clinical experience in the elderly. Clin Interv Aging 2013;8:1289–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callaghan MJ, Whittaker PE, Grimes S, Smith L. An evaluation of pulsed shortwave on knee osteoarthritis using radioleucoscintigraphy: a randomised, double blind, controlled trial. Joint Bone Spine 2005;72:150–5 [DOI] [PubMed] [Google Scholar]
- 18.Thamsborg G, Florescu A, Oturai P. et al. Treatment of knee osteoarthritis with pulsed electromagnetic fields: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage 2005;13:575–81 [DOI] [PubMed] [Google Scholar]
- 19.Fukuda TY, Alves da Cunha R, Fukuda VO. et al. Pulsed shortwave treatment in women with knee osteoarthritis: a multicenter, randomized, placebo-controlled clinical trial. Phys Ther 2011; 91:1009–17 [DOI] [PubMed] [Google Scholar]
- 20.Jensen K. Quantification of tenderness by palpation and use of pressure algometers. Advances in pain research and therapy. New York: Raven Press Ltd, 1990: 165–80 [Google Scholar]
- 21.Brucini M, Duranti R, Galletti R. et al. Pain thresholds and electromyographic features of periarticular muscles in patients with osteoarthritis of the knee. Pain 1981;10:57–66 [DOI] [PubMed] [Google Scholar]
- 22.Gerecz-Simon EM, Tunks ER, Heale JA. et al. Measurement of pain threshold in patients with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and healthy controls. Clin Rheumatol 1989;8:467–74 [DOI] [PubMed] [Google Scholar]
- 23.Jolliffe VA, Anand P, Kidd BL. Assessment of cutaneous sensory and autonomic axon reflexes in rheumatoid arthritis. Ann Rheum Dis 1995;54:251–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maixner W, Fillingim R, Booker D. et al. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain 1995;63:341–51 [DOI] [PubMed] [Google Scholar]
- 25.Giesecke T, Gracely RH, Grant MA. et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum 2004;50:613–23 [DOI] [PubMed] [Google Scholar]
- 26.Bagnato G, De Andres I, Sorbara S. et al. Pain threshold and intensity in rheumatic patients: correlations with the Hamilton Depression Rating scale. Clin Rheumatol 2015;34:555–61 [DOI] [PubMed] [Google Scholar]
- 27.Shupak NM, Prato FS, Thomas AW. Human exposure to a specific pulsed magnetic field: effects on thermal sensory and pain thresholds. Neurosci Lett 2004;363:157–62 [DOI] [PubMed] [Google Scholar]
- 28.Sartucci F, Bonfiglio L, Del Seppia C. et al. Changes in pain perception and pain-related somatosensory evoked potentials in humans produced by exposure to oscillating magnetic fields. Brain Res 1997;769:362–6 [DOI] [PubMed] [Google Scholar]
- 29.Uzunca K, Birtane M, Taştekin N. Effectiveness of pulsed electromagnetic field therapy in lateral epicondylitis. Clin Rheumatol 2007;26:69–74 [DOI] [PubMed] [Google Scholar]
- 30.Weintraub MI, Cole SP. A randomized controlled trial of the effects of a combination of static and dynamic magnetic fields on carpal tunnel syndrome. Pain Med 2008;9:493–504 [DOI] [PubMed] [Google Scholar]
- 31.Challis L. Mechanisms for interaction between RF fields and biological tissue. Bioelectromagnetics 2005;7S98–S106 [DOI] [PubMed] [Google Scholar]
- 32.Shupak NM, Hensel JM, Cross-Mellor SK. et al. Analgesic and behavioral effects of a 100 microT specific pulsed extremely low frequency magnetic field on control and morphine treated CF-1 mice. Neurosci Lett 2004;354:30–3 [DOI] [PubMed] [Google Scholar]
- 33.Alkan BM, Fidan F, Tosun A, Ardıçoğlu O. Quality of life and self-reported disability in patients with knee osteoarthritis. Mod Rheumatol 2014;24:166–71 [DOI] [PubMed] [Google Scholar]
- 34.Jobanputra P, Nuki G. Nonsteroidal anti-inflammatory drugs in the treatment of osteoarthritis. Curr Opin Rheumatol 1994;6:433–9 [DOI] [PubMed] [Google Scholar]