Abstract
The Social Environment and Biomarkers of Aging Study (SEBAS) is a nationally representative longitudinal survey of Taiwanese middle-aged and older adults. It adds the collection of biomarkers and performance assessments to the Taiwan Longitudinal Study of Aging (TLSA), a nationally representative study of adults aged 60 and over, including the institutionalized population. The TLSA began in 1989, with follow-ups approximately every 3 years; younger refresher cohorts were added in 1996 and 2003. The first wave of SEBAS, based on a sub-sample of respondents from the 1999 TLSA, was conducted in 2000. A total of 1023 respondents completed both a face-to-face home interview and, several weeks later, a hospital-based physical examination. In addition to a 12-h (7 pm–7 am) urine specimen collected the night before and a fasting blood specimen collected during the examination, trained staff measured blood pressure, height, weight and waist and hip circumferences. A second wave of SEBAS was conducted in 2006 using a similar protocol to SEBAS 2000, but with the addition of performance assessments conducted by the interviewers at the end of the home interview. Both waves of SEBAS also included measures of health status (physical, emotional, cognitive), health behaviours, social relationships and exposure to stressors. The SEBAS data, which are publicly available at [http://www.icpsr.umich.edu/icpsrweb/NACDA/studies/3792/version/5], allow researchers to explore the relationships among life challenges, the social environment and health and to examine the antecedents, correlates and consequences of change in biological measures and health.
Key Messages.
By combining self-reported information with clinical data, assays from blood and urine specimens, and performance assessments, SEBAS allows researchers to elaborate the relationships among the social environment, life challenges and health in an older population and to examine how biological markers of stress and health enhance our understanding of these relationships.
SEBAS data show that higher socioeconomic status, more contact with friends, greater participation in social activities, and religious involvement are all associated with lower physiological dysregulation and better health, but family social ties reveal little, if any, association with physiological dysregulation or cognitive function.
Findings based on SEBAS data provide only modest support for the theory of allostatic load, showing a weak association between exposure to stress and physiological dysregulation.
Biomarkers derived from blood and urine specimens, especially markers of inflammation, improve morbidity and mortality prediction compared with self-reports alone, as do interviewer-administered performance assessments (e.g. peak expiratory flow and grip strength).
Why was the cohort set up?
The social environment, encompassing position in social hierarchies as well as linkages within social networks and support systems, interacts with life challenges and stress exposure to influence physical and mental well-being. The Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan was initially developed to explore how understanding the relationships among life challenges, the social environment and health can be enhanced by examining biological markers of health and stress. Incorporating biological data into large-scale social surveys not only expands the range and depth of research topics that can be pursued, but can also enhance study findings based solely on self-reported data (which may suffer from misreporting) or on smaller clinical studies that are often not generalizable to the larger population.1
Specific aims of the initial study included: (i) investigating the extent to which biological markers of stress and chronic illness are related to reports of life events; (ii) examining the extent to which biological markers help to explain variation in health across social hierarchies and networks; and (iii) exploring the associations among the biological markers, data from physicians' examinations and self-reported health status, and their links to survival. With the addition of longitudinal data, the goals of the project have expanded to understanding the antecedents, correlates and consequences of levels and changes in biological measures, health and survival.
Who is in the cohort?
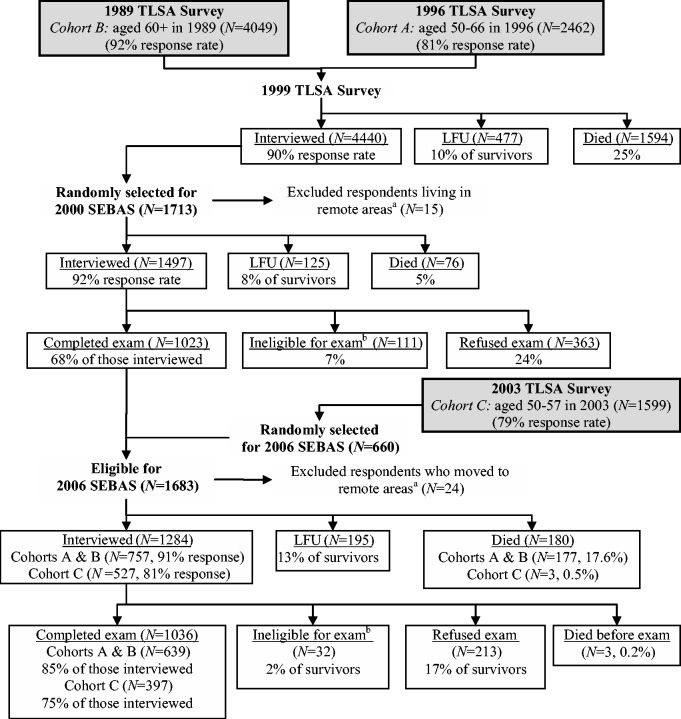
SEBAS is an extension of the Taiwan Longitudinal Study of Aging (TLSA: also called the Survey of Health and Living Status of the Near Elderly and Elderly). Here we give a brief summary of the survey design and protocol; more detailed information is provided elsewhere.2 The TLSA began in 1989 with follow-ups approximately every 3 years; younger refresher cohorts were added in 1996 and 2003. The original 1989 sample was nationally representative, including the institutionalized population, and comprised 4049 people aged 60 and older (see Figure 1).
Figure 1.
Overview of sample selection, participation, and attrition for SEBAS, 2000 and 2006. TLSA, Taiwan Longitudinal Survey of Aging (also known as the Survey of Health and Living Status of the Near–Elderly and Elderly in Taiwan); SEBAS, Social Environment and Biomarkers of Aging Study; LFU, lost-to-follow-upaA few respondents living in remote areas were excluded from the subsample because they lived too far from the hospitals contracted to do the physical examination portion of the study.bSome respondents were not asked to participate in the hospital examination due to their health condition (i.e. living in an institution, seriously ill, catheter or diaper, kidney dialysis, other health condition that precludes blood draw). Source: SEBAS User’s Guide2.
A three-stage sampling design that mirrored the original TLSA design was used to select from the respondents who were interviewed in 1999 a national sub-sample for the SEBAS 2000 cohort. Two visits were made to the SEBAS respondents’ homes between July and December 2000: one to administer a face-to-face questionnaire and the second, several weeks later, to deliver urine collection containers and make final arrangements for a hospital examination the following morning. As shown in Figure 1, 1497 persons (92% of those eligible) were interviewed in their home and 1023 (68% of those interviewed) participated in the physical examination, which was generally equivalent to an annual physical examination offered by the national health insurance programme.
Table 1 shows that respondents participating in the 2000 hospital examination were younger, more likely to be male, and better educated than those who did not participate. Compared with non-participants, examination participants were also less likely to report difficulty with any activity of daily living (ADL), which was not surprising given that respondents with serious health conditions were ineligible for the examination. The distribution by self-assessed health status suggests that respondents at both extremes were less likely to participate in the examination: those who reported being in poor/not-so-good and those who reported excellent health were under-represented among participants. As a result of these offsetting influences, the mean self-reported health status for participants and non-participants was the same (3.1 on a five-point scale where 5 = excellent, P ∼0.84 based on a t test).
Table 1.
Participation in the 2000 SEBAS and the 2006 SEBAS follow-up (unweighted %)
| Variables | 2000 SEBAS |
2006 SEBAS |
||||||
|---|---|---|---|---|---|---|---|---|
| Exam Participation |
Exam Participation |
|||||||
| Home Interview(N = 1497) | Non-Participants(N = 474) | Participants(N = 1023) | P-valuedifferencea | Home Interview(N = 1284) | Non-Participants(N = 248) | Participants(N = 1036) | P-valuedifferencea | |
| Age (years) | ||||||||
| 53–59 | 19.0 | 12.9 | 21.9 | <0.001 | 39.1 | 50.8 | 36.3 | <0.001 |
| 60–69 | 29.1 | 23.8 | 31.5 | 24.6 | 14.5 | 27.0 | ||
| 70–79 | 40.9 | 46.0 | 38.5 | 22.5 | 16.1 | 24.0 | ||
| 80+ | 11.0 | 17.3 | 8.1 | 13.8 | 18.6 | 12.6 | ||
| Female | 44.2 | 48.1 | 42.3 | 0.036 | 47.1 | 50.4 | 46.3 | 0.249 |
| Education | ||||||||
| No formal education | 35.9 | 41.4 | 33.3 | 0.011 | 20.6 | 23.4 | 19.9 | 0.279 |
| Primary education | 38.9 | 35.7 | 40.4 | 43.3 | 44.4 | 43.1 | ||
| Secondary education or higher | 25.3 | 23.0 | 26.3 | 36.1 | 32.3 | 37.1 | ||
| Mainlander (vs other ethnicitiesb) | 17.5 | 18.6 | 17.0 | 0.461 | 11.8 | 8.1 | 12.6 | 0.044 |
| Any ADLc difficulty | 9.4 | 20.0 | 4.4 | <0.001 | 9.2 | 15.7 | 7.6 | <0.001 |
| Self-assessed health status | ||||||||
| Poor | 4.6 | 7.4 | 3.5 | 0.001 | 4.6 | 7.8 | 3.8 | 0.008 |
| Not so good | 23.9 | 24.8 | 23.5 | 20.9 | 16.0 | 22.0 | ||
| Average | 45.6 | 39.7 | 48.0 | 41.4 | 41.0 | 41.5 | ||
| Good | 12.6 | 11.5 | 13.0 | 21.2 | 19.4 | 21.6 | ||
| Excellent | 13.4 | 16.7 | 12.0 | 12.1 | 16.0 | 11.2 | ||
aChi-square test for differences by whether participated in the physical examination.
bOther ethnicities include Fukienese, Hakka and Aboriginal.
cADL (Activities of Daily Living) include: bathing, dressing, eating, getting out of bed, moving about the house and using the toilet.
How often have they been followed-up?
A follow-up study was fielded between August 2006 and the end of January 2007, using a protocol similar to the 2000 SEBAS. The targeted sample included the 1023 respondents who had completed both the home interview and the health examination in 2000 as well as a refresher cohort of 660 respondents aged 53–60 in 2006 who were first interviewed in the 2003 TLSA. Thus, the 2006 SEBAS comprised a representative cross-section of the Taiwanese population aged 53 and older.
For the 2006 SEBAS, 1284 were interviewed in their homes (87% response rate; see Figure 1) and 1036 (81% of those interviewed) participated in the physical examination. Both the youngest (those aged 53–59) and oldest (80+) age groups were less likely to participate in the examination. Participants were also more likely to be Mainlanders (i.e. originally from Mainland China) and less likely to have difficulty with one or more ADLs than non-participants (Table 1). However, because of lower participation rates among both the healthiest and the least healthy individuals, the average self-reported health status among participants was very close to that of non-participants (3.2 vs 3.1 on a five-point scale where 5 = excellent, P ∼0.49 based on a t test).
Table 2 focuses on the longitudinal SEBAS sample (i.e. the 2000 study participants who were followed in 2006), showing that 757 of the examination participants were re-interviewed in 2006, 89 were lost to follow-up and 177 had died. Those who were lost to follow-up, and particularly those who had died, were older and more likely to have difficulty with ADLs than those who completed the 2006 interview. Compared with those interviewed, decedents were less educated, whereas those lost to follow-up were more educated.
Table 2.
Attrition of SEBAS 2000 examination participants (N = 1023; unweighted %)
| Variables | Interviewed (N = 757) | LFUa (N = 89) | Died before survey (N = 177) | P-value differenceb |
|---|---|---|---|---|
| Age (years) | ||||
| 54–59 | 24.7 | 28.1 | 6.8 | <0.001 |
| 60–69 | 35.8 | 28.1 | 14.7 | |
| 70–79 | 35.3 | 34.8 | 54.2 | |
| 80+ | 4.2 | 9.0 | 24.9 | |
| Female | 43.7 | 44.9 | 35.0 | 0.094 |
| Education | ||||
| No formal education | 31.6 | 30.2 | 42.4 | 0.015 |
| Primary education | 41.5 | 34.8 | 38.4 | |
| Secondary education or higher | 27.0 | 34.8 | 19.2 | |
| Mainlander (vs other ethnicitiesc) | 16.0 | 16.9 | 21.5 | 0.217 |
| Any ADL difficultyd | 2.9 | 4.5 | 10.7 | <0.001 |
aLFU, lost to follow-up
bChi-square test for differences by whether participated in the physical examination.
cOther ethnicities include Fukienese, Hakka, and Aboriginal.
dADL (activities of daily living) include: bathing, dressing, eating, getting out of bed, moving about the house and using the toilet.
What has been measured?
The SEBAS 2000 in-home interview was conducted by a local public health nurse and lasted for 1 h on average.2 In 2006, trained interviewers conducted the in-home interview and administered a new module of health assessments, which included in-home measurement of blood pressure (in addition to the measurements in hospital) and a set of performance-based tests (i.e. grip strength, lung capacity, walking speed and chair stands). Together, the 2006 in-home interview and health assessments took about 1.25 h.2
Table 3 summarizes the data collected during the home interview; Table 4 summarizes the clinical health indicators derived from the physical examination and the blood and urine samples. Comparisons of the laboratory assay results for duplicate specimens sent to the laboratory in Taiwan (Union Clinical Laboratory) indicate high intra-laboratory correlations (>= 0.9) for most markers tested, with a few notable exceptions (e.g. IGF-1, sICAM-1). Based on comparisons with results from a laboratory in the USA (Quest Diagnostics), inter-laboratory correlations were also generally high (>= 0.9), but in some cases (e.g. HbA1c, IGF-1 and urinary dopamine in 2000; urinary creatinine and urinary cortisol in 2006), the correlations were much lower (0.60–0.69); for details, see Table 2.2 in the SEBAS User’s Guide.2 Unless otherwise noted, measures were collected in both waves of SEBAS. Although most measures are self-explanatory, we highlight a few of them here. Additional information on study variables can be found at [http://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/3792?q=SEBAS&searchSource=icpsr-landing].
Table 3.
Summary of data collected in the SEBAS home interview (collected in both years unless indicated)
| Demographic data | Health behaviours |
| Agea | Relaxation practice (e.g. Tai Chi) (2006) |
| Sexa | Sleep habits (2006)c |
| Ethnicitya | Exercise |
| Marital status | Smoking statusd |
| Living arrangements | Alcohol consumptione |
| Length of time lived at current residence | Chews betel nute |
| Satisfaction with current living situation | Use of medical services |
| Employment status | Use of medicationsd |
| Socioeconomic status | Exposure to stressors / perceived stress |
| Subjective social status | Difficulty meeting living expenses |
| Educationa | Security-related stressors |
| Major life-time occupationa | Effects of 1999 earthquakes (2000) |
| Caregiver stress (2006) | |
| Social relationships | Daily hassles (2006) |
| Participation in clubs/organizations | Major life events in the past 12 months (2006) |
| Participation in other social activities (2006) | Traumatic events in lifetime (2006) |
| Number of friends/neighbours in regular contact | Perceived stress regarding various situations |
| (2006) | (health, finances, job, family members) |
| Social demands (2006) | Perceived Stress Scale, 10 items (2006)30 g |
| Physical health | Personality |
| Self-rated health status | Personal mastery, Pearlin scale31 |
| Interviewer-rated health status (2006) | Optimism (2006)f |
| Chronic conditions (ever had; current) | |
| Mobility limitations | 2006 home health assessment |
| ADL and IADL limitations | Systolic blood pressure (mmHg), 3 readings |
| Fall/injury in the past year | Diastolic blood pressure (mmHg), 3 readings |
| Chronic pain (2006)b | Grip strength (kg), 3 trials each hand |
| Gait speed (3-m walking test), 2 trials | |
| Emotional/cognitive well-being | Repeated chair stands (time to complete 5) |
| CES-D, 10-item subset | Peak expiratory flow (l/min), 3 trials |
| Cognitive function |
ADL, activities of daily living; CES-D, Center for Epidemiologic Studies Depression scale; IADL, instrumental activities of daily living.
aThese background variables were generated from the parent study (TLSA, a proprietary survey) and made available by the Bureau of Health Promotion, Taipei, Taiwan for inclusion in the SEBAS public use file.2
cSelected items from the Pittsburgh Sleep Quality Index.6
dIn 2000, these questions were asked during the hospital visit.
eThese questions were asked during the hospital visit.
fOne item from the Life Orientation Test-Revised (LOT-R).34
gAsked during the 2006 hospital exam.
Table 4.
Clinical health indicators collected in SEBAS 2000 and 2006 (both years unless indicated)
| Biological markers from fasting blood samples | Biomarkers from 12-h urine samples |
| White blood cell (WBC) count | Total volume of 12-h urine |
| WBC distributiona | Creatinine, urine |
| Red blood cell count | Free cortisol, urine |
| Haemoglobin | Norepinephrine, urine |
| Haematocrit | Epinephrine, urine |
| Mean cell volume (MCV) | Dopamine, urine |
| Mean cell haemoglobin (MCH) | |
| Mean cell haemoglobin concentration (MCHC) | Genetic markers |
| Platelet count | Apolipoprotein E (APOE) genotype |
| Total protein | Serotonin transporter gene-linked promoter |
| Albumin | region(5-HTTLPR) |
| Globulin | Telomere length (2000) |
| Aspartate aminotransferase (AST) | |
| Alanine transaminase (ALT) | Measures from physical examination |
| Total cholesterol | Anthropometry (height, weight, waist, hips) |
| High-density lipoprotein (HDL) cholesterol | Blood pressure (systolic, diastolic), 3 readings |
| Triglycerides | Pulse |
| Uric acid (mg/dl) | Abnormality of the lymph and thyroid glands, chest, heart, breasts, abdomen, limbs |
| Creatinine | Results from abdominal ultrasound |
| Blood urea nitrogen (BUN) | Visual acuity (2006) |
| Glucose | Abnormality of ear/nose/throat/oral cavity (2006) |
| Glycosylated haemoglobin (HbA1c) | Abnormality of the rectum (2006) |
| Dehydroepiandrosterone sulfate (DHEAS) | Physician-rated health status (2006) |
| Insulin-like growth factor 1 (IGF-1) | |
| Interleukin-6 (IL-6) | |
| High-sensitivity C-reactive protein (CRP) | |
| Fibrinogen (2006) | |
| Soluble E-selectin | |
| Soluble inter-cellular adhesion molecule 1 | |
| Soluble IL-6 receptor | |
| Folate | |
| Homocysteine |
aPercentage of each type of WBC including agranulocytes (i.e. lymphocytes and monocytes) and granulocytes (i.e. neutrophils, eosinophils, basophils). It is also referred to as the WBC differential.
In addition to standard measures of socioeconomic status (SES), such as education, occupational status and income, SEBAS included the MacArthur Scale of Subjective Social Status (Table 3).3 Respondents were shown a picture of a ladder with 10 rungs and told that the ladder represented where people stood in relation to each other in Taiwan, with the top of the ladder representing people who were the best off and the bottom of the ladder representing people who were the worst off. Respondents were asked to place themselves on the ladder according to where they thought they belonged in the hierarchy. The 2000 SEBAS contained a second measure that asked respondents to rank themselves in their communities rather than in Taiwan.4
As a complement to the simple self-rated health question, which is widely used and has been shown to be a good predictor of mortality, questions in the 2006 SEBAS asked the interviewer and the examining physician to assess the respondent’s health on the same five-point scale (excellent, good, average, not so good and poor). In an analysis of how well these questions predicted 5-year survival, two unexpected findings emerged: (i) physicians’ ratings were weak predictors of survival; and (ii) interviewer ratings were more powerful predictors of survival than self-ratings.5 The results suggest that including a simple question at the end of face-to-face interviews, asking interviewers to provide an assessment of the respondents’ overall health, may be a powerful and inexpensive addition to household surveys. Testing in other socio-cultural settings is needed to further evaluate the utility of such a measure.
Also in 2006, selected items from the Pittsburgh Sleep Quality Index (PSQI)6 were added to the survey. Three of the seven components of the PSQI were included in full (subjective sleep quality, sleep duration and habitual sleep efficiency). SEBAS included one of two items from each of two other components (sleep latency, daytime dysfunction). Items from the remaining two components (sleeping disturbances, use of sleeping medication) were excluded.
What has been found?
SEBAS has spurred more than 70 publications, primarily in social science, health and epidemiology journals. Analyses using the SEBAS data have covered a range of substantive and methodological questions; we highlight a few here. For a list of SEBAS-based publications, see [http://cph.georgetown.edu/taiwan.html#2].
Testing the allostatic load framework
The theory underlying allostatic load implies that the experience or perception of repeated stressful situations can cause dysregulation in multiple physiological systems which, in turn, can lead to poor health outcomes.7 Numerous SEBAS analyses have tested hypotheses based on the theory of allostatic load. In general these studies find, at most, only modest links between stress and physiological dysregulation.8–13 Thus, based on the measures of stressful experience and biomarkers typically included in large-scale biosocial surveys, SEBAS data have provided little evidence to support the allostatic load framework. Stronger relationships might have emerged if SEBAS contained more detailed measures of lifetime exposure and response to stressors or more comprehensive measures of biological risk. A comparative study between Taiwan, the USA and Russia suggests that the connection between perceived stress and physiological dysregulation is stronger in Russia than in the USA or Taiwan.14 The expected association was observed in Muscovites of both sexes, who also reported the highest levels of perceived stress among the three populations, but not in the Taiwanese, who reported the lowest levels of perceived stress. This finding raises the possibility that the adverse effects of perceived stress on biomarkers become evident only when the level of stress reaches some threshold.
Is the blood worth the toil, tears and sweat?
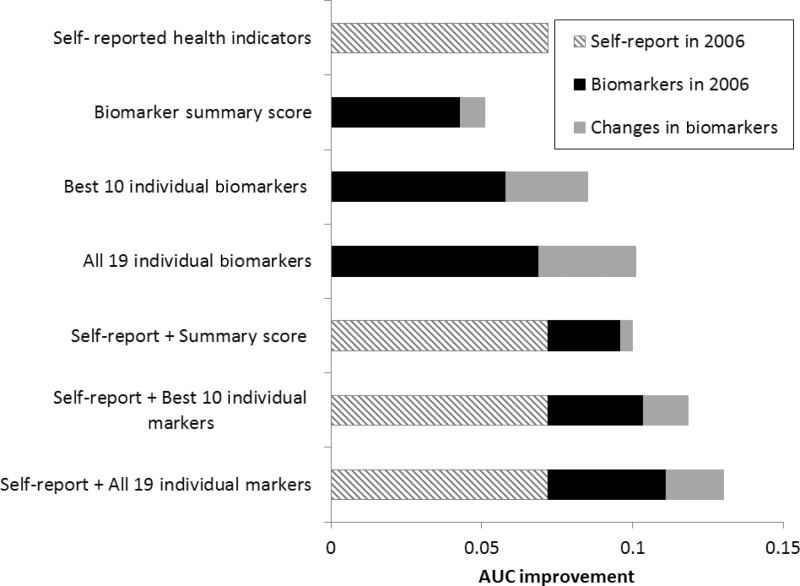
In light of the cost, complex logistics and considerable respondent burden imposed by adding biomarkers to large-scale surveys, a number of analyses using SEBAS data have explored whether biomarkers offer incremental value beyond self-reported measures, which are easier to collect and impose less respondent burden. Results demonstrate that biomarkers improve mortality prediction compared with self-reports alone, but a collection of individual markers performs better than a conventional biomarker summary score (see Figure 2).15 That study also suggests that collecting a second round of biological measures may improve mortality prediction compared with one-time measurement, although the incremental gain may be small.15 Another study shows that interviewer-administered performance assessments, particularly peak expiratory flow and grip strength, also predict mortality above and beyond self-reported physical functioning.16 Such tests are less invasive than collection of biofluids (and may be less expensive), but might be equally powerful in predicting downstream health and mortality.
Figure 2.
AUC improvements over baseline model from models predicting 5-year mortality as a function of self-reported indicators and biomarkers. In addition to age, the baseline model controls for sex, ethnicity (Mainlander vs Taiwanese), urban residence, education, social integration and perceived availability of social support. The self-reported health indicators include global self-assessed health, an index of mobility limitations, history of diabetes, history of cancer, number of hospitalizations in the past 12 months and smoking status. The biomarkers comprise eight standard cardiovascular/metabolic risk factors, four inflammatory markers, four neuroendocrine markers and three other markers that do not represent a common biological subsystem. The biomarker summary score counts the number of markers (out of 19) for which the respondent exhibits a high-risk level, which is defined by established cutoffs for the standard cardiovascular/metabolic factors and C-reactive protein (CRP) and by the high-risk quartile for the remaining markers. AUC, area under the receiver operating characteristic (ROC) curve. Source: Glei et al.15
In a related line of research, analyses of SEBAS data show that some biomarkers have greater prognostic power than others. For instance, with the exception of DHEAS, neuroendocrine markers (i.e. stress hormones) make little contribution to predicting mortality, whereas inflammatory markers yield the most prognostic value.15 These results bolster earlier findings suggesting that inflammatory markers have greater power than standard clinical (i.e. cardiovascular and metabolic) measures for predicting survival.17,18 Inflammatory markers also account for a higher proportion of sex differences in life expectancy at older ages than standard clinical markers, although no marker comes close to smoking in this regard.18
Social environment, biomarkers, and health
Additional SEBAS-based analyses show that the social environment, encompassing position in social hierarchy as well as linkages within social networks and support systems and social participation, is related to health in anticipated and unanticipated ways. For instance, contact with friends, participation in social activities and/or religious involvement are associated with lower levels of allostatic load12 and better health outcomes.19,20 However, even though the extended family system and filial piety play important roles in Taiwanese society,21 family social ties reveal little, if any, association with physiological dysregulation12 or cognitive function.19 The SEBAS data reveal weak and inconsistent associations between social relationship indicators and inflammatory markers.22
Other analyses have focused on the relationships among position in the social hierarchy, biological markers and health. These studies show the expected inverse relationship between education, income and health23 and that higher perceived social position is associated with better health, even after controlling for objective measures of SES.24,25 The use of cross-sectional data, however, may overestimate the relationship between subjective social status and health because the relationship between perceived social status and subsequent health is greatly attenuated when controlling for baseline health.25
An ongoing discussion in the literature concerns how ‘SES gets under the skin’ to affect health. In this regard, SEBAS data have been used to examine whether biological markers mediate the relationship between SES and health: several studies indicate that biomarkers have accounted for only a small part of the social disparities in health in Taiwan,23,26,27 Costa Rica27 and the USA.27 However, a similar analysis of education, biomarkers and health in Russia reveals exceptionally large social disparities in health among Muscovites; biomarkers account for a larger share of those disparities than in some countries that have been studied.28
What are the main strengths and weaknesses?
Perhaps the most notable strength of the data is the detail and breadth of the available indicators related to health, the social environment and life challenges. First, as seen in Table 4, the data contain an unusually large array of biological markers, including several genetic markers. Current work includes assays for additional genes and single nucleotide polymorphisms (SNPs); the public-use data file will be updated for these markers when the work is complete. Second, self-reported measures cover: multiple dimensions of health status (physical, emotional and cognitive); several health-related behaviours; and use of biomedical and traditional health-care facilities and providers. Third, interviewer-administered health assessments offer yet another method for evaluating the respondent’s health. Fourth, the data include high quality information for determining survival status. Finally, SEBAS includes indicators of both objective and subjective socioeconomic status, social relationships and exposure to stressors ranging from daily hassles to trauma.
Other strengths include: a relatively large, nationally representative sample; an age range that includes persons as young as 53; high participation rates; and longitudinal follow-up with low loss to follow-up. Nonetheless, the data also have limitations. As with any study of an older population, those who died at relatively early ages are not represented; yet, the proportion of the Taiwanese population dying before age 53 is small at 7.5% in 2006.29 Even so, surveying people in middle or older ages limits our ability to obtain reliable information about events that occurred much earlier in life (e.g. childhood). In addition, the subset of respondents for whom longitudinal biomarker data are available is much smaller than the sample of respondents interviewed in either wave (N = 639 participated in both the 2000 and 2006 physical examination, see Figure 1). Lastly, as with other general surveys of health and ageing, in order to obtain breadth of information, SEBAS must sacrifice depth of detail collected about any particular area. For instance, psychologists might mourn the limited data on personality, whereas others might pine for more detailed economic or contextual measures.
Can I get hold of the data? Where can I find out more?
The data and documentation are maintained and distributed by the National Archive of Computerized Data on Aging (NACDA) within the Inter-university Consortium of Political and Social Research (ICPSR) at the University of Michigan, Ann Arbor, MI, USA (persistent URL: http://www.icpsr.umich.edu/icpsrweb/NACDA/studies/3792/version/5). The dataset includes information from the: (i) 2000 SEBAS for the N = 1023 respondents who completed the home interview and the physical examination; and (ii) 2006 SEBAS for the N = 1284 respondents who completed the home interview (including clinical data for those who also participated in the examination: N = 1036). Users interested in obtaining and using these data must complete a Data Use Agreement form available by contacting ICPSR User Support (1-734-647-2200) or by downloading the form at the website noted above. Upon receipt of the data use agreement and supporting documents, a copy of the data will be sent to the primary user.
The public-use dataset includes limited data from the parent study (TLSA). Additional information about accessing the TLSA [http://www.hpa.gov.tw/english/ClassShow.aspx?No=200803270009] is available from the Surveillance and Health Research Division, Health Promotion Administration, Ministry of Health and Welfare at: 7F., No. 95, Mincyuan Rd., West District, Taichung City, 40341, Taiwan. E-mail: yuhsuanl@hpa.gov.tw
Acknowledgments
We gratefully acknowledge the hard work and dedication of the staff at the Center for Population and Health Survey Research (Bureau of Health Promotion), who were instrumental in the design and implementation of SEBAS and supervised all aspects of the fieldwork and data processing.
Funding
Funding for the SEBAS (2000 and 2006) was provided by the Behavioral and Social Research Division of the U.S. National Institute on Aging [grant numbers R01 AG16790, R01 AG16661]. The Bureau of Health Promotion (Department of Health, Taiwan) provided additional financial support for SEBAS 2000. Initial funding for the TLSA came from the Taiwan Department of Health, the Taiwan National Health Research Institute (grant number DD01-86IX-GR601S), and the Taiwan Provincial Government.
Conflict of interest: None declared.
References
- 1.Weinstein M, Willis R. Stretching social surveys to include bioindicators: possibilities for the Health and Retirement Study, experience from the Taiwan Study of the Elderly. In: Finch CE, Vaupel JW, Kinsella K, (eds). Cells and Surveys: Should Biological Measures Be Included in Social Science Research? Washington, DC: National Academy Press, 2000. [Google Scholar]
- 2.Chang M, Lin H, Chuang Y, et al. Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan, 2000 and 2006. Main documentation for SEBAS longitudinal public-use data (released 2012). Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2012. [Google Scholar]
- 3.Adler NE, Stewart J, in collaboration with the Psychosocial Working Group. The MacArthur Scale of Subjective Social Status. 2007. http://macses.ucsf.edu/Research/Psychosocial/subjective.php (31 March 2014, date last accessed). [Google Scholar]
- 4.Goldman N, Cornman JC, Chang MC. Measuring subjective social status: a case study of older Taiwanese. J Cross Cult Gerontol 2006;21:71–89. [DOI] [PubMed] [Google Scholar]
- 5.Todd MA, Goldman N. Do interviewer and physician health ratings predict mortality? A comparison with self-rated health. Epidemiology 2013;24:913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Int Med 1993;153:2093–101. [PubMed] [Google Scholar]
- 8.Gersten O. Neuroendocrine biomarkers, social relations, and the cumulative costs of stress in Taiwan. Soc Sci Med 2008;66:507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glei DA, Goldman N, Chuang YL, Weinstein M. Do chronic stressors lead to physiological dysregulation? Testing the theory of allostatic load. Psychosom Med 2007;69:769–76. [DOI] [PubMed] [Google Scholar]
- 10.Glei DA, Goldman N, Wu CH, Weinstein M. Does exposure to stressors predict changes in physiological dysregulation? Ann Behav Med 2013;46:121–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman N, Glei DA, Seplaki C, Liu IW, Weinstein M. Perceived stress and physiological dysregulation in older adults. Stress 2005;8:95–105. [DOI] [PubMed] [Google Scholar]
- 12.Seeman T, Glei D, Goldman N, Weinstein M, Singer B, Lin YH. Social relationships and allostatic load in Taiwanese elderly and near elderly. Soc Sci Med 2004;59:2245–57. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein M, Goldman N, Hedley A, Yu-Hsuan L, Seeman T. Social linkages to biological markers of health among the elderly. J Biosoc Sci 2003;35:433–53. [DOI] [PubMed] [Google Scholar]
- 14.Glei DA, Goldman N, Shkolnikov VM, et al. Perceived stress and biological risk: is the link stronger in Russians than in Taiwanese and Americans? Stress 2013;16:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glei DA, Goldman N, Rodríguez G, Weinstein M. Beyond self reports: changes in biomarkers as predictors of mortality. Popul Dev Rev 2014;40:331–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman N, Glei DA, Rosero-Bixby L, Chiou S, Weinstein M. Self-reported versus performance-based measures of physical function: prognostic value for survival. Demogr Res 2014;30:227–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman N, Turra CM, Glei DA, Seplaki CL, Lin YH, Weinstein M. Predicting mortality from clinical and nonclinical biomarkers. J Gerontol A Biol Sci Med Sci 2006;61:1070–74. [DOI] [PubMed] [Google Scholar]
- 18.Goldman N, Glei DA, Lin YH, Weinstein M. Improving mortality prediction using biosocial surveys. Am J Epidemiol 2009;169:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glei DA, Landau DA, Goldman N, Chuang YL, Rodriguez G, Weinstein M. Participating in social activities helps preserve cognitive function: an analysis of a longitudinal, population-based study of the elderly. Int J Epidemiol 2005;34:864–71. [DOI] [PubMed] [Google Scholar]
- 20.Yeager DM, Glei DA, Au M, Lin HS, Sloan RP, Weinstein M. Religious involvement and health outcomes among older persons in Taiwan. Soc Sci Med 2006;63:2228–41. [DOI] [PubMed] [Google Scholar]
- 21.Fricke T, Chang JS, Yang LS. Historical and ethnographic perspectives on the Chinese family. In: Thornton A, Lin HS. (eds). Social Change and the Family in Taiwan . Chicago, IL: University of Chicago Press, 1994. [Google Scholar]
- 22.Glei DA, Goldman N, Ryff CD, Lin YH, Weinstein M. Social relationships and inflammatory markers: an analysis of Taiwan and the U.S. Soc Sci Med 2012;74:1891–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu P, Wagle N, Goldman N, Weinstein M, Seeman TE. The associations between socioeconomic status, allostatic load and measures of health in older Taiwanese persons: Taiwan Social Environment and Biomarkers of Aging Study. J Biosoc Sci 2007;39:545–56. [DOI] [PubMed] [Google Scholar]
- 24.Hu P, Adler NE, Goldman N, Weinstein M, Seeman TE. Relationship between subjective social status and measures of health in older Taiwanese persons. J Am Geriatr Soc 2005;53:483–88. [DOI] [PubMed] [Google Scholar]
- 25.Collins AL, Goldman N. Perceived social position and health in older adults in Taiwan. Soc Sci Med 2008;66:536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowd JB, Goldman N. Do biomarkers of stress mediate the relation between socioeconomic status and health? J Epidemiol Community Health 2006;60:633–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman N, Turra CM, Rosero-Bixby L, Weir D, Crimmins E. Do biological measures mediate the relationship between education and health: A comparative study. Soc Sci Med 2011;72:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glei DA, Goldman N, Shkolnikov VM, et al. To what extent do biomarkers account for the large social disparities in health in Moscow? Soc Sci Med 2013;77:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.University of California, Berkeley (USA), Max Planck Institute for Demographic Research (Germany). Human Mortality Database 2006. www.mortality.org (2 April 2014, date last accessed). [Google Scholar]
- 30.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S. (eds). The Social Psychology of Health; Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage, 1988. [Google Scholar]
- 31.Pearlin LI, Lieberman EG, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav 1981;22:337–56. [PubMed] [Google Scholar]
- 32.Caraceni A, Cherny N, Fainsinger R, et al. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage 2002;23:239–55. [DOI] [PubMed] [Google Scholar]
- 33.Wang XS, Mendoza TR, Gao SZ, Cleeland CS. The Chinese version of the Brief Pain Inventory (BPI-C): its development and use in a study of cancer pain. Pain 1996;67:407–16. [DOI] [PubMed] [Google Scholar]
- 34.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol 1994;67:1063–78. [DOI] [PubMed] [Google Scholar]