Abstract
Saccharum spontaneum L. is a crucial wild parent of modern sugarcane cultivars whose ploidy clones have been utilized successfully in improving the stress resistance and yield related traits of sugarcane cultivars. To establish knowledge regarding the genetic variances and evolutional relationships of ploidy clones of Saccharum spontaneum collected in China, the rDNA-ITS sequences of 62 ploidy clones including octaploid clones (2n = 64), nonaploid clones (2n = 72), decaploid clones (2n = 80), and dodecaploid clones (2n = 96), were obtained and analyzed. The rDNA-ITS sequences of four species from Saccharum and Sorghum bicolor selected as controls. The results showed that decaploid clones (2n = 80) possess the most abundant variances with 58 variable sites and 20 parsim-informative sites in ITS sequences, which were then followed by octaploid clones with 43 variable sites and 17 parsim-informative sites. In haplotype diversity, all four population exhibited high diversity, especially nonaploid and decaploid populations. By comparing the genetic distances among four ploidy populations, the dodecaploid population exhibited the closest relationship with the nonaploid population, and then the relationship strength decreased successively for the decaploid population and then for the octaploid population. Population differentiation analysis showed that the phenomena of population differentiation were not found among different ploidy populations, and low coefficient of gene differentiation(Gst) and high gene flow(Nm) occur among these populations possessing close genetic relationship. These results mentioned above will contribute to the understanding of the evolution of different ploidy populations of Saccharum spontaneum and provide vital knowledge for their utilization in sugarcane breeding and innovation.
Introduction
Saccharum spontaneum L. is a crucial wild parent for modern sugarcane cultivars that can improve cultivars with regards to tolerances to abiotic or biotic stress and yield related traits. More notably it has the widest ecogeographical distribution among Saccharum spp. and different clones show wide morphological variation [1]. Clones vary from short bushy types with reduced leaves to midrib and practically no formation to tall, erect, broad-leaved forms with long internodes [2]. In China, the plant is distributed mainly in the central, southern, and southwest parts [3].
S. spontaneum also belongs to the complex polyploid plants like sugarcane, whose chromosome number has been reported to range from 2n = 40 to 128 with basic number of chromosome x = 8, and five types of chromosome number (2n = 64, 80, 96, 112, and 128) appear to be distributed highly frequency [4–6]. Studies using GISH and FISH techniques dissected the chromosome composition of model sugarcane cultivars revealed that approximately 10–20% of total chromosomes of cultivars come from S. spontaneum and that about 10% of these occurred in the inter-specific exchange between S. spontaneum and S. officinarum [7–9]. These studies have confirmed that S. spontaneum has become an important component of modern sugarcane cultivars.
During the past several years, research communities around the world have mainly focused their studies on the genetic diversity of clones of S. spontaneum collected from different areas. This studies have proved that S. spontaneum possess very rich genetic variances in phenotypic and molecular level traits [10–15]. In addition, because S. spontaneum is easy to cross with S. officinarum & sugarcane cultivars and their offspring exhibit good performance in stress resistance, adaptability, and ratoon capability S. spontaneum is regarded as one of the most valuable wild specie for exploring sugarcane breeding [1].
When reviewing the history of sugarcane breeding, it is unfortunate that only limited euploid clones of S. spontaneum, including Glagah (2n = 112), Indian (2n = 64), and Yacheng (2n = 64, 80), have been utilized successfully in sugarcane breeding and were used to make series of elite parents such as the POJ series, the Co series and the Yacheng series. To date, these parents still play a critical role in breeding [1, 16].
Since the 1970s, the collecting work of S. spontaneum has been carried out in China. At present about 700 clones, which were collected from Yunnan, Guangxi, Guangdong, Fujian, Sichuan, and Hainan, were conserved in the China National Nursery of Sugarcane Germplasm Resources (CNNSGR) in Kaiyuan, Yunnan province. By identifying the chromosome number of 247 clones conserved in CNNSGR, Cai et al. [17] found 11 chromosome types that are 2n = 60, 64, 70, 72, 74, 78, 80, 92, 96, 104, 108 and 4 which were euploid types (2n = 64, 72, 80, 96) make up a high percentage in all identified clones. Currently, the genetic background and evolutionary relationships for these euploid clones still remain unclear, which has limited their utilization in sugarcane breeding.
At present, some sequences such as rDNA-ITS, rbcl, apha-tubulin, rpl16 and rpoC2 have been using in the genetic relationship analysis of these species belong to Saccharum L. and other related genus as Miscanthus Anderss., Erianthus Michaux and Narenga Bor. [18–23]. These previous studies demonstrated that these sequences except rDNA-ITS are very conserved among different species, which suit for evolutionary analysis of different genus. For rDNA-ITS, the characters of rich variances, rapid evolutionary rate and easy PCR amplification make it a very important marker used in the evolutionary analysis of "sugarcane complex", and it also is often used to evaluate evolutionary relationships at the subspecies level [24]. In view of this, 62 ploidy clones belonging to 4 euploid types of S. spontaneum, were evaluated in this study for genetic variances and phylogenetic relationships via the rDNA-ITS sequences. The results will provide informative knowledge for utilization in sugarcane breeding and innovation.
Materials and Methods
Ethics Statement
S. spontaneum is not considered an endangered species, collecting is allowed in field environment. These S. spontaneum clones in this study were collected in recent decades by Yunnan Sugarcane Research Institute (YSRI). At present, these clones were conserved in the CNNSGR (China National Nursery of Sugarcane Germplasm Resources), which was built by China's Ministry of Agriculture in Kaiyuan city, Yunan province in 1995. The YSRI was entrusted with managing the routine works of CNNSGR. We were assigned to responsible for management, evaluation of these resources by YSRI. Finally, we confirm that no specific permits were required for the present studies.
Plant materials
A total of 62 different ploidy clones of S. spontaneum were selected from CNNSGR, of which 45 clones (23 decaploid clones and 22 octaploid clones) were chosen according to the standard of one clone per county with reference to their collection location. Because there were only 7 nonaploid clones and 10 dodecaploid clones conserved in CNNSGR, all of these clones were chosen for this study. And 31 rDNA-ITS sequences from five species (Saccharum officinarum, Saccharum barberi, Saccharum sinense, Saccharum robustum and Sorghum bicolor) downloaded from GenBank were regarded as controls. All clones and control sequences were listed in Tables 1 and 2 in detail.
Table 1. The list of clones of S. spontaneum used in this study.
| No. | Sample name | Ploidy type/Chromosome number | Collected location | GenBank accession No. |
|---|---|---|---|---|
| 1 | Yunnan82-59 | Octaploid/2n = 64 | Binchuan county, Yunnan | KJ934283 |
| 2 | Yunnan82-149 | Octaploid/2n = 64 | Changning county, Yunnan | KJ934287 |
| 3 | Yunnan83-238 | Octaploid/2n = 64 | Dayao county, Yunnan | KJ934293 |
| 4 | Yunnan75-2-2 | Octaploid/2n = 64 | Eshan county, Yunnan | KJ934276 |
| 5 | Yunnan82-79 | Octaploid/2n = 64 | Gengma county, Yunnan | KJ934285 |
| 6 | Yunnan83-160 | Octaploid/2n = 64 | Hekou county, Yunnan | KJ934288 |
| 7 | Yunnan4 | Octaploid/2n = 64 | Honghe county, Yunnan | KJ934274 |
| 8 | Yunnan82-20 | Octaploid/2n = 64 | Lianghe county, Yunnan | KJ934280 |
| 9 | Yunnan83-227 | Octaploid/2n = 64 | Liuku county, Yunnan | KJ934291 |
| 10 | Yunnan83-225 | Octaploid/2n = 64 | Lushui county, Yunnan | KJ934290 |
| 11 | Yunnan75-1-10 | Octaploid/2n = 64 | Mang city, Yunnan | KJ934275 |
| 12 | Yunnan84-268 | Octaploid/2n = 64 | Mang city, Yunnan | KJ934294 |
| 13 | Yunnan Mengzi | Octaploid/2n = 64 | Mengzi county, Yunnan | KJ934273 |
| 14 | Yunnan82-63 | Octaploid/2n = 64 | Nanjian county, Yunnan | KJ934284 |
| 15 | Yunnan82-9 | Octaploid/2n = 64 | Ruili city, Yunnan | KJ934278 |
| 16 | Yunnan82-25 | Octaploid/2n = 64 | Tengchong county, Yunnan | KJ934281 |
| 17 | Yunnan83-213 | Octaploid/2n = 64 | Yangbi county, Yunnan | KJ934289 |
| 18 | Yunnan82-14 | Octaploid/2n = 64 | Yingjiang county, Yunnan | KJ934279 |
| 19 | Yunnan83-228 | Octaploid/2n = 64 | Yongping county, Yunnan | KJ934292 |
| 20 | Yunnan82-58 | Octaploid/2n = 64 | Rongsheng county, Yunnan | KJ934282 |
| 21 | Yunnan75-2-11 | Octaploid/2n = 64 | Yuanjiang county, Yunnan | KJ934277 |
| 22 | Yunnan82-140 | Octaploid/2n = 64 | Yuanyang county, Yunnan | KJ934286 |
| 23 | Fujian89-1-11 | Nonaploid/2n = 72 | Gutian county, Fujian | KJ934297 |
| 24 | Fujian89-1-1 | Nonaploid/2n = 72 | Songxi county, Fujian | KJ934296 |
| 25 | Guizhou78-1-11 | Nonaploid/2n = 72 | Xishui county, Guizhou | KJ934298 |
| 26 | Yunnan76-1-016 | Nonaploid/2n = 72 | Miyi county, Sichuan | KJ934300 |
| 27 | Sichuan92-42 | Nonaploid/2n = 72 | Leshan city, Sichuan | KJ934299 |
| 28 | Yunnan82-50 | Nonaploid/2n = 72 | Huaping county, Yunnan | KJ934295 |
| 29 | Yunnan83-201 | Nonaploid/2n = 72 | Yanjing county, Yunnan | KJ934301 |
| 30 | Fujian Dongshan | Decaploid/2n = 80 | Dongshan county, Fujian | KJ934334 |
| 31 | Fujian92-1-11 | Decaploid/2n = 80 | Fuzhou city, Fujian | KJ934333 |
| 32 | Fujian87-1-14 | Decaploid/2n = 80 | Lizhi,Putian city, Fujian | KJ934332 |
| 33 | Fujian89-1-21 | Decaploid/2n = 80 | Xiamen city, Fujian | KJ934303 |
| 34 | Guangdong16 | Decaploid/2n = 80 | Guangzhou city, Guangdong | KJ934304 |
| 35 | Guangdong35 | Decaploid/2n = 80 | Huazhou city, Guangdong | KJ934307 |
| 36 | Guangdong31 | Decaploid/2n = 80 | Luhe county, Guangdong | KJ934306 |
| 37 | Guangdong Shaoguan | Decaploid/2n = 80 | Ruiyuan county, Guangdong | KJ934311 |
| 38 | Guizhou78-2-4 | Decaploid/2n = 80 | Rongjiang county, Guizhou | KJ934312 |
| 39 | Guizhou78-1-31 | Decaploid/2n = 80 | Sinan county, Guizhou | KJ934338 |
| 40 | Guizhou78-1-5 | Decaploid/2n = 80 | Xishui county, Guizhou | KJ934337 |
| 41 | Guizhou84-260 | Decaploid/2n = 80 | Xingyi city, Guizhou | KJ934302 |
| 42 | Hainan Ledong1 | Decaploid/2n = 80 | Huangliu county, Hainan | KJ934340 |
| 43 | Sichuan79-1-26 | Decaploid/2n = 80 | DA county, Sichuan | KJ934313 |
| 44 | Sichuan88-41 | Decaploid/2n = 80 | Jitang county, Sichuan | KJ934343 |
| 45 | Sichuan79-2-1 | Decaploid/2n = 80 | Lushui county, Sichuan | KJ934341 |
| 46 | Yunnan75-2-35 | Decaploid/2n = 80 | Hekou county, Yunnan | KJ934346 |
| 47 | Yunnan76-3-2 | Decaploid/2n = 80 | Jinghong city, Yunnan | KJ934324 |
| 48 | Yunnan82-12 | Decaploid/2n = 80 | Longchuan county, Yunnan | KJ934325 |
| 49 | Yunnan82-44 | Decaploid/2n = 80 | Zhongdian county, Yunnan | KJ934326 |
| 50 | Chongqing76-1-024 | Decaploid/2n = 80 | Miyi county, Chongqing | KJ934347 |
| 51 | Chongqing79-2-13 | Decaploid/2n = 80 | Wanzhou district, Chongqing | KJ934342 |
| 52 | Chongqing79-2-16 | Decaploid/2n = 80 | Yunyang county, Chongqing | KJ934316 |
| 53 | Fujian Huian | Dodecaploid/2n = 96 | Huian county, Fujian | KJ934358 |
| 54 | Fujian88-1-12 | Dodecaploid/2n = 96 | Nanjing county, Fujian | KJ934353 |
| 55 | Fujian88-1-13 | Dodecaploid/2n = 96 | Nanjing county, Fujian | KJ934354 |
| 56 | Fujian89-1-16 | Dodecaploid/2n = 96 | Putian city, Fujian | KJ934355 |
| 57 | Fujian89-1-17 | Dodecaploid/2n = 96 | Putian city, Fujian | KJ934356 |
| 58 | Fujian89-1-18 | Dodecaploid/2n = 96 | Putian city, Fujian | KJ934357 |
| 59 | Fujian Xianyou | Dodecaploid/2n = 96 | Putian city, Fujian | KJ934359 |
| 60 | Guangdong30 | Dodecaploid/2n = 96 | Haifeng county, Guangdong | KJ934360 |
| 61 | Guizhou78-2-28 | Dodecaploid/2n = 96 | Sanjiang county, Guizhou | KJ934361 |
| 62 | Sichuan79-2-11 | Dodecaploid/2n = 96 | Zhong county, Chongqing | KJ934352 |
Table 2. The list of control rDNA-ITS sequences.
| Specie name | Sample name | GenBank accession No. |
|---|---|---|
| Saccharum officinarum | Mangeer, Orambo, genotype104, R3, R1, R2, Skendzic5068, Karia | AB250692.1, AB250691.1, AF345231.1, AF345229.1, AF345230.1, DQ005064.1, AB250693.1 |
| Saccharum barberi | Nargori, PutjeeKhajee, Dhaurkinara, R5, R4, R6 | AB281150.1, AB281148.1, AB281149.1, AF345199.1, AF331657.1, AF345200.1 |
| Saccharum sinense | Khelia, Tukya1, Khakai, R8, R10, R9, R7 | AB281153.1, AB281154.1, AB281152.1, AF345242.1, AF345240.1, AF345243.1, AF345241.1 |
| Saccharum robustum | NG-77-27, R12, R13, R11 | AB281156.1, AF345238.1, AF345239.1, AF345237.1 |
| Sorghum bicolor | Vu12, Vu11, B1, B2, B3, B4 | DQ190421.1, DQ190420.1, GQ856358.1, GQ121748.1, GQ121745.1, GQ121744.1, GQ121743.1 |
DNA extraction and PCR amplification
Considering all stalks per clone arise from these rhizome buds through vegetative propagation, the mixed young tender leaves from multiple stalks per clone were powdered with liquid nitrogen, then the genomic DNA of which was extracted by using the traditional CTAB method, the quality and concentration of DNA were respectively tested with 0.8% agarose gel and Thermo Nanodrop 2000, and then obtained DNA samples were diluted to the concentration of 20 ng/μl with deionized water for PCR amplification.
The rDNA-ITS region of all samples, which contain ITS1, 5.8s, and ITS2 regions, were amplified through using the universal primers ITS4 and ITS5(ITS4 primer sequence: 5’-TCCTCCGCTTATTGATATGC-3’, ITS5 primer sequence: 5’-GGAAGTAAAAGTCGTAACAAGG-3’) [25]. In view of lots of clones and shorter amplification sequence length, the High Fidelity TransTaq DNA Polymerase from Transgen biotech company, whose fidelity of PCR amplification is 18 times than common Taq polymerase, was employed for amplifying these short sequences instead of using PCR replication experiment to reduce the PCR amplification error. The PCR reaction system and procedures were performed according to Chen et al. [22]. PCR was performed on a Mastercycler gradient thermocycler (Eppendorf, Germany). The PCR products were tested by 1.0% agarose gel electrophoresis and then were purified using the OMEGAEZNA Gel extraction Kit. The purified PCR product was cloned into a PMD19-T vector, and the recombinant plasmids were transformed into a DH5α competent cell. In order to further increase the accuracy of sequence, five transformed clones per sample were selected for bi-directional sequencing by the BGI Company, China, then the sequence occupying the highest proportion among five sequences each sample was used for analysis. Finally, all obtained ITS sequences were uploaded to GenBank, the sequence accession No. per sample was list in Table 1.
Sequence alignment and analyses
All obtained right sequences were aligned using the Clustal W program [26] with default settings. The basic sequence statistics including GC content, variable sites, and parsim-informative sites were counted through MEGA 6.06 software [27]. In view of DnaSP5.0 [28] and Arlequin3.11 [29] softwares successfully used to estimate nucleotide diversity of DNA or gene sequences and population differentiation of ployploid plants such as wheat [30–32] and potato [33,34], the two softwares were also used for rDNA-ITS sequence analysis of S. spontaneum clones. The haplotype diversity, nucleotide diversity, average number of nucleotide difference, gene flow(Nm) and coefficient of gene differentiation (Gst) were calculated according to these formulas (equation 8.4, equation 10.5 and equation 5) from Nei’s reports [35,36] by using DnaSP5.0 software; and the analysis of molecular variance among populations were implemented by using Arlequin 3.11 software to calculate the Variance of components, Percentage of variation, fixation Index according to the standard AMOVN computations method with choosing haplotypic data and DNA type as data parameter type.
The genetic distances among four different ploidy populations were calculated according to Kimura 2-Parameter model using MEGA6.06 software. Differences in genetic distance between intra-population and inter-population were assessed by using independent-samples T test at P<0.05. The maximum-likelihood (ML) and neighbor-joining (NJ) method were used to construct a haplotype phylogenetic tree according to the Kimura 2-Parameter model using MEGA6.06 software, and all branches were evaluated with 1000 bootstrap replications and the trees with bootstrap confidence values >50% appear in the phylogenetic tree.
Results
Component and variance analysis of ITS sequences
Regarding the length of ITS sequences, there was only a types of sequences length in ITS1 sequences (207 bp) and 5.8S rDNA sequences (164 bp), and three length types (218 bp, 219 bp, and 220 bp) in ITS2 sequences. With regards to GC content, the value of GC content in ITS2 sequences with a mean of 69.3% is higher than that in ITS1 sequences with a mean of 63.5% (Table 3). 5.8S rDNA sequences exhibited the lowest GC content with a mean of 57.1%. Among different ploidy populations, there are no significant differences found in GC content.
Table 3. The GC content analysis of composition of ITS sequence of different ploidy populations of S. spontaneum.
| ITS1 GC content (%) | 5.8s rDNA GC content (%) | ITS2 GC content (%) | ||||
|---|---|---|---|---|---|---|
| Population | Range | Mean | Range | Mean | Range | Mean |
| Octaploid | 61.8–64.7 | 63.7 | 56.1–57.3 | 57.2 | 68.5–69.7 | 69.4 |
| Nonaploid | 61.4–64.3 | 63.1 | 56.1–57.3 | 57.1 | 67.9–69.7 | 69.1 |
| Decaploid | 61.8–64.7 | 63.4 | 56.1–57.9 | 57.1 | 67.6–69.9 | 69.1 |
| Dodecaploid | 62.3–64.7 | 63.4 | 56.7–57.3 | 57.0 | 68.3–70.3 | 69.4 |
| Mean | 63.5 | 57.1 | 69.3 | |||
According to the results of ITS sequences aligned using the Clustal W program, every ploidy population had 207 sites found in ITS1 sequences. However, there were differences in the number of sites for ITS2 sequences among different ploidy populations with 222 in an octaploid population, 220 in a decaploid population, and 219 in nonaploid and dodecaploid populations. For ITS sequences variable sites, the decaploid population had more rich variable sites with total 58 variable sites and 20 parsim-informative sites (20 variable sites and 13 parsim-informative sites in ITS1 sequences, 11 variable sites and 1 parsim-informative sites in 5.8S rDNA sequences, 27 variable sites and 6 parsim-informative sites in ITS2 sequences), which made up 9.81% and 3.38% of total sites respectively (Table 4). The ranked second for variances of ITS sequences is the octaploid population with total 43 variable sites and 17 parsim-informative sites. Then the dodecaploid and nonaploid populations exhibited low number of variable sites. As mentioned above, the largest variances of ITS sequences arise in the decaploid population, followed by the octaploid population. This may be due to the number of clones selected in this study.
Table 4. The analysis of variable sites of ITS sequence of different ploidy populations of S. spontaneum.
| Population | Site name | ITS1 | 5.8s rDNA | ITS2 | Total | Percentage of total sites (%) |
|---|---|---|---|---|---|---|
| Octaploid | Variable sites | 20 | 7 | 16 | 43 | 7.25 |
| Parsim-informative sites | 9 | 1 | 7 | 17 | 2.87 | |
| Nonaploid | Variable sites | 12 | 2 | 11 | 25 | 4.24 |
| Parsim-informative sites | 8 | 1 | 4 | 13 | 2.20 | |
| Decaploid | Variable sites | 20 | 11 | 27 | 58 | 9.81 |
| Parsim-informative sites | 13 | 1 | 6 | 20 | 3.38 | |
| Dodecaploid | Variable sites | 14 | 1 | 11 | 26 | 4.41 |
| Parsim-informative sites | 7 | 1 | 3 | 11 | 1.86 |
Haplotype diversity analysis of population
The results of haplotype diversity analysis among four populations showed that total 51 haplotypes were found in four ploidy populations (Table 5), there were 20 haplotypes in octaploid population, 7 in nonaploid population, 22 in decaploid population and 8 in dodecaploid population. Hap2 and Hap3 were shared by three populations; Hap4 and Hap18 were shared by two populations. In the aspect of haplotype diversity, all four populations exhibited high diversity, the haplotype diversity (Hd) value ranged from 0.9333 to 1.0000 (Table 5). Nonaploid population performed the highest diversity, followed by decaploid population. Similarly, the high diversity in nonaploid and decaploid populations was also found in nucleotide diversity (Pi) because of high Pi value (0.0174 and 0.0177). Moreover, the two populations also appear big nucleotide difference, varying from 10.1905–10.3795.
Table 5. Haplotype diversity, nucleotide diversity of different ploidy populations of S. spontaneum according to rDNA-ITS haplotype data.
| Population | Haplotype | Haplotype diversity (Hd) | Nucleotide diversity (Pi) | Average number of Nucleotide difference (k) |
|---|---|---|---|---|
| Octaploid | Hap3,18,34–51 | 0.9870±0.077 | 0.0154 | 9.0260 |
| Nonaploid | Hap2,3,29–33 | 1.0000±0.077 | 0.0174 | 10.1905 |
| Decaploid | Hap1-22 | 0.9961±0.014 | 0.0177 | 10.3795 |
| Dodecaploid | Hap2,4,23–28 | 0.9333±0.077 | 0.0141 | 8.2444 |
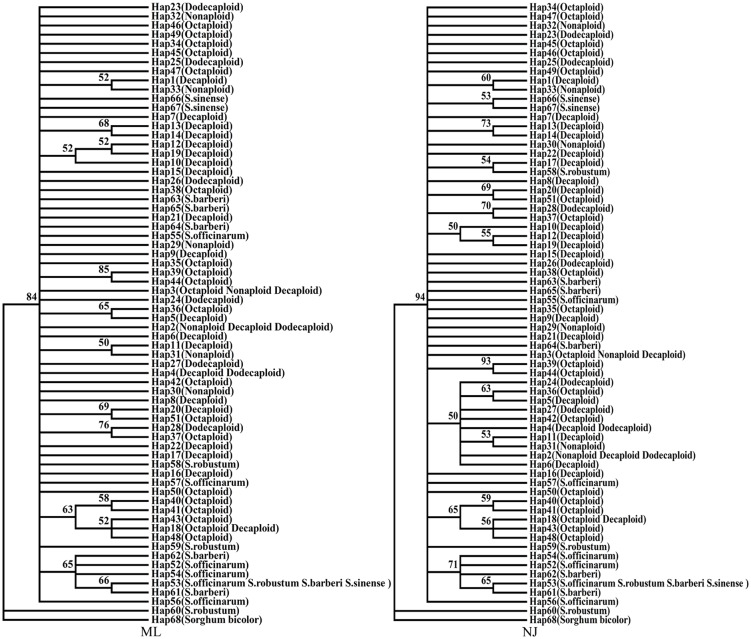
Using 17 haplotype data of rDNA-ITS sequence as outgroup, 16 of which from four species of Saccharum (S.officinarum, S.robustum, S.barberi and S.sinense) and 1 from Sorghum bicolor. Two phylogenetic trees with bootstrap confidence values >50% were constructed based on a Kimura 2-parameter model using the maximum-likelihood (ML) and neighbor-joining (NJ) methods (Fig 1). The results showed that the NJ tree was similar to the ML tree. For the two trees, the Hap68 from Sorghum bicolor and Hap60 from S.robustum separated firstly from the largest group consisting of 66 remained haplotypes. In the big group, 5 haplotypes from S.officinarum, S.robustum, S.barberi and S.sinense were clustered together with 71% and 65% bootstrap value in NJ and ML, and 5 haplotypes from octaploid and decaploid populations were assigned into another small group with 65% or 63% bootstrap value. Because the haplotypes from same population did not cluster together instead of exhibiting confused clustering relationships, these haplotypes from different ploidy populations were not obvious differentiation.
Fig 1. The ML and NJ phylogenetic tree based on rDNA-ITS haplotype data of different polyploid clones of S. spontaneum.
Genetic distance among populations
By using a Kimura 2-parameter model of MEGA6.06 software, the mean genetic distances among different ploidy populations were obtained. The results are listed in Table 6. Four populations showed a close genetic relationship, of which nonaploid population and dodecaploid population exhibited the closest relationship with the smallest genetic distance of 0.0156. The genetic distances (0.0162) among dodecaploid population and decaploid or octaploid population were ranked as second. However, octaploid population and nonaploid displayed the farthest genetic relationship with the biggest genetic distance of 0.0171.
Table 6. The T test of genetic distance difference between inter-population and intra-population obtained using Kimura 2-parameter model.
| Inter-population type | Mean pairwise distance among individuals of inter-population | Mean pairwise distance among individuals of intra-population | T test of pairwise distances between inter-population and intra-population |
|---|---|---|---|
| Octaploid and Nonaploid | 0.0171(N = 154) | Octaploid: 0.0150(N = 231) | 0.004* |
| Nonaploid: 0.0177(N = 21) | 0.737 | ||
| Octaploid and Decaploid | 0.0170(N = 506) | Octaploid: 0.0150(N = 231) | 0.000* |
| Decaploid: 0.0178(N = 253) | 0.157 | ||
| Octaploid and Dodecaploid | 0.0163(N = 220) | Octaploid: 0.0150(N = 231) | 0.029* |
| Dodecaploid: 0.0143(N = 45) | 0.127 | ||
| Nonaploid and Decaploid | 0.0170(N = 161) | Nonaploid: 0.0177(N = 21) | 0.713 |
| Decaploid: 0.0178(N = 253) | 0.122 | ||
| Nonaploid and Dodecaploid | 0.0156(N = 70) | Nonaploid: 0.0177(N = 21) | 0.321 |
| Dodecaploid: 0.0143(N = 45) | 0.396 | ||
| Decaploid and Dodecaploid | 0.0162(N = 230) | Decaploid: 0.0178(N = 253) | 0.024* |
| Dodecaploid: 0.0143(N = 45) | 0.168 |
Note: N stands for pairwise distance number;
* indicates a statistically significant difference at p<0.05
In order to determine whether a reliable phylogenic tree of four populations can be constructed successfully according to ITS sequence data. The differences of genetic distance between inter-population and intra-population were assessed using independent-samples T test. The results exhibited that the genetic distances of inter-populations have no significant bigger than that of intra-population at P<0.05 (Table 6), which means that the reliability of population phylogenic tree may be interfered by intra-population variation. According to the situation above, a reliable phylogenic tree among four populations cannot be constructed.
Population differentiation
The coefficient of gene differentiation (Gst), Gene flow and molecular variance were computed by using DnaSP5.0 and Arlequin 3.11 softwares. the results exhibited that the lowerest Gst value (0.0191), the highest Nm value (12.83) were obtained between nonaploid and decaploid populations (Table 7), this result indicated that two populations have high frequency gene exchanging, followed by the Gst (0.0314)and Nm (7.71) value between decaploid and dodecaploid populations. Between octaploid and dodecaploid populations, the biggest Gst value (0.0814) and the lowest Nm value (2.82) implied that low genetic exchanging occurred between two populations, similar result also appeared between octaploid and nonaploid populations. AMOVA analysis indicated that there was no significant differentiation among four ploidy populations at significance level of 0.001 with a low fixation index (0.0403) (Table 8). And the most of the variation (95.97%) was from within populations, only 4.03% variation from among populations. On comparison the percentage of variation of among population, the biggest value of 10.96% between octaploid and dodecaploid populations implied that there were more genetic differences between two populations, followed by between octaploid and nonaploid populations with a value of 8.26%. The results were consistent with the analysis of coefficient of gene differentiation (Gst) and Gene flow.
Table 7. Pairwise Gst (above the diagonal) and Nm (below the diagonal) among different ploidy populations according to rDNA-ITS data.
| Population | Octaploid | Nonaploid | Decaploid | Dodecaploid |
|---|---|---|---|---|
| Octaploid | 0.0621 | 0.0436 | 0.0814 | |
| Nonaploid | 3.78 | 0.0191 | 0.0544 | |
| Decaploid | 5.49 | 12.83 | 0.0314 | |
| Dodecaploid | 2.82 | 4.35 | 7.71 |
Table 8. Molecular variance (AMOVA) analysis among different ploidy populations according to rDNA-ITS haplotype data.
| Group | Source of variation | df | Sum of squares | Variance of components | Percentage of variation (%) | Fixation index |
|---|---|---|---|---|---|---|
| Octaploid and Nonaploid | among populations | 1 | 10.48 | 0.48 | 8.26 | 0.0826 |
| within populations | 27 | 144.69 | 5.36 | 91.74 | ||
| Total | 28 | 155.17 | 5.84 | |||
| Octaploid and Decaploid | among populations | 1 | 11.49 | 0.26 | 4.56 | 0.0456 |
| within populations | 43 | 238.29 | 5.54 | 95.44 | ||
| Total | 44 | 249.78 | 5.81 | |||
| Octaploid and Dodecaploid | among populations | 1 | 13.70 | 0.63 | 10.96 | 0.1096 |
| within populations | 30 | 152.65 | 5.09 | 89.04 | ||
| Total | 31 | 166.34 | 5.71 | |||
| Nonaploid and Decaploid | among populations | 1 | 3.22 | -0.23 | -4.24 | -0.0424 |
| within populations | 28 | 159.88 | 5.71 | 104.24 | ||
| Total | 29 | 163.10 | 5.48 | |||
| Nonaploid and Dodecaploid | among populations | 1 | 4.52 | -0.05 | -1.06 | -0.0106 |
| within populations | 15 | 74.24 | 4.95 | 101.06 | ||
| Total | 16 | 78.77 | 4.90 | |||
| Decaploid and Dodecaploid | among populations | 1 | 5.34 | -0.01 | -0.09 | -0.0010 |
| within populations | 31 | 167.84 | 5.41 | 100.09 | ||
| Total | 32 | 173.18 | 5.41 | |||
| Total | among populations | 3 | 25.96 | 0.23 | 4.03 | 0.0403 |
| within populations | 58 | 312.53 | 5.39 | 95.97 | ||
| Total | 61 | 338.48 | 5.61 |
Discussion
S. spontaneum is a very complex polyploid plant which possess approximately 26 types of chromosome number (2n = 40–128) [4]. In China, about 16 types have been reported with chromosome number ranging from 54 to 108, but only four ploidy clones (2n = 64, 72, 80, 96) appear to be distributed with high frequency [17,37–38]. However, the questions of how these ploidy clones evolved, their genetic relationships, and which ploidy clones have high breeding value for improving of sugarcane cultivar still remain unanswered. In this study, the analysis result of variable site analysis and haplotype diversity showed that decaploid and octaploid performed rich genetic variances. For the genetic relationship of four euploid populations of S. spontaneum, it was first illustrated according to rDNA ITS sequences. No obvious population differentiations appeared among four ploidy populations because of their small coefficient of gene differentiation and high gene flow value. This may be due to overlapping of their distribution regions, natural crossing with each other lead to high gene exchanging among populations.
Regarding the origin of S. spontaneum in china, Chen et al. [11] hypothesized that S. spontaneum might have originated from southern regions of Yunnan in China which has low altitude and latitude. They conjectured that it then spread to northwest regions of Yunnan with a higher altitude and latitude, then through Sichuan and Guizhou, and finally extended to other provinces such as Guangxi, Guangdong, Fujian, Jiangxi, and Zhejiang. Because octaploid clones are mainly distributed in possible origin regions such as Yunnan [17, 37–38], we inferred that octaploid clones might belong to a primitive chromosome type. According to chromosome number of nonaploid clone (2n = 72), we presumed that nonaploid clones may have arisen from a crossing of offspring between the octaploid clones (2n = 64) and decaploid clones (2n = 80) due to the overlap in their distribution regions. Because of 40 chromosomes from decaploid and 32 from octaploidy, the nonaploid should have a more close genetic relationship with decaploid than with octaploid. The genetic distance of three ploidy populations in this study is consistent with our assumption.
For Dodecaploid, it only distributed in Fujian provinces in China. Because its distribution region belongs to the extended regions of the evolution of S. spontaneum, we conjectured that dodecaploid clones may belong to evolutional types. Sreenivasan [39] hypothesized that it may originate from a triploid seedling from an octaploid, but the theory not be supported by our study. Actually, dodecaploid has a more close relationship with nonaploid rather than octaploid and decaploid, it means that dodecaploid may derived from nonaploid. But how they evolve still remains unknown, we presumed that the odd ploidy clone may produce a kind of six ploidy gamete containing 48 chromosomes, then crossing with each other form dodecaploid clone possessing 96 chromosomes. More research about the evolution of different ploidy of S. spontaneum should be carried out in future.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Data Availability
All sequence data have been uploaded to GenBank, their acession No. are KJ934283, KJ934287, KJ934293, KJ934276, KJ934285, KJ934288, KJ934274, KJ934280, KJ934291, KJ934290, KJ934275, KJ934294, KJ934273, KJ934284, KJ934278, KJ934281, KJ934289, KJ934279, KJ934292, KJ934282, KJ934277, KJ934286, KJ934297, KJ934296, KJ934298, KJ934300, KJ934299, KJ934295, KJ934301, KJ934334, KJ934333, KJ934332, KJ934303, KJ934304, KJ934307, KJ934306, KJ934312, KJ934311, KJ934338, KJ934337, KJ934302, KJ934340, KJ934313, KJ934343, KJ934341, KJ934346, KJ934324, KJ934325, KJ934326, KJ934347, KJ934342, KJ934316, KJ934358, KJ934353, KJ934354, KJ934355, KJ934356, KJ934357, KJ934359, KJ934360, KJ934361, KJ934352.
Funding Statement
This work was supported by the Yunnan Natural Science Foundation (2011FB120) funder name: Xinlong Liu, conceived and designed the experiments, the National Natural Science Foundation of China (31360359) funder name: Xinlong Liu, conceived and designed the experiments; the Earmarked Fund for the Modern Agriculture Technology of China (CARS-20-1-5), funder name: Zuhu Deng, conceived and designed the experiments; Candidates of the Young and Middle Aged Academic Leaders of Yunnan Province (2014HB038), funder name: Xinlong Liu, conceived and designed the experiments.
References
- 1.Henry R, Kole C. Genetics, Genomics and Breeding of Sugarcane. New Hampshire: Science Publishers; 2010. [Google Scholar]
- 2.Guo BZ. The Flora of China. Beijing: China Science Press; 1987. [Google Scholar]
- 3.Mukherjee SK. Origin and distribution of Saccharum. Bot Gaz. 1957; 119:55–61. [Google Scholar]
- 4.Panje R, Babu C. Studies in Saccharum spontaneum distribution and geographical association of chromosome numbers. Cytologia. 1960; 25:152–172. [Google Scholar]
- 5.da Silva J, Honeycutt RJ, Burnquist W, Al-Janabi SM, Sorrells ME, Tanksley SD, et al. Saccharum spontaneum L.‘SES 208’genetic linkage map combining RFLP-and PCR-based markers. Mol Breeding. 1995; 2:165–179. [Google Scholar]
- 6.Ha S, Moore PH, Heinz D, Kato S, Ohmido N, Fukui k. Quantitative chromosome map of the polyploid Saccharum spontaneum by multicolor fluorescence in situ hybridization and imaging methods. Plant Molecular Biology. 1999; 39(6):1165–1173. [DOI] [PubMed] [Google Scholar]
- 7.D'hont A, Grivet L, Feldmann P, Glaszmann JC, Rao S, Berding N, et al. Characterisation of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. Molecular and General Genetics MGG. 1996; 4:405–413. [DOI] [PubMed] [Google Scholar]
- 8.Cuadrado A, Acevedo R, de la Espina SMD, Jouve N, De La Torre C. Genome remodelling in three modern S. officinarum× S. spontaneum sugarcane cultivars. Journal of Experimental Botany. 2004; 55(398):847–854. [DOI] [PubMed] [Google Scholar]
- 9.D'hont A. Unraveling the genome structure of polyploids using FISH and GISH; examples of sugarcane and banana. Cytogenetic & Genome Research. 2005; 109(1–3):27–33. [DOI] [PubMed] [Google Scholar]
- 10.Tai PYP, Miller JD, Legendre BL. Evaluation of Saccharum spontaneum germplasm in the world collection of sugarcane and related grasses maintained at national germplasm repository, Miami, Forida. Sugar Cane. 1997; 1:15–17. [Google Scholar]
- 11.Chen H, Fan YH, Shi XW, Cai Q, Zhang M, Zhang YP, et al. Research on genetic diversity and systemic evolution in Saccharum spontaneum L. Acta Agronomica Sinica. 2001; 27:645–652. [Google Scholar]
- 12.Pan YB, Burner DM, Legendre BL, Grisham MP, White WH. An assessment of the genetic diversity within a collection of Saccharum spontaneum L. with RAPD-PCR. Genetic Resources & Crop Evolution. 2005; 51(8):895–903. [Google Scholar]
- 13.Mary S, Nair NV, Chaturvedi PK, Selvi A. Analysis of genetic diversity among Saccharum spontaneum L. from four geographical regions of India, using molecular markers. Genetic Resources & Crop Evolution. 2006; 53(6):1221–1231. [Google Scholar]
- 14.Zhang GM, Yang RZ, Liu HB, Fang WK. Principal component analysis for 7 quantitative traits and cluster analysis based on 7 quantitative traits of Saccharum spontaneum L. Southwest China Journal of Agricultural Sciences. 2006; 19(6):1127–1131. [Google Scholar]
- 15.Liu XL, Su HS, Ying XM, Ma L, Lu X, Liu HB, et al. Phenotypic correlation and genetic diversity of decaploids of Saccharum spontaneum. Journal of Hunan Agricultural University. 2013; 38(6):574–579. [Google Scholar]
- 16.Liu SM, Wang QN, Huang ZX, Zhang CM, Hu HX, Cheng FU, et al. Utilization of sugarcane parents of Yacheng series in sugarcane breeding of China. Sugarcane & Canesugar. 2011; 4:5–10. [Google Scholar]
- 17.Cai Q, Wen JC, Fan YH, Wang LP, Ma L. Chromosome analysis of Saccharum L. and related plants. Southwest China Journal of Agricultural Sciences. 2002; 15(2):16–19. [Google Scholar]
- 18.Takahashi S, Furukawa TT, Terajima Y, Shimada H, Sugimoto A, Kadowaki K. Very close relationship of the chloroplast genomes among Saccharum species. Tag.theoretical & Applied Genetics.theoretische Und Angewandte Genetik. 2005; 110(8):1523–1529. [DOI] [PubMed] [Google Scholar]
- 19.Hodkinson TR, Chase MW, Lledo DM, Salamin N, Renvoize SA. Phylogenetics of Miscanthus, Saccharum and related genera (Saccharinae, Andropogoneae, Poaceae) based on DNA sequences from its nuclear ribosomal DNA and plastid trnl intron and trnl-f intergenic spacers. Journal of Plant Research. 2002; 115(5):381–392. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YW, Long HS, Fang YH, Yao YG, Cai Q, Zhang YP. Sequence variation of rbcl gene and evolution of Saccharum and related species. Acta Botanica Yunnanica. 2002; 24(1):29–36. [Google Scholar]
- 21.Bacci M, Miranda VFO, Martins VG, Figueira AVO. A search for markers of sugarcane evolution. Genetics & Molecular Biology. 2001; 24(1–4):169–174. [Google Scholar]
- 22.Chen H, Fan YH, Xiang YJG, Cai Q, Zhang YP. Phylogenetic relationships of Saccharum and related species inferred from sequence analysis of the nrDNA ITS region. Acta Agronomica Sinica. 2003; 29(3):379–385. [Google Scholar]
- 23.Liu XL, Su HS, Ma L, Lu X, Ying XM, Cai Q. Phylogenetic relationships of sugarcane related genera and species based on ITS sequences of nuclear ribosomal DNA. Acta Agronomica Sinica. 2010; 36(11):1853–1863. [Google Scholar]
- 24.Qian J, Sun Y, Duan YH. Internal transcribed spacer region of rDNA in common wheat and its Genome origins. Acta Agronomica Sinica. 2009; 35(6):1021–1029. [Google Scholar]
- 25.Hsiao C, Chatterton NJ, Asay KH, Jensen KB. Phylogenetic relationships of the monogenomic species of the wheat tribe, triticeae (Poaceae), inferred from nuclear rDNA (internal transcribed spacer) sequences. Genome. 1995; 38(2):211–223. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994; 22(22):4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology & Evolution. 2013; 30(4):2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Librado P, Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009; 25(11):1451–1452. 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- 29.Excoffier L, Laval G, Schneider S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online. 2005; 1:47–50. [PMC free article] [PubMed] [Google Scholar]
- 30.Giles RJ, Brown TA. Gludy allele variations in Aegilops tauschii and Triticum aestivum: implications for the origins of hexaploid wheats. Theoretical & Applied Genetics. 2006; 112(8):1563–1572. [DOI] [PubMed] [Google Scholar]
- 31.Qiu JW, Schürch AC, Yahiaoui N, Dong LL, Fan HJ, Zhang ZJ, et al. Physical mapping and identification of a candidate for the leaf rust resistance gene lr1 of wheat. Theoretical & Applied Genetics. 2007; 115(2):159–168. [DOI] [PubMed] [Google Scholar]
- 32.Guzmán C, Caballero L, Martín LM, Alvarez JB. Waxy genes from spelt wheat: new alleles for modern wheat breeding and new phylogenetic inferences about the origin of this species. Annals of Botany. 2012; 110(6):1161–1171. 10.1093/aob/mcs201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivan S, Haynes KG, Jones RW. Assessment of linkage disequilibrium in potato genome with single nucleotide polymorphism markers. Engineering Fracture Mechanics. 2006; 173(4):2237–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speranskaya AS, Krinitsina AA, Kudryavtseva AV, Poltronieri P, Santino A, Oparina NY, et al. Impact of recombination on polymorphism of genes encoding kunitz-type protease inhibitors in the genus Solanum. Biochimie. 2012; 94(8):1687–1696. 10.1016/j.biochi.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 35.Nei M. Evolution of human races at the gene level. Progress in Clinical & Biological Research. 1982; 103(Pt A):167–181. [PubMed] [Google Scholar]
- 36.Nei M. Molecular Evolutionary Genetics. New York: Columbia University Press; 1987. [Google Scholar]
- 37.Wang SQ, Wang ZL, Guo CF. Studies on the chromosome of Saccharum spontaneum from Fujian. Sugarcane and Canesugar. 1996; 5:9–13. [Google Scholar]
- 38.Yang QH, He SC. Analysis of chromosome number and distributing of Saccharum spontaneum in province, China Sugarcane. 1996; 1: 10–13. [Google Scholar]
- 39.Sreenivasan TV. Cytogenetical studies in Saccharum spontaneum. Proceedings of the Indian Academy of Sciences-Section B. 1975; 81(3):131–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequence data have been uploaded to GenBank, their acession No. are KJ934283, KJ934287, KJ934293, KJ934276, KJ934285, KJ934288, KJ934274, KJ934280, KJ934291, KJ934290, KJ934275, KJ934294, KJ934273, KJ934284, KJ934278, KJ934281, KJ934289, KJ934279, KJ934292, KJ934282, KJ934277, KJ934286, KJ934297, KJ934296, KJ934298, KJ934300, KJ934299, KJ934295, KJ934301, KJ934334, KJ934333, KJ934332, KJ934303, KJ934304, KJ934307, KJ934306, KJ934312, KJ934311, KJ934338, KJ934337, KJ934302, KJ934340, KJ934313, KJ934343, KJ934341, KJ934346, KJ934324, KJ934325, KJ934326, KJ934347, KJ934342, KJ934316, KJ934358, KJ934353, KJ934354, KJ934355, KJ934356, KJ934357, KJ934359, KJ934360, KJ934361, KJ934352.